Introduction
Head and neck (HN) squamous cell carcinoma (SCC) is
the sixth most common type of malignancy worldwide accounting for
~39,000 new cases in the United States annually (1). Early-stage cancer frequently manifests
with minimal or no clinical findings and symptoms, resulting in a
delayed diagnosis and poor survival rate. Approximately 40% of
patients with HNSCC present with early stage disease, and either
surgical resection or radiotherapy is recommended as the single
treatment modality. The majority of patients (60%) present with a
locally advanced disease (2), which
requires a multidisciplinary approach, combining surgery,
radiotherapy and chemotherapy. Significant additional concerns are
second primary tumors (synchronous or later occurring) and distant
metastases. Second primary tumors in HNSCC have an incidence rate
of 6–20% (3).
The identification of reliable tumor markers for
HNSCC is expected to improve the diagnosis and prognosis of
patients. Cytogenetic and immunohistochemical analyses have
revealed that the overall prognosis in HNSCC patients is correlated
with DNA aneuploidy and Ki-67 score (4). Furthermore, human papillomavirus,
epidermal growth factor receptor and the mutation status of p53
have been shown to have prognostic value (5). However, currently the only molecular
marker that has been established for use in the clinical setting is
the SCC antigen (6). Therefore,
there is an urgent requirement for novel prognostic markers to
guide therapeutic decision-making and improve patient outcome.
The functional role of fascin remains unclear,
although experimental data indicates a role in cell motility and
the detachment of tumor cells (7).
Fascin is involved in the cross-binding of actin bundles to form
membrane protrusions and, thus, is significant in cell motility and
the migratory changes in carcinogenesis (7). Fascin appears to provide cancer cells
with an efficient mechanism to assemble stable long-living invasive
protrusions, which allow tumor invasion into the extracellular
matrix and disrupt epithelial junctions (8).
Fascin has emerged as an interesting potential
biomarker due to its low or absent expression in the majority of
normal adult epithelia; colonic (9), breast (10), ovarian (11), stomach (12), pancreas (13), oral cavity (14,15),
oropharynx (15), nasopharynx
(16) and larynx (17), yet fascin upregulation has been
reported in all types of human carcinoma that has been studied to
date (17,18). Consistently, primary carcinomas,
with high levels of fascin, correlate with a clinically more
aggressive disease and poor prognosis (19). However, the number of available
studies on fascin expression in upper aerodigestive tract cancers
is limited (14–17) and no comparative analysis of the
expression in HNSCC-associated macroscopically-normal tissue and
HNSCC-metastases has been performed.
In the present prospective study, fascin was
analyzed to evaluate its potential as a clinically relevant
biomarker in HNSCC.
Patients and methods
Patients and tissue specimens
A total of 25 primary tumors collected from 25 adult
patients (males, n=22; females, n=3) with histologically confirmed
HNSCC were included in this explorative prospective study. The
patients underwent surgery between 2004 and 2009 at the Department
of Otorhinolaryngology, in the Head and Neck Surgery of a tertiary
referral center (University Medical Center of the Johannes
Gutenberg University Mainz, Mainz, Germany). The exclusion criteria
were as follows: Recurrent HNSCC tumors at the same site; second
primary tumors in the presence of previous HNSCC in neighboring
sites; and cases of in situ carcinoma. The median follow-up
time was 48.8 months. Regarding the tumor samples, a particular
specimen slide was selected based on whether the transition area
(invasion front), between the tumor and healthy tumor-adjacent
epithelial tissues, could be observed, however, the two could be
clearly differentiated from each other.
The present study was reviewed and approved by the
Institutional Review Board of the University Medical Center of
Johannes Gutenberg University Mainz (Mainz, Germany) and performed
in accordance with the Declaration of Helsinki. Written informed
consent was obtained from all of the patients.
