Introduction
RbAp48, a member of the WD-40 protein family that is
characterized by its ability to bind to the retinoblastoma protein
(Rb), was first identified from the HeLa cell lysate (1). RbAp48 is involved in the regulation of
cytoskeletal organization. The overexpression of RbAp48 in breast
cancer cells results in profound changes of cellular morphology,
including cell size reduction, decreased cellular protrusions and a
circular cell shape (2). In
addition, small interfering RNA-mediated depletion of RbAp48 in
HPV16-immortalized human cervical mucosa epithelial H8 cells
dramatically stimulates cell growth and colony formation (3). Previously, numerous studies have
demonstrated that the level of RbAp48 is changed in liver cancer
(4), thyroid carcinoma (5) and acute myeloid leukemia (6) cells when compared with normal cells.
These studies indicated that RbAp48 may play significant roles in
cell cycle and tumor formation. RbAp48, together with the
hematopoietic transcription factor, GATA-1, and several other
proteins functions early to repress the genes required to maintain
G1E cells in the undifferentiated state, which contributes to
terminal erythroid differentiation (7). Additionally, GATA-1 is able to mediate
the transcription of erythroid-specific genes and repress the
proliferation-related c-Myc gene in G1E-ER4 cells (8). c-Myc plays key roles in normal,
non-transformed cells in regulating cell growth, differentiation
and apoptosis.
However, little is known about the function of
RbAp48 in the regulation of cell differentiation. Our previous
experiments discovered that the expression level of RbAp48 in fetal
livers was increased during terminal erythroid differentiation
through comparative proteomic analysis (data not published). In the
present study, the changes in the cellular level and localization
of RbAp48 during terminal erythroid maturation were examined.
Murine erythroleukemia (MEL) differentiation in culture induced by
SB provides an ideal model for studying differentiation-associated
proteins. By means of this model, the present study attempted to
preliminarily elucidate the function of RbAp48 in MEL
differentiation.
Materials and methods
Cell and cell culture
HEK293T and MEL cells were purchased from the Type
Culture Collection of the Chinese Academy of Science (Shanghai,
China) and were cultured in Dulbecco’s modified Eagle’s medium
(Gibco; Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Hyclone; Thermo Fisher
Scientific, Rockford, IL, USA) at 37°C in a humidified atmosphere
of 5% CO2.
Benzidine staining
Erythroid differentiation was evaluated by the
expression of hemoglobin, which can be detected by benzidine
staining. The MEL cells were suspended in a benzidine staining
solution (0.4 mg/ml benzidine in 0.6% H2O2,
3% acetic acid and 8.5 g/l NaCl). Following 2 min of staining, the
differentiated cells were stained blue and images were captured by
an inverted fluorescence microscope (Nikon, Tokyo, Japan). The
percentage of benzidine-positive cells was the number of blue cells
divided by the total number of cells.
MTT assay
To measure cell proliferation activity, each group
of MEL cells was seeded in 96-well plates at a density of
3×103 cells per well. The MTT assay was performed at 24,
48, 72, 96 and 120 h. To each well, 10 μl MTT (5 mg/ml) was added
and the cells were incubated for an additional 4 h in the cell
culture incubator. The medium in each well was then replaced with
150 μl dimethylsulfoxide and the absorbance was measured at 570 nm
on a spectrophotometer (Thermo Fisher Scientific).
Semi-quantitative polymerase chain
reaction (PCR)
Total RNA was extracted from each cell sample. The
cDNA was synthesized from 0.5 μg total RNA using a reverse
transcription kit (Fermentas; Thermo Fisher Scientific). The number
of PCR cycles was optimized in each case to guarantee that the
product intensity fell within the linear range of amplification.
