Introduction
Gastrinoma is a type of neuroendocrine tumor with an
incidence of approximately one to three cases per year for every
1,000,000 individuals (1). At
present, ~50–60% of gastrinoma cases are malignant (2) and a five-year survival rate of
patients with metastatic gastrinoma is possible for 20–40% of these
cases (3). To the best of our
knowledge, 90% of gastrinomas are located in the gastrinoma
triangle comprising the duodenum (60–80%) and the pancreas
(10–40%), or in the adjacent lymph nodes (1). The first common signs of gastrinoma
include secretory diarrhea, peptic telephium, severe esophagitis
and hypercalcemia (4). Ectopia and
asymptomatic gastrinoma are rarely reported in the literature. In
the current study, a rare case report of gastrinoma is presented
and the underlying clinical and pathological factors that may have
resulted in the misdiagnosis are investigated. The aim of the
present study was to focus the attention of clinicians on this type
of cancer by providing preliminary clinical experience in order to
improve its diagnosis and treatment.
Case report
On December 15, 2006, a 39-year-old female was
admitted to the Anhui Provincial Hospital (Anhui, China) following
the observation of a pancreatic body and a tail lesion in an annual
periodic health examination. The patient had not experienced any
discomfort and was hemodynamically stable. A benign abdominal
examination was conducted and all of the blood and biochemical
parameters were within their respective reference ranges. The
family and personal medical histories were unremarkable. Written
informed consent was obtained from the patient for publication of
the present study and any accompanying images. Enhanced computed
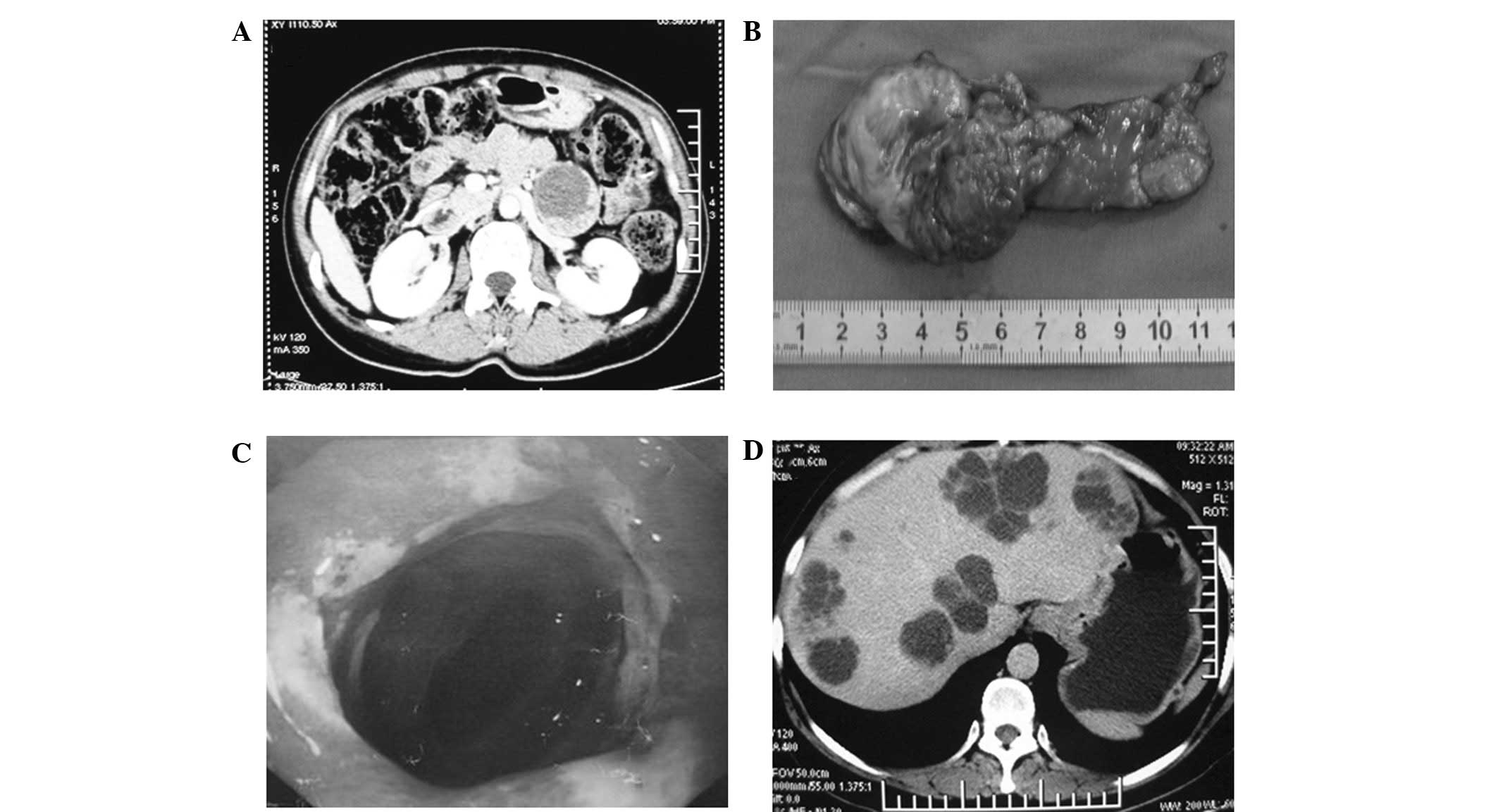
tomography (CT) revealed a cystic mass in the pancreatic body and
tail (Fig. 1A). Considering these
observations, the patient was diagnosed with pancreatic body and
tail space-occupying lesions. The patient also underwent a distal
pancreatectomy (Fig. 1B). A solid