Immunohistochemistry
Immunohistochemical analysis of formalin-fixed,
paraffin-embedded specimens was performed using standard procedures
(20). Heat-induced antigen
retrieval was performed, using microwave treatment (3×5 min each;
600 W in 10 mM citrate buffer, pH 6.0), on all of the slides
following dewaxing and rehydration; blocking of endogenous
peroxidase with 3% H2O2 methanol was
subsequently performed. Following pre-incubation with 10% normal
serum in 2% bovine serum albumin (BSA)/phosphate-buffered saline
(PBS) for 20 min (to avoid unspecific binding), primary antibodies
were incubated overnight at 4°C. A monoclonal antibody raised in
mice against the epitope fascin (1:50; Dako Deutschland GmbH,
Hamburg, Germany) was used. The slides were incubated with a
biotinylated secondary antibody (1:100; DAKO Deutschland GmbH),
streptavidin peroxidase (1:100; Dianova GmbH, Hamburg, Germany) and
3,3′-diaminobenzidine/H2O2 (1.85 mM). All of
the washing procedures were performed in PBS and dilutions of
antibodies were prepared in 2% BSA/PBS at room temperature. The
slides were counterstained with hematoxylin and eosin. The primary
antibody was substituted with PBS and served as the negative
control, and Hodgkin lymphoma tissue was used for positive control
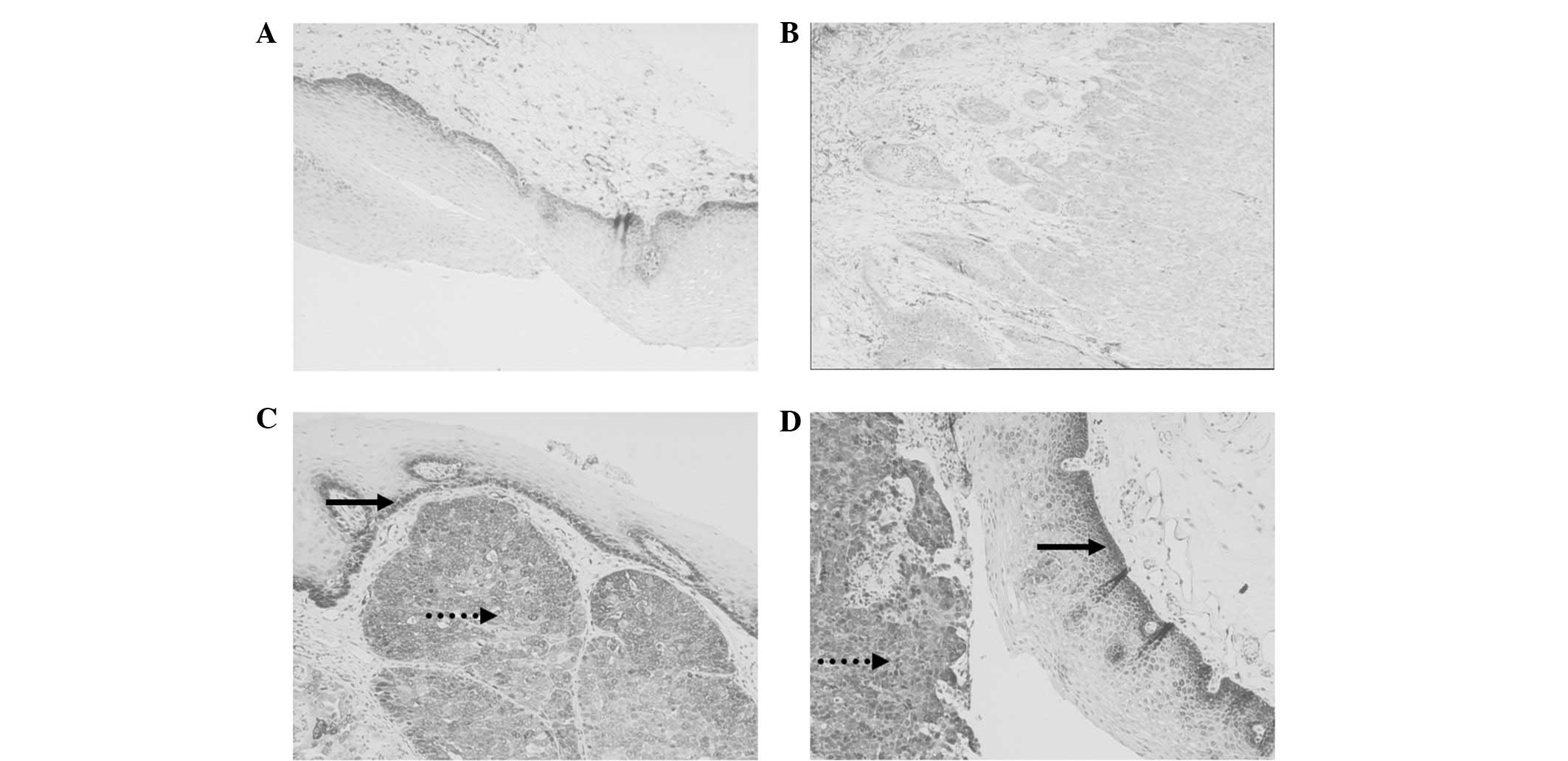
staining (Fig. 1A). The staining
reaction was quantified using a scoring system that was modified
according to Bittinger et al (21). Immunostained specimens were
independently examined by two investigators and, in the instance of
a discrepancy, by a third individual; the investigators were
blinded to the origin of the specimen. Briefly, specimens were
scored according to the intensity of staining (0, none to weak; 1,
weak; 2, moderate; 3, strong) and the percentage of tumor cells
stained (0, 0–24% positive; 1, 25–50% positive; 2, 51–80% positive;
3, >81% positive), or the cell layers of the healthy epithelial
tissue specimens that were stained (0, negative; 1, only basal
cells; 2, all the cells beyond superficial cells; 3, all the
cells). The scores for the intensity of staining and the percentage
of stained cells were summated to yield an integrated staining
score. The four groups were compared for statistical analysis.