PCR amplification was performed as follows: initial denaturation at
94°C for 5 min, followed by additional denaturation at 94°C for 30
sec, annealing at 50°C for 30 sec, extension at 72°C for 30 sec. At
the end of the cycle, the reaction mixtures were maintained at 72°C
for a further 3 min. The primers are listed in Table I. The PCR products were analyzed by
agarose gel electrophoresis with β-actin as an internal
control.
 | Table ISequence of PCR primers |
Table I
Sequence of PCR primers
| Gene | Primer sequence |
|---|
| α-globin | F:
AAGCAACATCAAGGCTGCCT
R: ACCTTCTTGCCGTGACCCTT |
| β-globin | F:
AACTCTGGGAAGGCTCCTGA
R: TGCAGCTCACTGAGATGAGC |
| GPA | F:
GCTGCTGTGACAACATCAGG
R: CAGTAGGGGCCGTGTGATAA |
| β-actin | F:
GAGACCTTCAACACCCCAGC
R: ATGTCACGCACGATTTCCC |
Western blot analysis
The cells were washed with ice-cold
phosphate-buffered saline (PBS) and lysed by lysis buffer (50 mM
Tris, 150 mM NaCl, 1 mM EDTA, 1% Triton 100 and 0.1% SDS; pH 8.0)
for 20 min on ice. Protein concentrations were determined by
Bradford assay. Equal amounts of proteins were subjected to 12%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes.
The membranes were blocked in Tris-buffered saline and Tween 20
(TBST; 50 mM Tris, 150 mM NaCl and 0.1% Tween-20; pH 7.5)
containing 5% skimmed dry milk for 2 h at room temperature, and
then incubated with primary anti-RbAp48 rabbit monoclonal
(Epitomics, Inc., Burlingame, CA, USA), anti-c-Myc (Epitomics,
Inc.), anti-GATA-1 rabbit monoclonal (CST, Boston, MA, USA) or
anti-β-actin rabbit monoclonal (Sigma-Aldrich, St. Louis, MO, USA)
antibodies overnight at 4°C. The membranes were then washed and
incubated with goat anti-rabbit immunoglobulin G (IgG)-horseradish
peroxidase antibody (Sigma-Aldrich) for 2 h at room temperature.
The proteins were visualized by chemiluminescence using an enhanced
chemiluminescence kit (Advansta, Inc., Menlo Park, CA, USA).
Immunoblots were quantified by Quantity One software (Bio-Rad,
Hercules, CA, USA).
Immunofluorescence assay
The MEL cells were harvested at 0, 24, 48, 72, and
96 h following treatment with 1.25 mM SB. Subsequent to being fixed
in 4% paraformaldehyde for 15 min, the cells were permeabilized
with methanol for 10 min, stained with DAPI for 15 min at 37°C and
blocked in 5% bovine serum albumin for 30 min at room temperature.
The cells were incubated with anti-RbAp48 antibody overnight at
4°C, washed with PBS Tween-20 and then incubated with cyanine dye
3-conjugated goat anti-rabbit IgG antibody (Invitrogen Life
Technologies) for 2 h at room temperature. The images of stained
cells were captured using confocal fluorescence microscopy
(Nikon).
Generation of the stable RbAp48-knockdown
cell line
For knockdown of the RbAp48 gene by RNA
interference, the following oligonucleotide pair was designed:
Small hairpin (sh)RNA sense, 5′-CCG GCC CTG CAT CAT TGC AAC AAA GCT
CGA GCT TTG TTG CAA TGA TGC AGG GTT TTT G-3′ and antisense, 5′-AAT
TCA AAA ACC CTG CAT CAT TGC AAC AAA GCT CGA GCT TTG TTG CAA TGA TGC
AGG G-3′. As a control, a scramble sequence of shRNA was also
designed: shRNA-negative control sense, 5′-CCG GCC TAA GGT TAA GTC
GCC CTC GCT CGA GCG AGG GCG ACT TAA CCT TAG GTT TTT G-3′ and
antisense, 5′-AAT TCA AAA ACC TAA GGT TAA GTC GCC CTC GCT CGA GCG
AGG GCG ACT TAA CCT TAG G-3′. Two double-stranded oligonucleotides
were inserted into the plasmid vector, pLKO.1-TRC (Addgene,
Cambridge, MA, USA), via AgeI and EcoRI restriction
sites. All different lentiviruses were produced by co-transfection
of 293T cells with pLKO.1-RbAp48-shRNA or pLKO.1-shRNA-NC vectors
and the ecotropic packaging vectors, pCMV-VSVG and pCMV-dR8.2,
using Lipofectamine 2000 (Invitrogen Life Technologies), and then
the lentiviruses were used to transfect the MEL cells. Stable
RbAp48-knockdown cells were selected in 1 μg/ml puromycin
(Invitrogen Life Technologies).