pseudopapillary tumor of the pancreas (SPTP) was suspected as a
result of postoperative pathology (World Health Organisation, 2010
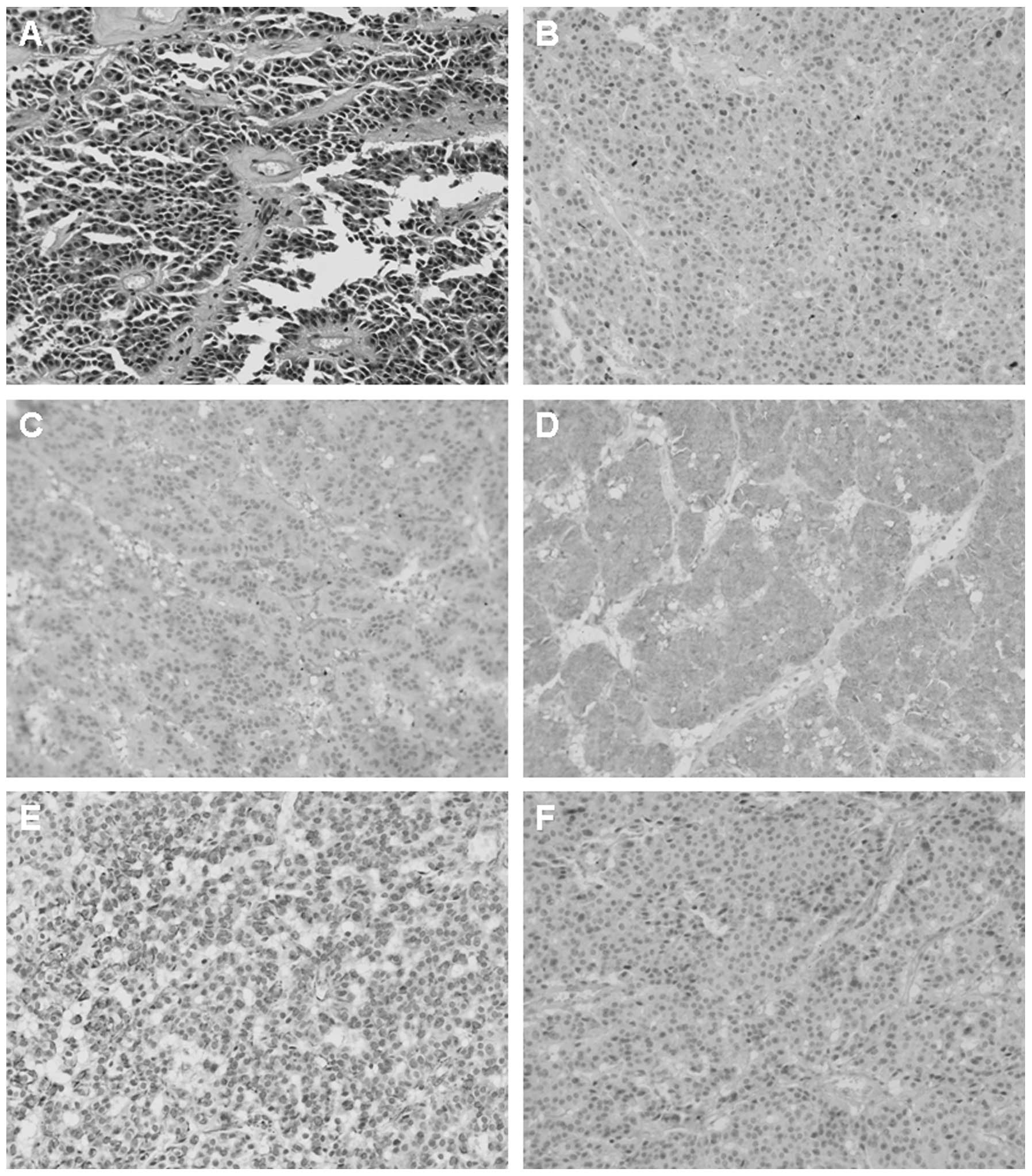
guideline: Low malignant potential) (5). Immunohistochemistry results indicated
that the tumor cells were positive for α1-antichymotrypsin,
synaptophysin (Syn), vimentin and neuron-specific enolase (Fig. 2). In the following year, the patient
remained well and no apparent symptoms occurred. However, the
patient was referred to the Anhui Provincial Hospital after
presenting with stomachache in December 2008. After 24 months of
follow-up, a gastroscopy revealed a duodenal bulbar ulcer and
hemorrhagic gastric body inflammation (Fig. 1C). B-scan ultrasonography of the
abdomen also revealed a low-density liver lesion (size, 1 cm). The
patient was diagnosed with a peptic ulcer (active stage) and was
administered proton pump inhibitor (PPIs; including omeprazole)
treatment. Following the second hospitalization, the condition and
ulcer symptoms of the patient improved during the follow-up period.
Although the ulcer healed, an additional gastroscopy in June 2009
showed gastrointestinal erosion. B-scan ultrasonography revealed
that multiple, small low-density liver lesions in the left lobe of
the liver. The patient was continuously treated with the PPI
(omeprazole). However, the patient rejected further treatment, as
the low-density liver lesion was asymptomatic. In October 2010, a
gastroscopy demonstrated the presence of an ulcer that was
affecting the stomach and duodenum. Although the PPI was
continuously administered, the patient exhibited symptoms,
including belching and acidic reflux. Enhanced CT scans of the
abdomen revealed multiple round lesions located in the left and
right lobes of the liver (Fig. 1D).
Positron emission tomography-CT showed pancreatic malignant lesions
and multiple metastatic neoplasia of the liver. A gastrinoma with
liver metastasis was suspected on the basis of the patient’s
medical history. The serum gastrin level of the patient (237.97