Specimens with an integrated score of 0, 1–2, 3–4 and 5–6 were
included in groups 0, 1, 2 and 3, respectively.
Statistical analysis
The association between categorical variables was
analyzed using contingency tables and Fisher’s exact tests
(two-sided). P≤0.05 was considered to indicate a statistically
significant difference and the P-values were considered to be
descriptive as they were not adjusted for multiplicity. Survival
(overall and event-free) is described by Kaplan-Meier estimates and
the statistical analysis was performed using SAS 9.2 (SAS Institute
Inc., Cary, NC, USA) and SPSS 15.0 (SPSS Inc., Chicago, IL,
USA).
The present study aimed to determine whether fascin
expression in tumors or healthy epithelia is a predictor of
survival by investigating overall, relapse-free and event-free
survival. In the first case, only mortalities were considered to be
events, in the second case only relapses were considered and in the
third case, mortalities, relapses and second primary tumors were
considered to be events. In addition, gender, pathological (p)
tumor-node-metastasis (TNM), tumor site, smoking, alcohol,
chemotherapy, radiation therapy and tumor grade were examined as
further potential explanatory variables. The possible predictors
were assessed separately by computing Kaplan-Meier estimates for
each stratum and compared survival with strata using the log-rank
test.
In order to assess whether one type of tissue
exhibited systematically higher fascin expression compared with
another, a sign test was performed comparing tumor tissue, cervical
lymph node metastases and healthy tissue.
Results
Clinical data
In total, tissue samples from 25 patients were
analyzed, including tumor samples from 23 patients, healthy
epithelial tissue samples from 20 patients and cervical lymph node
metastases from eight patients. The median age was 62 years (range,
39–77 years). Tobacco consumption and alcohol abuse history were
positive in 20 and 17 patients, respectively. The patients with IDs
1, 6, 5 and 13 had a HNSCC of the paranasal sinuses, larynx,
hypopharynx and oropharynx, respectively. Only one of the 25
patients developed a second primary tumor and four patients
experienced recurrence (Table
I).
 | Table IClinical and histological data of
patients. |
Table I
Clinical and histological data of
patients.
| | | | Integrated score
groups 0–3 | |
|---|
| | | |
| |
|---|
| ID | Age at first surgery
(years) | Gender | pTNM | Fascin (tumor) | Fascin (healthy
