Introduction
Metastatic cancer of the bone marrow develops when
malignant tumor cells of non-hematopoietic systems metastasize to
the bone marrow by means of hematogenous dissemination or direct
invasion. Micrometastasis in the bone marrow is detected in 30–40%
of breast cancer patients (1,2) and
this metastasis is manifested as the progressive aggravation of
anemia in the short term, thrombocytopenia and a significantly
declined performance status in the majority of patients. Notably,
the median survival of patients with metastatic cancer of the bone
marrow presenting with thrombocytopenia is only one month and
chemotherapy is usually ineffective (3). The presence of micrometastasis in the
bone marrow and its effect on prognosis has been shown in patients
with identical stages of breast cancer, as defined by tumor size,
histological grade, the presence or absence of lymph node
metastasis and the expression of hormone receptors (4,5).
However, the clinical treatment of such micrometastasis is limited
due to the low statistical power of published studies and lack of
clinical trials (6). The bone
marrow metastasis of breast cancer is common and the current study
presents a case of bone marrow metastasis of breast cancer whose
peripheral blood cell counts completely returned to within the
normal ranges following monotherapy with trastuzumab. This study
was approved by the Ethical Committee of the General Hospital of
Shenyang Military Region (Shenyang, China) and the patient provided
written informed consent.
Case report
The current study presents the case of a 41-year-old
female who underwent modified radical mastectomy due to left breast
infiltrating ductal cancer (pathological stage, IIA; pT1aN1M0) on
May 30, 2010 at the General Hospital of Shenyang Military Region
(Shenyang, China). The patient was negative for estrogen and
progesterone receptors. In addition, the human epidermal growth
factor 2 (HER-2) immunohistochemical analysis showed ++/+++
staining and fluorescence in situ hybridization (FISH)