Statistical analysis
SPSS Statistics 19 software (IBM, Armonk, NY, USA)
was used for statistical analysis. Data were presented as the mean
± standard deviation for three different determinations.
Statistical significance was analyzed using the one-way analysis of
variance test followed by Fisher’s least significant difference
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Erythroid differentiation of MEL cells
induced by SB
Expression of hemoglobin was the most evident
feature of erythroid differentiation. A preliminary study showed
that the optimum concentration of the inducer, SB, was 1.25 mM,
which was used in subsequent experiments (data now shown).
Benzidine staining was used to detect the expression of hemoglobin
in MEL cells (Fig. 1A). Almost 80%
of the cells had differentiated following treatment with SB for 72
h (Fig. 1B). However, no
benzidine-positive cells were observed in the untreated cells. The
MEL cells, in the presence of SB, could initiate erythroid
differentiation at the expense of proliferation (Fig. 1C). During MEL differentiation, the
erythroid maturation-related mRNA expression of α-globin, β-globin
and GPA was increased markedly, and reached a plateau at 72 h
(Fig. 1D).
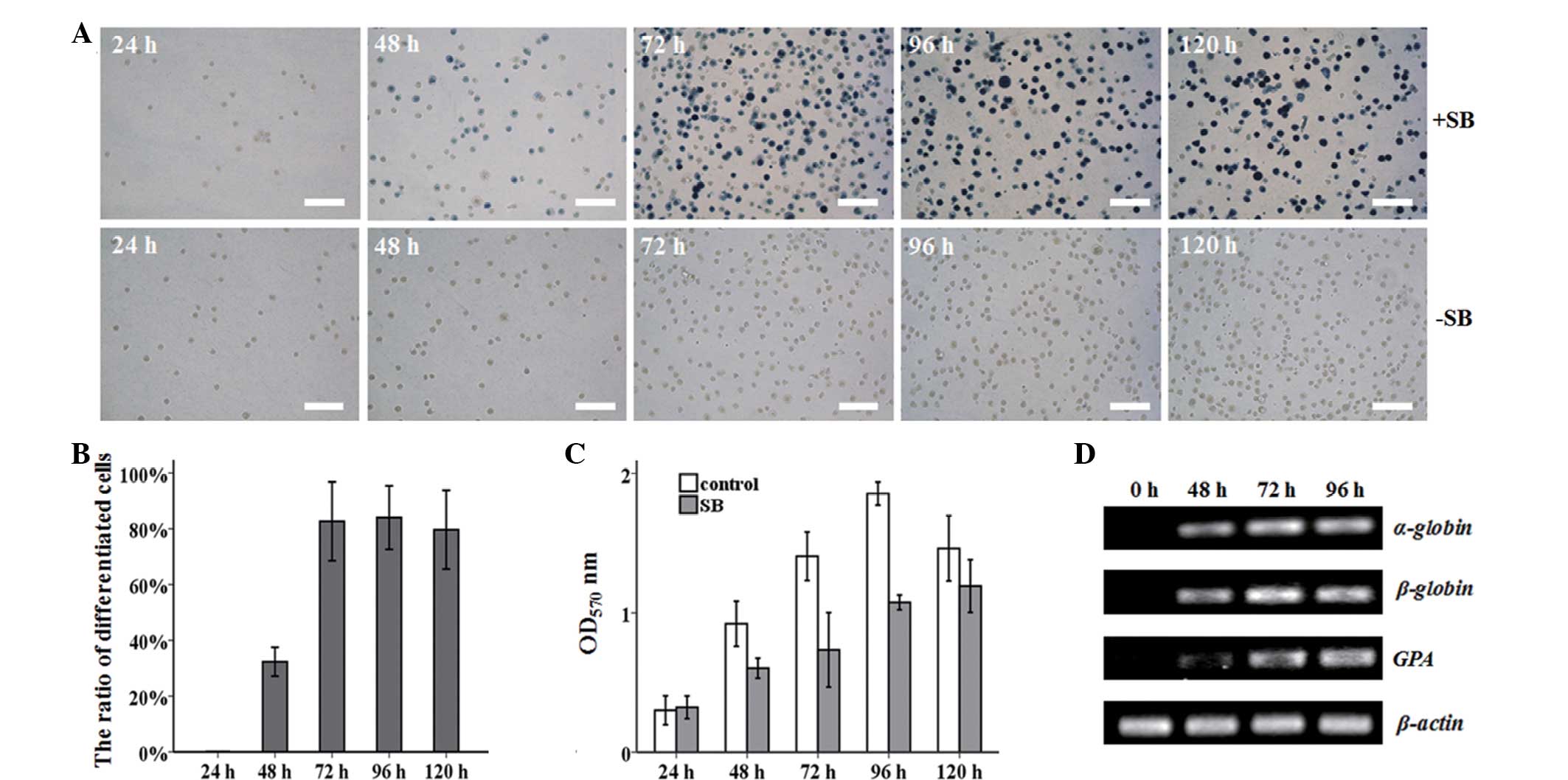 | Figure 1SB-induced erythroid differentiation
of MEL cells. (A) MEL cells were harvested after 24, 48, 72, 96,