epithelium) | Fascin (cervical
lymph node metastasis) | Recurrence |
|---|
| 1 | 52 | F | T2N1M0 | n.a. | n.a. | 3 | No |
| 2 | 50 | M | T1N2M0 | 3 | 1 | n.a. | Yes |
| 3 | 58 | M | T1N2M1 | 3 | n.a. | 2 | No |
| 4 | 63 | F | T3N1M1 | 2 | 2 | 3 | No |
| 5 | 39 | F | T2N2M0 | 2 | n.a. | n.a. | No |
| 6 | 65 | M | T1N2M0 | 3 | 1 | n.a. | No |
| 7 | 73 | M | T3N0M0 | 3 | 1 | n.a. | No |
| 8 | 52 | M | T3N2M0 | 2 | 2 | n.a. | Yes |
| 9 | 62 | M | T4N2M0 | 3 | n.a. | n.a. | No |
| 10 | 53 | M | T3N0M0 | 2 | 2 | n.a. | No |
| 11 | 61 | M | T1N0M0 | 2 | 2 | n.a. | Yes |
| 12 | 65 | M | T2N2M0 | 3 | 2 | 3 | No |
| 13 | 63 | M | T1N1M0 | 2 | 1 | 3 | No |
| 14 | 68 | M | T1N2M0 | 3 | 1 | 3 | No |
| 15 | 77 | M | T4N0M0 | 2 | n.a. | n.a. | No |
| 16 | 49 | M | T2N2M0 | 3 | 1 | 2 | No |
| 17 | 76 | M | T1N2M0 | 2 | 2 | 3 | No |
| 18 | 69 | M | T4N2M1 | 3 | 1 | n.a. | No |
| 19 | 65 | M | T2N2M0 | 2 | 2 | n.a. | No |
| 20 | 45 | M | T2N1M0 | 2 | 1 | n.a. | No |
| 21 | 45 | M | T2N0M0 | 2 | 1 | n.a. | No |
| 22 | 53 | M | T2N0M0 | 2 | 1 | n.a. | Yes |
| 23 | 46 | M | T2N0M0 | 1 | 2 | n.a. | No |
| 24 | 64 | M | T3N0M0 | n.a. | 1 | n.a. | No |
| 25 | 71 | M | T1N2M0 | 3 | 1 | n.a. | No |
Immunohistochemical analysis of fascin
expression and survival
The specimens from healthy tumor-adjacent epithelial
tissue, tumor tissues and cervical lymph node metastases tested
positive for fascin. Increased fascin expression levels were found
in tumor tissue and cervical lymph node metastases samples when
compared with the expression levels in the healthy epithelial
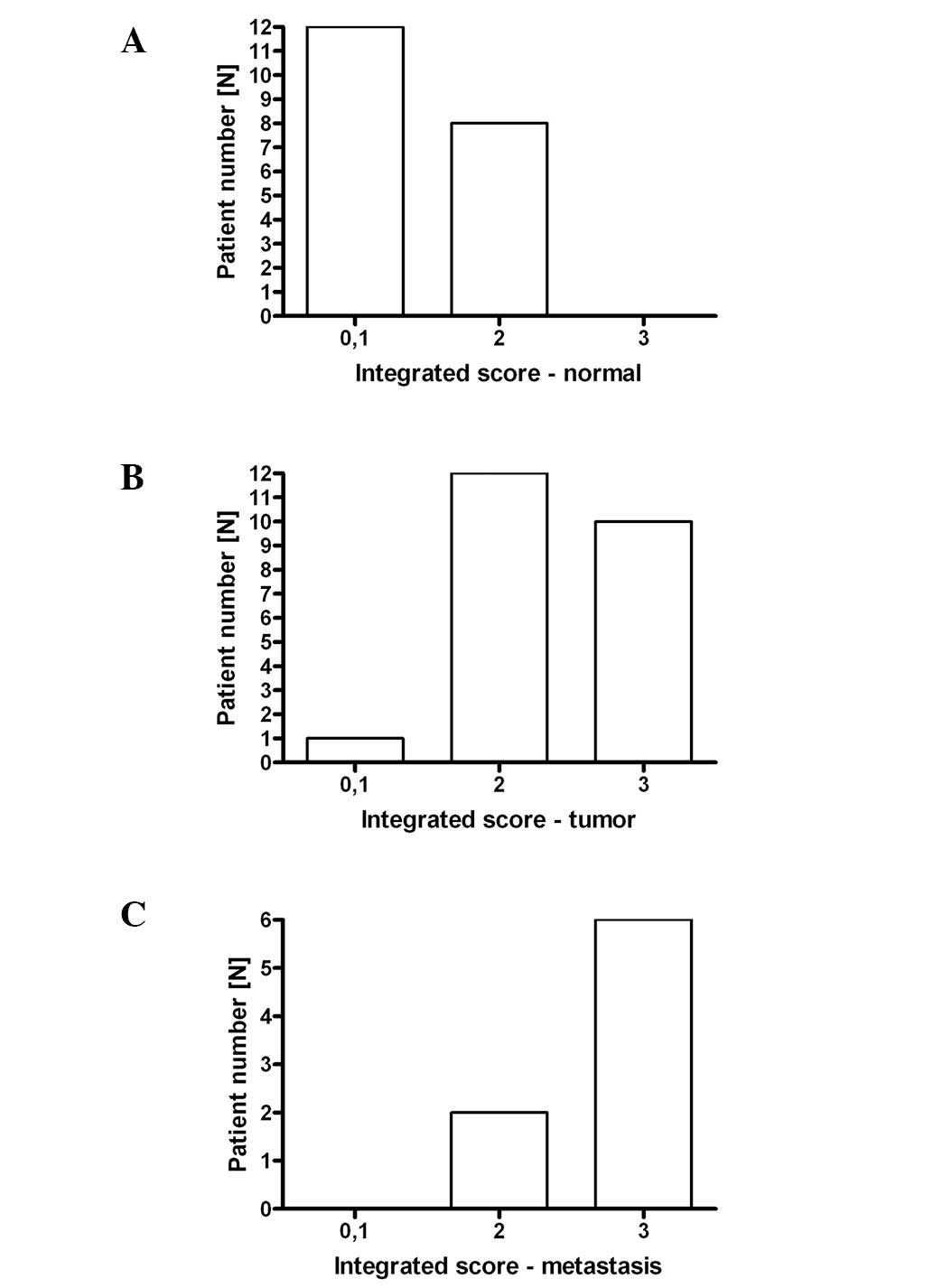
tissue. From the 20 available healthy epithelial tissue specimens,
12 had an integrated score of 1–2 (group 1) and eight of 3–4 (group
2; Fig. 2A). From the 23 available
tumor specimens, one had an integrated score of 1–2 (group 1), 12
of 3–4 (group 2) and 10 of 5–6 (group 3; Fig. 2B). Among the eight available
cervical lymph node metastases, two (one of which is depicted in
Fig. 1B) had an integrated score of
3–4 (group 2) and six of 5–6 (group 3; Fig. 2C). The expression levels of fascin
were significantly increased in the tumor tissue (P=0.03) and lymph
node regional metastasis (P=0.03) compared with the normal tissue,
as detected via the sign test, while there was no systematic
difference (P=1.00) when comparing between the tumor tissue and
lymph node metastases. In addition, increased fascin expression was
observed at the invasion front of the tumor in all of the samples
(Fig. 1C and D).