pg/ml) was abnormal (normal, <100 pg/ml). A new biopsy specimen
from the pancreatic body and tail mass (obtained at the Zhongshan
Hospital, Fudan University, Shanghai, China) indicated a
neuroendocrine carcinoma. Immunohistochemical staining for gastrin,
chromogranin (CgA) and Ki-67 was positive. Gastrinoma of the
pancreatic body and tail with liver metastases was confirmed. The
patient subsequently received treatment with octreotide acetate (20
mg/month) and sunitinib (37.5 mg/day), which had a poor effect.
Finally, a liver transplant was successfully performed for the
patient on June 1, 2011. The patient currently maintains a good
overall condition.
Discussion
The current study presents a rare case of a female
patient with malignant pancreatic neuroendocrine carcinoma with
liver metastases, who was initially misdiagnosed with SPTP. The
potential underlying clinical and pathological factors that may
have led to misdiagnosis are analyzed and listed in the present
study.
First, the majority of gastrinomas are located in
the gastrinoma triangle (comprising of the duodenum and the
pancreas) or in the adjacent lymph nodes (1), whereas the present case of gastrinoma
occurred in an unusual location, the pancreatic body and tail,
which is outside the typical triangle. Therefore, the location
complicated the diagnosis.
Secondly, it is known that gastrohelcosis and
diarrhea are common first signs of a gastrinoma, particularly in
non-functioning cases, while SPTP are generally characterized in
young females with no clinical symptoms prior to the onset of the
illness, negative tumor markers and without a history of peptic
ulcers (6). Combined with the
clinical data of the patient, it is particularly difficult to
initially differentiate between gastrinomas and SPTP. This was
another significant cause of erroneous diagnosis during the early
stage of the disease in the present case.
Thirdly, pathological assessment is the most
reliable diagnostic basis and considered to be the gold standard.