indicated HER-2 gene amplification. Following the surgery, six
cycles of adjuvant chemotherapy (docetaxel plus epirubicin regimen)
in combination with sequential adjuvant radiotherapy were
conducted. In May 2011, the patient felt pain at multiple sites on
the body and subsequent positron emission tomography/computed
tomography (CT) examination indicated the following: Left cervical,
left supraclavicular, mediastinal, retroperitoneal and pelvic lymph
node metastases; superior lobe metastasis of the left lung;
peritoneal metastasis; multiple osseous metastases; and tumors in
the right appendix region. The patient had an Eastern Cooperative
Oncology Group (ECOG) performance status score of 3 and numeric
rating scale (NRS) pain score of 8. The results of the routine
blood test showed that the lowest peripheral blood cell counts were
1.6×109 cells/l for white blood cells (WBCs), 54 g/l for
hemoglobin (Hb) and 26×109 cells/l for platelets (PLTs).
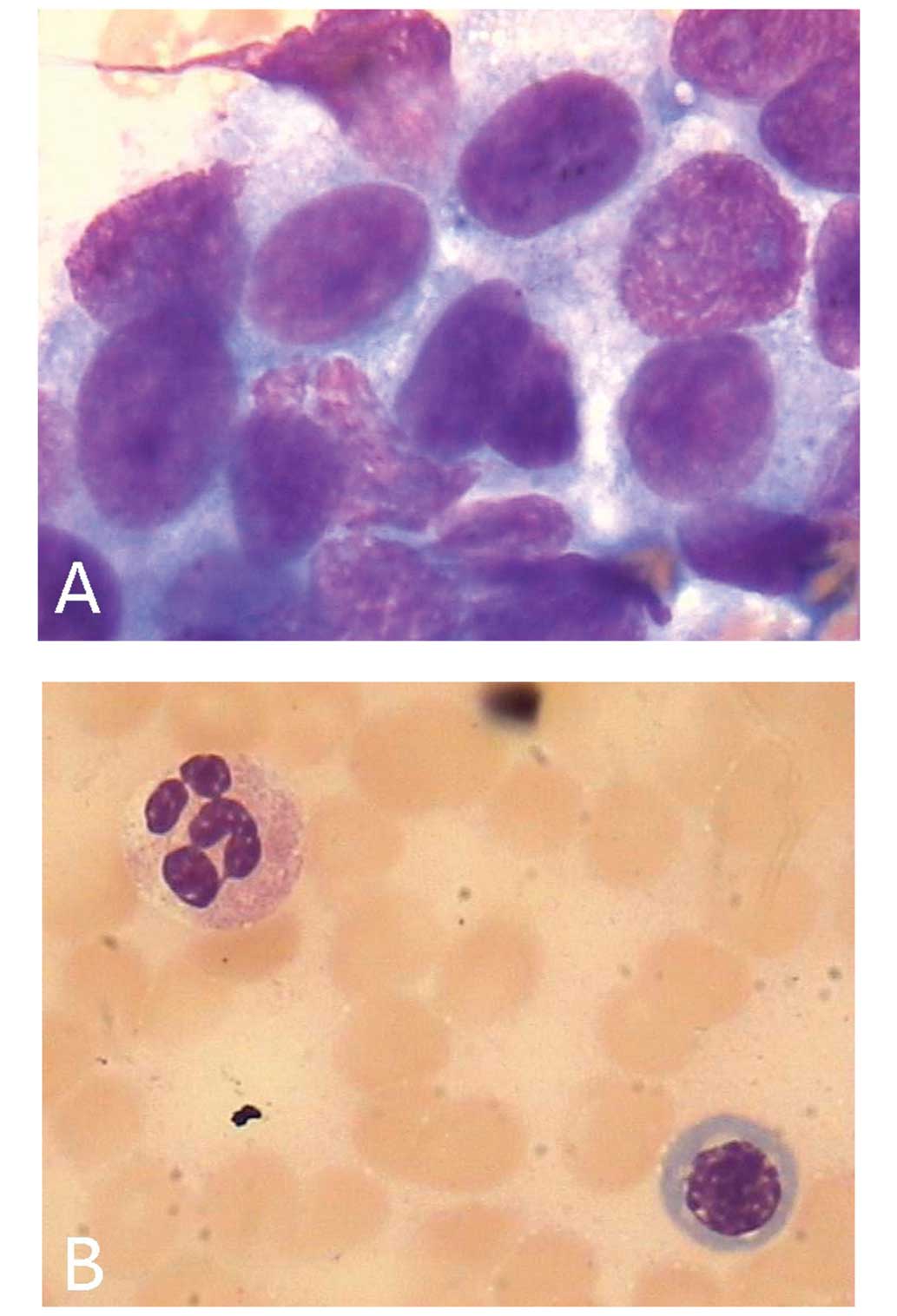
In addition, a bone marrow smear test revealed the presence of
metastatic cancer cells (Fig. 1A).
Trastuzumab monotherapy was subsequently initiated on June 1, 2011,
at a loading dose of 8 mg/kg and a maintenance dose of 6 mg/kg
every three weeks. During the night following administration of the
initial dose of trastuzumab, the patient experienced fever (body
temperature of 39.0°C) and more severe pain, which were alleviated
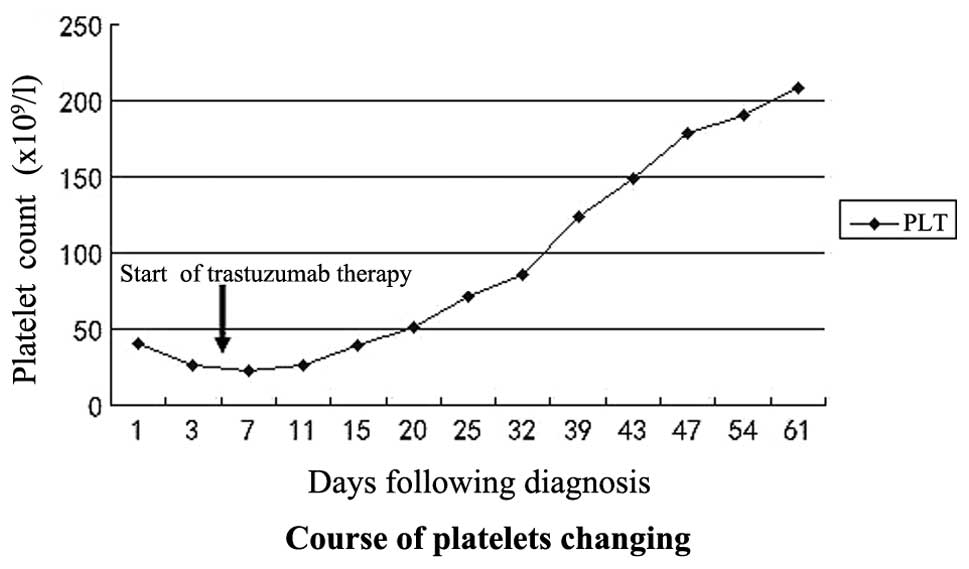
by symptomatic treatment. Following trastuzumab treatment, the
patient’s PLT count markedly increased (Fig. 2), while the WBC count and Hb
concentration increased gradually. Furthermore, following four
doses of trastuzumab treatment, the patient’s performance status
score (ECOG) decreased to 1 and pain score (NRS) decreased to 2. On
August 17, 2011, taxol chemotherapy was initiated at a dose of 75
mg/m2 on days two and nine, and was administered on the
basis of trastuzumab. In total, seven cycles, each lasting 21 days,
were completed. No cancer cells were identified in the subsequent
bone marrow smear test (Fig. 1B).