The fascin expression levels in the tumor tissues
were not associated with pT (P=0.56) or pM stage (P=0.63), smoking
status (P=1.00), tumor grade (P=1.00), alcohol consumption
(P=0.18), gender (P=0.53) or tumor localization (P=0.07), however,
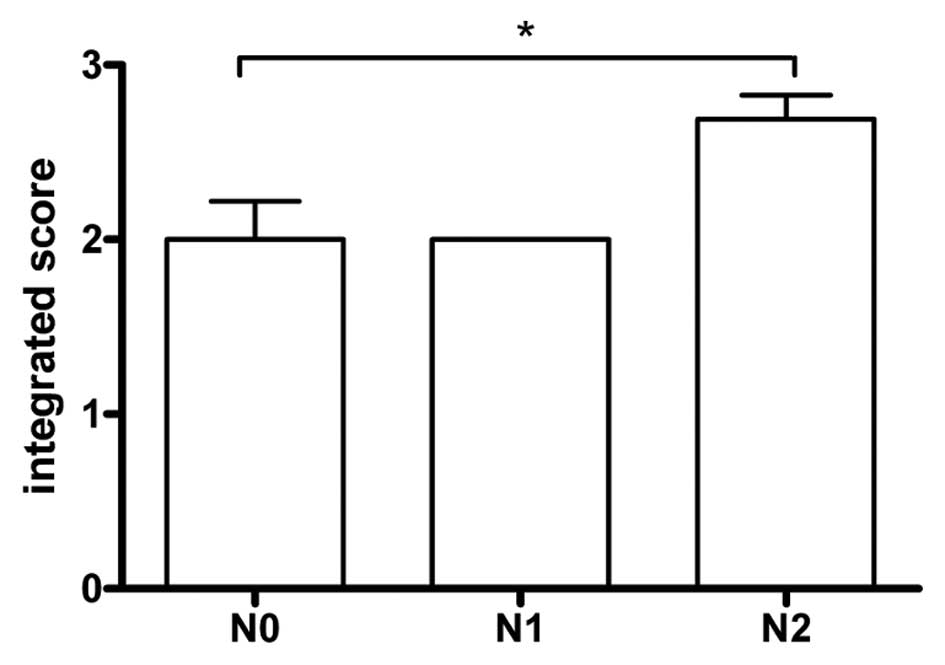
an association (P=0.05) was observed between fascin expression in
the tumor tissues and pN stage (Fig.
3). The association between fascin expression in lymph nodes
and other factors was not investigated due to the low sample
number.
The fascin expression levels in the healthy
epithelial tissue were not associated with pT (P=0.90), pN (P=1.00)
or pM (P=1.00) stage, smoking status (P=0.62), tumor grade
(P=1.00), alcohol consumption (P=0.64), gender (P=0.40) or tumor
localization (P=0.85).