However, it is worth noting that the characteristics of hematoxylin
and eosin (H&E) staining with gastrinoma and solid
pseudopapillary tumors are similar and may be confused, even when
specific typical immunohistochemical indicators, such as Syn or CgA
(7), are observed. Interstitial
hyaline degeneration and false papillary structure are the two
microscopically characterized changes of SPTP and corresponding
changes are observed in the pathological section when performing
H&E staining. Therefore, combined with the clinical data and
experiences of the patient, our pathologists were confident in the
initial diagnosis of SPTP. However, whether pancreatic
neuroendocrine tumors can be diagnosed with any certainty when the
abovementioned immunohistochemical indicators are positive remains
a controversial issue. The associated literatures on this issue
were reviewed and certain studies found that specific SPTP patients
were misdiagnosed with pancreatic neuroendocrine tumors even though
expression of Syn and CgA was positive (7–11).
Thus, the classic index is not completely reliable, however, the
studies also found that E-cadherin and β-catenin were potentially
effective in the differential diagnosis of the two diseases.
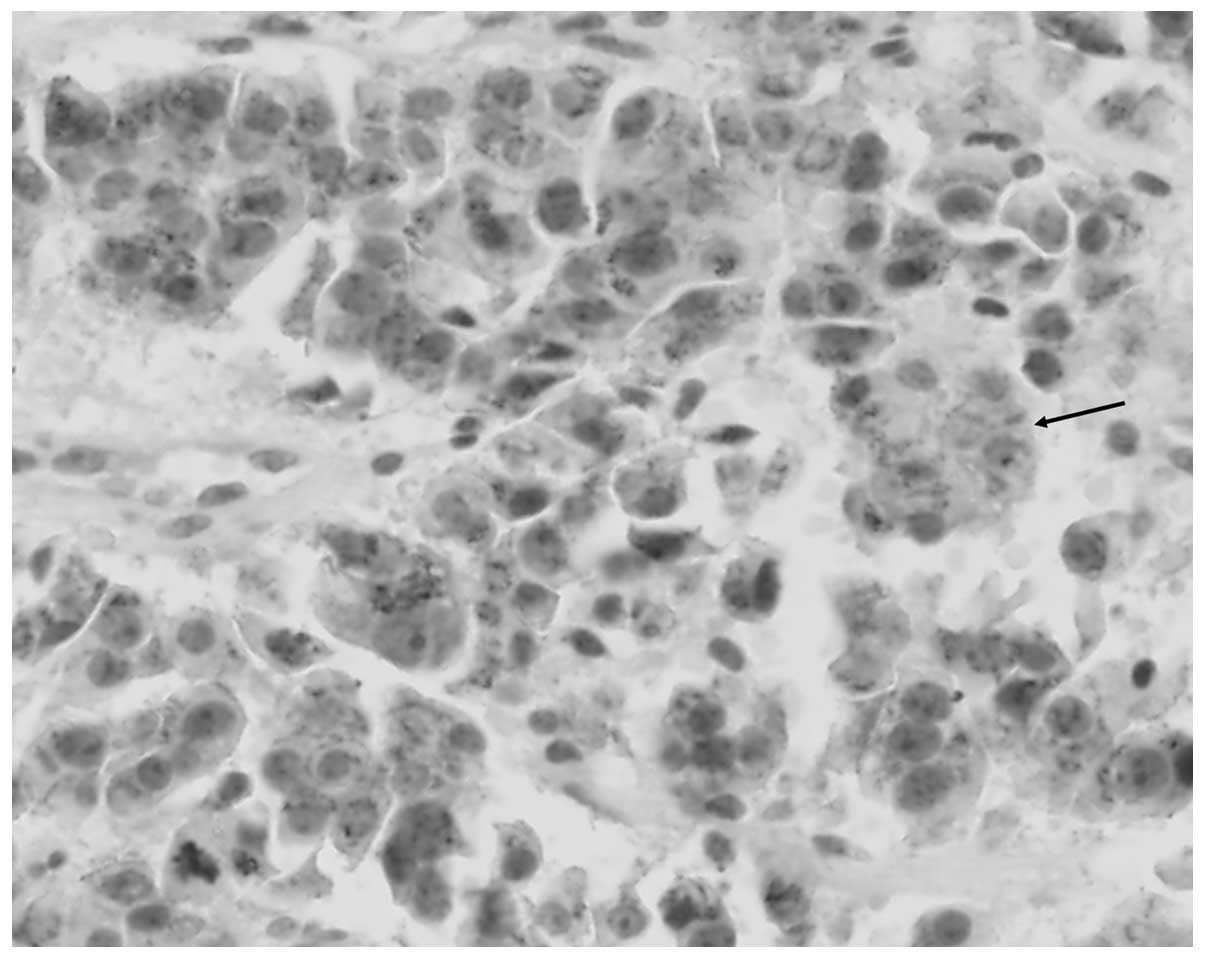
Therefore, in the present study the immunostaining of β-catenin was
conducted, which supplemented our results and were consistent with
previous studies (Fig. 3). However,
this conclusion requires support from large-scale clinical
trials.