and 120 h with or without 1.25 mM SB induction, and stained with
benzidine. Benzidine-positive cells turned blue. Untreated MEL
cells served as a control. White bar, 100 μm. (B) The percentage of
benzidine-positive cells was counted at different times.
*P<0.001 vs. control group. Control group, MEL cells
without SB treatment at various time points. (C) The effect of 1.25
mM SB on the MEL cell proliferation ability was measured by MTT
assay. *P<0.05 and **P<0.01 vs. control
group. Control group, MEL cells without SB treatment at various
time points. (D) The mRNA expression of α-globin, β-globin and GPA
was dramatically increased during MEL differentiation, as assayed
by semi-quantitative PCR. β-actin was used as an internal control.
Three independent experiments were performed. SB, sodium butyrate;
MEL, murine erythroleukemia; OD, optical density; PCR, polymerase
chain reaction; GPA, glycophorin A. |
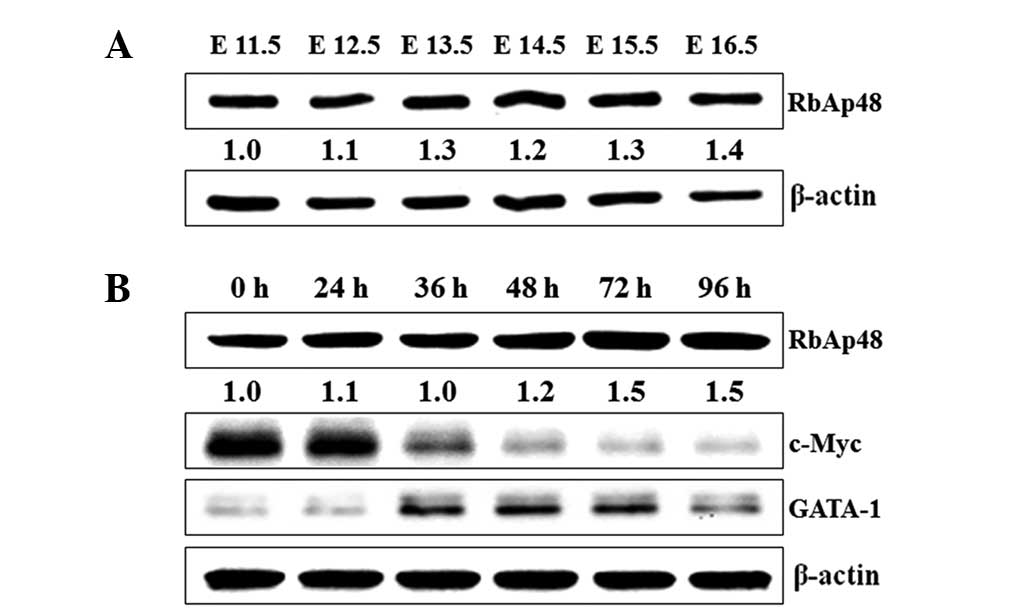
An elevation of RbAp48 level and the
changes of GATA-1 and c-Myc level during terminal erythroid
maturation
Since our previous experiments had indicated that
the level of RbAp48 in the fetal liver was changed during murine
embryonic development, we hypothesized that RbAp48 may function in
terminal erythroid maturation. To test this prediction, fetal
livers were isolated from imprinting control region (ICR) mouse
embryos of E11.5–16.5 stage, and the RbAp48 expression level was