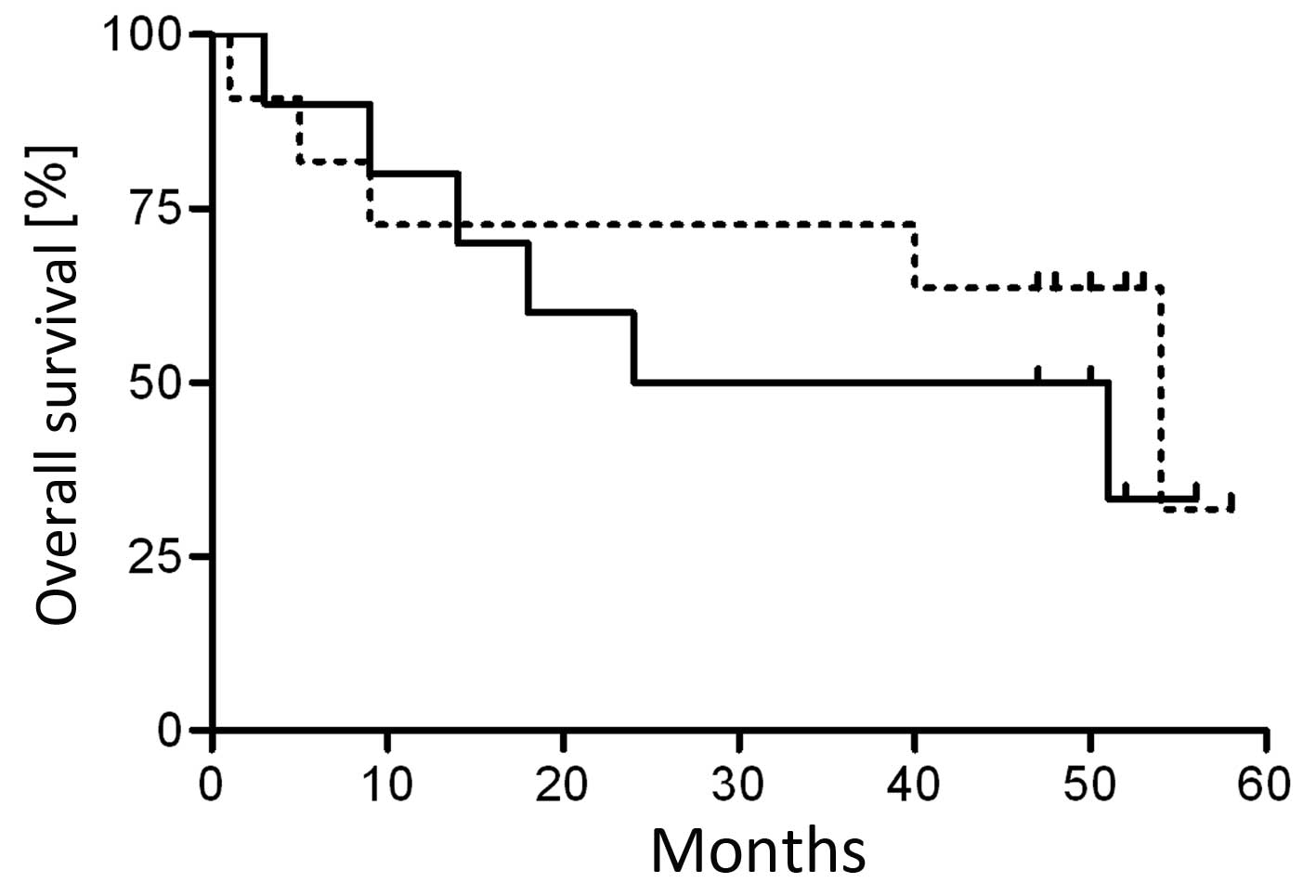
For overall survival, the univariate analyses did
not support the hypothesis that fascin expression, in tumor and
healthy epithelial tissues, is a predictor for overall,
relapse-free and event-free survival (Table II). To further analyze the affect
of fascin expression on overall survival, the tumor samples were
grouped as fascin high and fascin low. The high group included
tumor samples with fascin integrated scores of 5–6 (group 3) and
the low group included those with integrated scores of 0–4 (groups
0–2). Despite a median overall survival of only 38 months in the
fascin high group, compared with 54 months in the fascin low group,
no significant correlation was observed between fascin expression
and overall survival (Fig. 4 and
Table II).
 | Table IIP-values obtained from log-rank tests
for possible predictors of survival. |
Table II
P-values obtained from log-rank tests
for possible predictors of survival.
| Explanatory
variable (integrated score group) | Overall
survival | Relapse-free
survival | Event-free
survival |
|---|
| Fascin (1 vs. 2 vs.
3) | 0.58 | 0.74 | 0.71 |
| Fascin (0–2 vs.
3) | 0.38 | 0.78 | 0.78 |
| Healthy epithelium
(1 vs. 2) | 0.42 | 0.94 | 0.94 |
Discussion
The present study investigated fascin expression in
primary HNSCC, the tumor invasion front, surrounding healthy
tissues and lymph node metastases. A longer median overall survival
in the group with low fascin expression levels was observed,
however, was not identified to be statistically significant, which
is in accordance with previous studies associating fascin
expression with survival. Wu et al (16) reported that in nasopharyngeal SCC,
the overall survival and disease-free survival rate for patients
with high fascin expression was significantly lower compared with
patients exhibiting low fascin expression. Lee et al
(14) reported that the mean
overall survival of patients with oral SCC and high tumor fascin
expression was 34 months compared with 49 months in patients with
no or low fascin expression levels.