CT demonstrated metastatic foci in the left lung, however, the
enlarged lymph nodes had subsided, and the tumor in the right
appendix region had decreased in size by 50%. In April 2012, the
trastuzumab treatment was withdrawn due to brain metastasis and in
May 2012, the patient succumbed to disease progression.
Discussion
Bone marrow micrometastasis has already developed in
almost one-third of patients with breast cancer at presentation.
Furthermore, large tumor sizes, poor differentiation, lymph node
metastasis and negative hormone receptors are risk factors for bone
marrow metastasis (1). Severe bone
marrow metastasis leads to severe bone marrow suppression, which
restricts the efficacy of cytotoxic drugs in such patients. Few
individual cases of bone marrow metastasis of breast cancer have
been reported (7–10) and the clinical use of cytotoxic
drugs continue to pose considerable risks.
Trastuzumab is a humanized monoclonal antibody which
is directed against the extracellular domain of the HER-2 gene
(11). Combined chemotherapy with
trastuzumab has become a standard treatment for
HER-2-overexpressing breast cancer (12–14).
Despite the lack of high-level medical evidence supporting
trastuzumab monotherapy as a treatment for metastatic breast
cancer, significant clinical benefits have been highlighted in a
phase II clinical study in the treatment of advanced breast cancer
with HER-2 gene immunohistochemical scores of >3 or positive
FISH tests (15). Furthermore,
Tsutani et al (16) reported
one case of complete remission in a patient with lung metastasis of
breast cancer treated with trastuzumab alone. The individual in the
present case was a patient with HER-2-overexpressing breast cancer,
who developed bone marrow metastasis complicated by severe bone
marrow suppression one year following surgery. Such patients are
not sensitive to endocrine therapy and chemotherapy poses great
risks due to poor performance status and severe bone marrow
suppression. The tentative administration of trastuzumab
monotherapy in the present case was found to improve the patient’s
disease condition gradually and provided the patient with the
opportunity to accept combined chemotherapy, which was likely to
significantly prolong survival and improve the patient’s quality of
life.
Trastuzumab treatment is selected on the premise of
HER-2 gene amplification or overexpression. In the current case,
immunohistochemical or FISH tests were impractical due to the small
quantity of malignant cells identified in the bone marrow smears.
Therefore, it was not possible to directly evaluate the HER-2 gene
amplification in the bone marrow metastatic foci. This is a fairly
common problem in clinical practice, however, in this circumstance,
the trial use of trastuzumab was essentially the only available
treatment with the prospect of effectively controlling the tumor
progression. We considered an evaluation of the rationality of
trastuzumab treatment to be essential prior to administration. A
meta-analysis showed that the HER-2 gene inconsistency rate between
the metastatic and primary foci of breast cancer was ~5.54%
(17). According to this result, it
is reasonable to guide treatment based on the HER-2 test results of
primary foci when the HER-2 gene amplification of the metastatic
foci cannot be obtained.
In conclusion, when sufficient clinical evidence for
HER-2 gene amplification is available, trastuzumab may be
considered as a beneficial treatment option for breast cancer
patients with metastases in whom chemotherapy and endocrine therapy
are not suitable.