measured. A gradual increase in RbAp48 was detected in the fetal
liver from E11.5 to E16.5 (Fig.
2A). Furthermore, it was found that the RbAp48 expression also
increased in MEL cells induced by SB, and GATA-1 and c-Myc levels
were changed significantly. The GATA-1 level showed a rapid
increase in the early stage of differentiation, and the c-Myc level
was gradually downregulated during MEL differentiation (Fig. 2B). Therefore, RbAp48, GATA-1 and
c-Myc may play significant roles in erythroid differentiation.
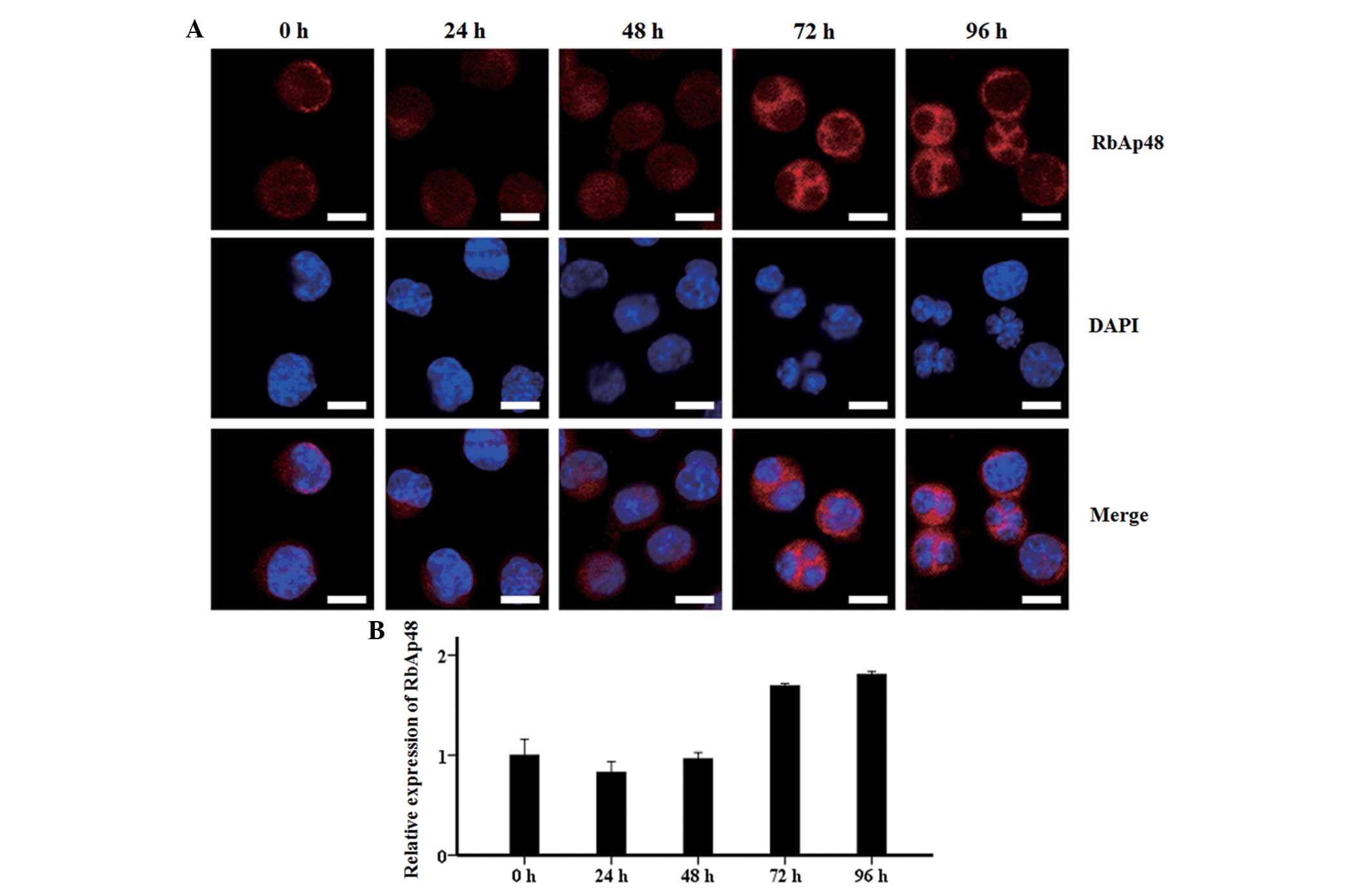
Cellular location of RbAp48 during
erythroid differentiation
Confocal microscopic observations showed that the
RbAp48 protein was mainly distributed in the nucleus within 48 h of
SB treatment, and rapidly accumulated in the cytoplasm from 72 to
96 h (Fig. 3A). Quantitative
analysis with EZ-C1 3.20 software illustrated that the fluorescence
intensity of RbAp48 in each cell remained stable and at a low level
from 0 to 48 h, prior to an increase to 1.7 fold at 72 and 96 h
(Fig. 3B). This was consistent with
the western blot analysis results of RbAp48.
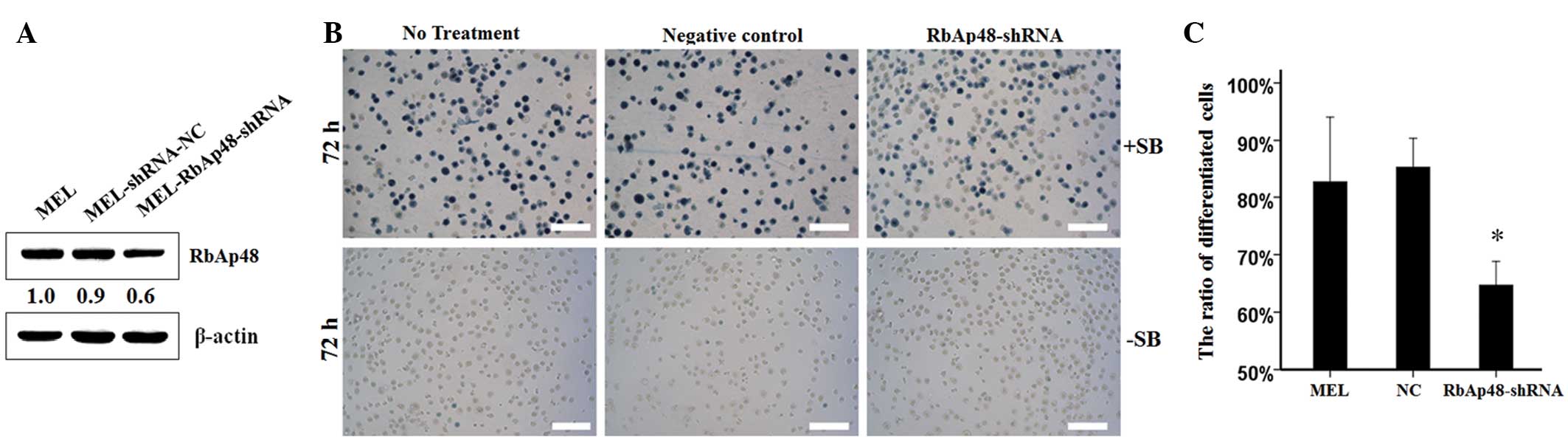
Suppression of RbAp48 expression results
in significant prevention of MEL cell differentiation induced by
SB
To determine whether a specific level of RbAp48 was
required for the erythroid differentiation of MEL cells, the