A notable observation in the present study was that
fascin expression in tumors is correlated with pN status, which is
in accordance with the decreased overall survival of patients with
high fascin-expressing primary tumors. Furthermore, Vignjevic et
al (22) found an increased
fascin level in primary colon cancer tissues was associated with
clinical distant metastases. Fascin participates in the regulation
of cell adhesion, interactions and migration and is an F-actin
interacting protein, which forms parallel actin bundles that are
found in leading edge protrusions of mesenchymal cells (23). In epithelial cells, de novo
expression of fascin induces protrusions and increases motility
(24). Previous in vitro
studies demonstrated that elevated levels of fascin increased the
speed of cell migration and emphasized the association between
fascin overexpression and the motility of transformed cells in
urothelial carcinoma (8).
Disruption of endogenous fascin expression in nasopharyngeal
carcinoma cells using the small interfering RNA technique
suppressed nasopharyngeal carcinoma cell invasiveness, and
decreased cell filopodia and lamellipodia, thus, indicating the
relevance of fascin to cancer cell invasiveness (16). These data may therefore indicate the
possible role of fascin in the pathogenesis of lymphatic
metastases.
Fascin expression has been found to be low or absent
in the majority of normal adult epithelia of varying origin
(9). However, increased fascin
expression in the basal layer of nasopharyngeal epithelial tissues
has previously been reported (16),
which supports our observation of frequent and increased fascin
expression in healthy, although tumor-adjacent, epithelial tissue.
The observed upregulated fascin expression may reflect a
tissue-specific expression pattern or an association between fascin
and the proliferating capacity of cells. Alternative explanations
may be that the epithelial tissue that was examined was directly
adjacent to the tumor and the tumor may condition its
microenvironment, or that the macroscopically-normal epithelial
tissue was not normal at a molecular level. The latter is supported
by our previous study regarding genetic alterations similar to
those found in the primary tumors in the tumor-adjacent normal
tissue (3,25). Previous studies have found increased
levels of fascin in dysplastic epithelia (26) or fascin levels increasing gradually
in the progression from normal epithelia to simple hyperplasia,
dysplasia, carcinoma in situ, to invasive esophageal SCC
(26), supporting the assumption
that unexpected observation of fascin expression in normal
epithelia may reflect pre-malignant changes at the molecular
level.
The findings of the present study support the
hypothesis that fascin is involved in HNSCC. A possible mechanism
may be the increased motility of fascin-expressing cancer cells. As
a consequence, patients with high tumor fascin levels may be at a
higher risk for a more aggressive tumor and therefore, should be
treated accordingly, i.e. fascin expression may have relevance for
therapeutic decision-making. For example, in patients with
questionable symptoms who may undergo neck lymph node surgery, such
as a marginal case when ultrasonography is not sufficient to
determine metastasis from enlarged cervical lymph nodes, a patient
exhibiting high fascin levels in the tumor tissues would be
classified as high risk and may receive surgery, while a patient
with low fascin levels would not. However, such an approach
requires further investigation.
In conclusion, the present study provides evidence
of the role of fascin in HNSCC metastasis. Thus, fascin should be
evaluated further as a potential molecular marker for the
prediction of regional lymphatic metastasis in HNSCC.
Acknowledgements
The preliminary data were presented, in part, at the
fourth European Conference on Head and Neck Oncology (Athens,
Greece) on 5th March 2010.
References
|
1
|
Jemal A, Murray T, Ward E, et al: Cancer
statistics, 2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar
|
|
2
|
Million RR: Cancer of the head and neck.
Cancer: Principles and Practice of Oncology. De Vita VT Jr, Hellman
S and Rosenberg SA: 1. 4th edition. JB Lippincott; Philadelphia,
PA: pp. 396–420. 1992
|
|
3
|
Brieger J, Jacob R, Riazimand HS, Essig E,
Heinrich UR, Bittinger F and Mann WJ: Chromosomal aberrations in
premalignant and malignant squamous epithelium. Cancer Genet
Cytogenet. 144:148–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Welkoborsky HJ, Hinni M, Dienes HP and
Mann WJ: Predicting recurrence and survival in patients with
laryngeal cancer by means of DNA cytometry, tumor front grading,
and proliferation markers. Ann Otol Rhinol Laryngol. 104:503–510.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar
|
|
6
|
Torre GC: SCC antigen in malignant and
nonmalignant squamous lesions. Tumour Biol. 19:517–526. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kureishy N, Sapountzi V, Prag S, Anilkumar
N and Adams JC: Fascins, and their roles in cell structure and
function. Bioessays. 24:350–361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karasavvidou F, Barbanis S, Pappa D,
Moutzouris G, Tzortzis V, Melekos MD and Koukoulis G: Fascin
determination in urothelial carcinomas of the urinary bladder: a
marker of invasiveness. Arch Pathol Lab Med. 132:1912–1915.