Finally and most significantly, a monism, rather
than pluralism, approach is required to diagnose the disease. The
patient in the present case experienced intractable recurrent
peptic ulcers and multiple low-density liver lesions developed
continuously during the postoperative follow-up period. If
clinicians are able to connect these novel conditions with previous
surgical history, it may allow a correct revised diagnosis to be
obtained promptly following surgery.
Therefore, a serious misdiagnosis emphasizes the
requirement for a more accurate differential diagnosis of such a
rare case of a disease without a typical clinical manifestation.
The aim of the present study was to focus the attention of
clinicians on this disease in order to improve its diagnosis and
treatment to a certain extent.
In conclusion, pancreatic gastrinoma should be
carefully differentiated from SPTP during diagnosis. Immunostaining
of E-cadherin and β-catenin may be necessary to aid with the
differential diagnosis.
Acknowledgements
This study was partly supported by the National
Natural Science Foundation of China (grant nos. 81172364 and
81201906).
References
|
1
|
Jensen RT, Niederle B, Mitry E, et al;
Frascati Consensus Conference; European Neuroendocrine Tumor
Society. Gastrinoma (duodenal and pancreatic). Neuroendocrinology.
84:173–182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pachera S, Yokoyama Y, Nishio H, et al: A
rare surgical case of multiple liver resections for recurrent liver
metastases from pancreatic gastrinoma: liver and vena cava
resection. J Hepatobiliary Pancreat Surg. 16:692–698. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamaguchi M, Yamada Y, Hosokawa Y, et al:
Long-term suppressive effect of octreotide on progression of
metastatic gastrinoma with multiple endocrine neoplasia type 1:
seven-year follow up. Intern Med. 49:1557–1563. 2010.PubMed/NCBI
|
|
4
|
Roy PK, Venzon DJ, Shojamanesh H, et al:
Zollinger-Ellison syndrome. Clinical presentation in 261 patients.
Medicine (Baltimore). 79:379–411. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bosman FT, Cameiro F, Hruban RH and Theise
ND: WHO Classification of Tumors of the Digestive system. 4th
edition. IARC Press; Lyon: 2010
|
|
6
|
Kosmahl M, Pauser U, Peters K, Sipos B,
Lüttges J, Kremer B and Klöppel G: Cystic neoplasms of the pancreas
and tumor-like lesions with cystic features: a review of 418 cases
and a classification proposal. Virchows Arch. 445:168–178. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu BA, Li ZM, Su ZS and She XL:
Pathological differential diagnosis of solid-pseudopapillary
neoplasm and endocrine tumors of the pancreas. World J
Gastroenterol. 16:1025–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka Y, Kato K, Notohara K, et al:
Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation
in pancreatic solid-pseudopapillary neoplasm. Cancer Res.
61:8401–8404. 2001.PubMed/NCBI
|
|
9
|
Kim MJ, Jang SJ and Yu E: Loss of
E-cadherin and cytoplasmic-nuclear expression of beta-catenin are
the most useful immunoprofiles in the diagnosis of
solid-pseudopapillary neoplasm of the pancreas. Hum Pathol.
39:251–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abraham SC, Klimstra DS, Wilentz RE, et
al: Solid-pseudopapillary tumors of the pancreas are genetically
distinct from pancreatic ductal adenocarcinomas and almost always
harbor beta-catenin mutations. Am J Pathol. 160:1361–1369. 2002.
View Article : Google Scholar
|
|
11
|
Tanaka Y, Notohara K, Kato K, et al:
Usefulness of beta-catenin immunostaining for the differential
diagnosis of solid-pseudopapillary neoplasm of the pancreas. Am J
Surg Pathol. 26:818–820. 2002. View Article : Google Scholar : PubMed/NCBI
|