expression of the RbAp48 gene in MEL cells was suppressed using the
RNA interference (RNAi) approach. The stable RbAp48-knockdown cell
line was verified by western blot analysis. MEL-RbAp48-shRNA
resulted in a 0.6-fold reduction of endogenous RbAp48 when compared
with MEL-shRNA-NC and parent MEL cells (Fig. 4A). To further investigate the effect
of the knockdown of RbAp48 on cell differentiation, benzidine
staining was performed on the cultured cells incubated with SB for
72 h, and there were no benzidine-positive cells in the absence of
SB (Fig. 4B). The results of the
present study revealed that the knockdown of RbAp48 expression in
the MEL cells decreased the differentiation ability by ~20%
(Fig. 4C).
Discussion
Leukemia, a malignant hematopoietic system disease,
is mainly the result of a disorder of the hematopoietic stem cells
in differentiation and apoptosis (9). However, studies have indicated that
SB, a histone deacetylase inhibitor, exhibits anticancer effects
via the apoptosis and differentiation of cancer cells (10). In the present study, MEL cells, when
cultured in the presence of SB, chose the differentiation pathway
and synthesized erythroid markers, including α-globin, β-globin and
GPA. The effect of SB on the proliferation characteristic of the
MEL cells was also detected. SB evidently suppressed the cell
proliferation ability. Therefore, SB induced MEL differentiation at