2008.PubMed/NCBI
|
|
9
|
Hashimoto Y, Skacel M, Lavery IC,
Mukherjee AL, Casey G and Adams JC: Prognostic significance of
fascin expression in advanced colorectal cancer: an
immunohistochemical study of colorectal adenomas and
adenocarcinomas. BMC Cancer. 6:2412006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoder BJ, Tso E, Skacel M, et al: The
expression of fascin, an actin-bundling motility protein,
correlates with hormone receptor-negative breast cancer and a more
aggressive clinical course. Clin Cancer Res. 11:186–192. 2005.
|
|
11
|
Hu W, McCrea PD, Deavers M, Kavanagh JJ,
Kudelka AP and Verschraegen CF: Increased expression of fascin,
motility associated protein, in cell cultures derived from ovarian
cancer and in borderline and carcinomatous ovarian tumors. Clin Exp
Metastasis. 18:83–88. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hashimoto Y, Shimada Y, Kawamura J,
Yamasaki S and Imamura M: The prognostic relevance of fascin
expression in human gastric carcinoma. Oncology. 67:262–270. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maitra A, Iacobuzio-Donahue C, Rahman A,
et al: Immunohistochemical validation of a novel epithelial and a
novel stromal marker of pancreatic ductal adenocarcinoma identified
by global expression microarrays: sea urchin fascin homolog and
heat shock protein 47. Am J Clin Pathol. 118:52–59. 2002.
View Article : Google Scholar
|
|
14
|
Lee TK, Poon RT, Man K, et al: Fascin
over-expression is associated with aggressiveness of oral squamous
cell carcinoma. Cancer Lett. 254:308–315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SF, Yang SF, Li JW, et al: Expression
of fascin in oral and oropharyngeal squamous cell carcinomas has
prognostic significance - a tissue microarray study of 129 cases.
Histopathology. 51:173–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu D, Chen L, Liao W, Ding Y, Zhang Q, Li
Z and Liu L: Fascin1 expression predicts poor prognosis in patients
with nasopharyngeal carcinoma and correlates with tumor invasion.
Ann Oncol. 21:589–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou J, Yang H, Chen F, et al: Prognostic
significance of fascin-1 and E-cadherin expression in laryngeal
squamous cell carcinoma. Eur J Cancer Prev. 19:11–17. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brieger J, Duesterhoeft A, Brochhausen C,
Gosepath J, Kirkpatrick CJ and Mann WJ: Recurrence of pleomorphic
adenoma of the parotid gland - predictive value of cadherin-11 and
fascin. APMIS. 116:1050–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hashimoto Y, Parsons M and Adams JC: Dual
actin-bundling and protein kinase C-binding activities of fascin
regulate carcinoma cell migration downstream of rac and contribute
to metastasis. Mol Biol Cell. 18:4591–4602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schuon R, Brieger J, Heinrich UR, Roth Y,
Szyfter W and Mann WJ: Immunohistochemical analysis of growth
mechanisms in juvenile nasopharyngeal angiofibroma. Eur Arch
Otorhinolaryngol. 264:389–394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bittinger F, Brochhausen C, Köhler H, et
al: Differential expression of cell adhesion molecules in inflamed
appendix: correlation with clinical stage. J Pathol. 186:422–428.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vignjevic D, Schoumacher M, Gavert N, et
al: Fascin, a novel target of beta-catenin-TCF signaling, is
expressed at the invasive front of human colon cancer. Cancer Res.
67:6844–6853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adams JC: Fascin protrusions in cell
interactions. Trends Cardiovasc Med. 14:221–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamashiro S, Yamakita Y, Ono S and
Matsumura F: Fascin, an actin-bundling protein, induces membrane
protrusions and increases cell motility of epithelial cells. Mol
Biol Cell. 9:993–1006. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brieger J, Kastner J, Gosepath J and Mann
WJ: Evaluation of microsatellite amplifications at chromosomal
locus 3q26 as surrogate marker for premalignant changes in mucosa
surrounding head and neck squamous cell carcinoma. Cancer Genet
Cytogenet. 167:26–31. 2006. View Article : Google Scholar
|
|
26
|
Takikita M, Hu N, Shou JZ, et al: Fascin
and CK4 as biomarkers for esophageal squamous cell carcinoma.
Anticancer Res. 31:945–952. 2011.PubMed/NCBI
|