References
|
1
|
Braun S, Vogl FD, Naume B, et al: A pooled
analysis of bone marrow micrometastasis in breast cancer. N Engl J
Med. 353:793–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Janni W, Rack B, Kasprowicz N, Scholz C
and Hepp P: DTCs in breast cancer: clinical research and practice.
Recent Results Cancer Res. 195:173–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kiliçkap S, Erman M, Dinçer M, Aksoy S,
Harputluoğlu H and Yalçin Y: Bone marrow metastasis of solid
tumors: Clinicopathological evaluation of 73 cases. Turk J Cancer.
37:85–88. 2007.
|
|
4
|
Yovtchev YP, Minkov GA, Petrov AT, Nikolov
SS and Vlaykova TI: Epithelial cells expressing cytokeratins-19 and
bone marrow micrometastases in patients with breast cancer at the
time of primary surgery: clinical outcome during long-term
follow-up. Breast Cancer. Oct 29–2012.(Epub ahead of print).
|
|
5
|
Singletary SE, Allred C, Ashley P, et al:
Revision of the American Joint Committee on Cancer staging system
for breast cancer. J Clin Oncol. 20:3628–3636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldhirsch A, Glick JH, Gelber RD, Coates
AS and Senn HJ: Meeting highlights: International Consensus Panel
on the Treatment of Primary Breast Cancer. Seventh International
Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin
Oncol. 19:3817–3827. 2001.
|
|
7
|
Bjelic-Radisic V, Stöger H, Winter R,
Beham-Schmid C and Petru E: Long-term control of bone marrow
carcinosis and severe thrombocytopenia with standard-dose
chemotherapy in a breast cancer patient: a case report. Anticancer
Res. 26:1627–1630. 2006.PubMed/NCBI
|
|
8
|
Ballot J, McDonnell D and Crown J:
Successful treatment of thrombocytopenia due to marrow metastases
of breast cancer with weekly docetaxel. J Natl Cancer Inst.
95:831–832. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodriguez-Kraul R, Hortobagyi GN, Buzdar
AU and Blumenschein GR: Combination chemotherapy for breast cancer
metastatic to bone marrow. Cancer. 48:227–232. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dady PJ, Gazet JC, Ford HT and Powles TJ:
Combination chemotherapy for thrombocytopenia with bone marrow
metastases from breast cancer. Br Med J. 1:5541977. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hudziak RM, Lewis GD, Winget M, Fendly BM,
Shepard HM and Ullrich A: p185HER2 monoclonal antibody has
antiproliferative effects in vitro and sensitizes human breast
tumor cells to tumor necrosis factor. Mol Cell Biol. 9:1165–1172.
1989.PubMed/NCBI
|
|
12
|
Smith I, Procter M, Gelber RD, et al:
2-year follow-up of trastuzumab after adjuvant chemotherapy in
HER2-positive breast cancer: a randomised controlled trial. Lancet.
369:29–36. 2007.PubMed/NCBI
|
|
13
|
Marty M, Cognetti F, Maraninchi D, et al:
Randomized phase II trial of the efficacy and safety of trastuzumab
combined with docetaxel in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer administered as
first-line treatment: the M77001 study group. J Clin Oncol.
23:4265–4274. 2005. View Article : Google Scholar
|
|
14
|
Slamon DJ, Leyland-Jones B, Shak S, et al:
Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vogel CL, Cobleigh MA, Tripathy D, et al:
Efficacy and safety of trastuzumab as a single agent in first-line
treatment of HER2-overexpressing metastatic breast cancer. J Clin
Oncol. 20:719–726. 2002. View Article : Google Scholar
|
|
16
|
Tsutani Y, Ohsumi S, Aogi K, et al: A case
of metastatic breast cancer with HER2 gene amplification that
responded completely to single agent trastuzumab. Breast Cancer.
13:374–377. 2006. View Article : Google Scholar
|
|
17
|
Houssami N, Macaskill P, Balleine RL,
Bilous M and Pegram MD: HER2 discordance between primary breast
cancer and its paired metastasis: tumor biology or test artefact?
Insights through meta-analysis. Breast Cancer Res Treat.
129:659–674. 2011. View Article : Google Scholar : PubMed/NCBI
|