the expense of proliferation, accompanied by the expression of
erythroid markers.
RbAp48 was found to be upregulated in the fetal
liver from E11.5 to E16.5. Furthermore, it also showed a steady
increase upon SB induction in the MEL cells. This specific
expression in certain phases indicated that RbAp48 was involved in
cell differentiation. During MEL differentiation, GATA-1 and c-Myc
levels were also changed significantly. The GATA-1 level showed a
rapid increase in the early stage of differentiation, and the c-Myc
level was gradually downregulated during MEL differentiation. The
GATA-1/RbAp48 complex has been proven to promote erythroid
differentiation in G1E cells (7).
GATA-1-mediated c-Myc transcriptional repression is due to the
direct interaction in the c-Myc promoter (8). Repression of c-Myc has been linked to
proliferation cessation, and evidence has demonstrated that the
downregulation of c-Myc is essential for terminal erythroid
maturation (11). Therefore,
RbAp48, GATA-1 and c-Myc may play significant roles in erythroid
differentiation.
Next, the present study investigated the cellular
localization of RbAp48 during erythroid differentiation. Prolonging
the SB induction time rapidly increased the content of RbAp48 in
the cytoplasm at 72 and 96 h. This indicated that RbAp48 showed
marked expression in the late stage of erythroid differentiation.
In short, through western blot analysis and immunofluorescence
assays, it was found that the RbAp48 level was upregulated during
MEL differentiation. Therefore, a high level of RbAp48 may
contribute to MEL differentiation. To further study the effect of
low level RbAp48 on MEL differentiation, a stable RbAp48-knockdown
cell line was isolated from the MEL cells by the RNAi method. The
results of the present study revealed that a low level of RbAp48
blocked the erythroid differentiation of the MEL cells. This result
further indicated that a high level of RbAp48 was essential for
erythroid differentiation, and that RbAp48 may be a significant
differentiation factor.
In the present study, MEL cells could re-enter the
erythroid program and obtain the capability to synthesize
hemoglobin and GPA in the presence of SB. The expression level of
RbAp48 was found to be upregulated during terminal erythroid
differentiation, and a relatively low expression level of RbAp48 in
MEL cells partly contributed to erythroid differentiation
cessation. This indicates the novel role of RbAp48 in regulating
MEL differentiation. Advances in the research of RbAp48 in
erythroid differentiation will extend our understanding of the
mechanisms of SB-induced MEL differentiation.
Acknowledgements
This study was supported by the Zhejiang Provincial
Opening Foundation of Biomedicine Engineering of China
(SWYX0902).
References
|
1
|
Qian YW, Wang YC, Hollingsworth RE Jr,
Jones D, Ling N and Lee EY: A retinoblastoma-binding protein
related to a negative regulator of Ras in yeast. Nature.
364:648–652. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scuto A, Zhang H, Zhao H, Rivera M,
Yeatman TJ, Jove R and Torres-Roca JF: RbAp48 regulates
cytoskeletal organization and morphology by increasing K-Ras
activity and signaling through mitogen-activated protein kinase.
Cancer Res. 67:10317–10324. 2007. View Article : Google Scholar
|
|
3
|
Kong L, Yu XP, Bai XH, Zhang WF, Zhang Y,
Zhao WM, Jia JH, Tang W, Zhou YB and Liu CJ: RbAp48 is a critical
mediator controlling the transforming activity of human
papillomavirus type 16 in cervical cancer. J Biol Chem.
282:26381–26391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song H, Xia SL, Liao C, Li YL, Wang YF, Li
TP and Zhao MJ: Genes encoding Pir51, Beclin 1, RbAp48 and aldolase
b are up or down-regulated in human primary hepatocellular
carcinoma. World J Gastroenterol. 10:509–513. 2004.PubMed/NCBI
|
|
5
|
Pacifico F, Paolillo M, Chiappetta G,
Crescenzi E, Arena S, Scaloni A, Monaco M, Vascotto C, Tell G,
Formisano S and Leonardi A: RbAp48 is a target of nuclear
factor-kappaB activity in thyroid cancer. J Clin Endocrinol Metab.
92:1458–1466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Casas S, Ollila J, Aventín A, Vihinen M,
Sierra J and Knuutila S: Changes in apoptosis-related pathways in
acute myelocytic leukemia. Cancer Genet Cytogenet. 146:89–101.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rodriguez P, Bonte E, Krijgsveld J,
Kolodziej KE, Guyot B, Heck AJ, Vyas P, de Boer E, Grosveld F and
Strouboulis J: GATA-1 forms distinct activating and repressive
complexes in erythroid cells. EMBO J. 24:2354–2366. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rylski M, Welch JJ, Chen YY, Letting DL,
Diehl JA, Chodosh LA, Blobel GA and Weiss MJ: GATA-1-mediated
proliferation arrest during erythroid maturation. Mol Cell Biol.
23:5031–5042. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Enver T and Greaves M: Loops, lineage, and
leukemia. Cell. 94:9–12. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin H, Lee YS and Lee YC: Sodium
butyrate-induced DAPK-mediated apoptosis in human gastric cancer
cells. Oncol Rep. 27:1111–1115. 2012.PubMed/NCBI
|
|
11
|
Jayapal SR, Lee KL, Ji P, Kaldis P, Lim B
and Lodish HF: Down-regulation of Myc is essential for terminal
erythroid maturation. J Biol Chem. 285:40252–40265. 2010.
View Article : Google Scholar : PubMed/NCBI
|