Introduction
Colorectal adenocarcinoma is one of the most common
types of malignant tumor, with an increasing prevalence over recent
years. It has the second highest incidence rate of all types of
cancers and it is the second most common cause of cancer-associated
mortality in Europe (1,2). Carcinogenesis of colorectal
adenocarcinoma includes numerous genomic alterations and is a
multi-step process. Thus far, a number of genes, including K-ras,
DCC, p53, nm23, β-catenin, c-Myc, cyclin-dependent kinases and the
caspase family, have been reported to be associated with the
tumorigenesis and development of colorectal adenocarcinoma
(3–6). However, the mechanism of tumorigenesis
remains unclear. Therefore, the genes that may be associated with
tumorigenesis require further investigation, as understanding the
molecular mechanism of this process may aid with cancer prevention,
early diagnosis and effective treatment.
The FBXL20 gene contains 10,381 base pairs (bps),
with a 1,308-bp open reading frame. It is located on the human
chromosome 17q21.2, and predicts to encode a 436-amino-acid protein
that contains an F-box motif, which is the key feature of F-box
proteins (FBPs). The essential role of FBPs has been demonstrated
in studies of various types of species for ubiquitin-mediated
degradation of cellular regulatory proteins. Welcker et al
(7) reported that the F-box and WD
repeat domain containing 7 (Fbw7) tumor suppressor, a member of the
F-Box family, regulates glycogen synthase kinase 3 (GSK3)
phosphorylation-dependent c-Myc protein degradation. Furthermore,
c-Myc proteins regulate cell growth and division in numerous types
of human cancer. The study showed that phosphorylation of c-Myc on
threonine-58 by GSK3 regulates the binding of Fbw7 to c-Myc.
Therefore, the activation of c-Myc is a significant oncogenic
consequence of the loss of Fbw7 in cancer (7). In another study, it was shown that the
accumulation of cyclin-dependent kinase inhibitor, p27 was caused
by S-phase kinase-associated protein 2, another member of the F-Box
family, and the upregulated p27 level may be a good indicator of
proliferative activity and poor prognosis (8). Similar types of studies have been
carried out to investigate the structure and function of the F-Box
family members. These studies demonstrated that the F-Box family
members are significant in tumorigenesis and development by
inducing the specific targeting proteins into the ubiquitin
proteasome process.
Our previous study on FBXL20 showed that the gene
was critical in the abnormal Wnt signaling pathway, as the
β-catenin expression level was significantly decreased after
silencing the FBXL20 gene in the colon adenocarcinoma SW480 and
SW620 cells (9). It was also
identified that FBXL20 was potentially involved in the
ubiquitin-mediated degradation process of E-cadherin and the SET
nuclear oncogene. The viability of the colon cells, that
transfected small interfering RNA targeted to the FBXL20 gene, was
significantly inhibited. In addition, the marked increase of the
E-cadherin expression level and the significant decrease of the
c-Myc expression level was due to the decreased β-catenin
expression level in the cytoplasm. The E-cadherin/catenin complex,
formed by β-catenin and E-cadherin, was significant in maintaining
the structural integrity of the epithelial cells, inhibiting the
migration of carcinoma cells and metabolism. It was also found that
the SET expression level was significantly increased subsequent to
knocking-down the FBXL20 expression level in the colon cell lines
(9). Additionally, Amold et
al (10) identified that
protein phosphatase-2A (PP2A) dephosphorylates Axin, which leads to
the destabilization and degradation of Axin. In our previous study,
SET expression was observed to be upregulated, whereas PP2A
expression was downregulated (9).
Therefore, the reduced level of PP2A resulted in a low level of
β-catenin due to an accumulation of the Axin-adenomatous polyposis
coli (APC)-casein kinase 1-GSK3β complex.
To the best of our knowledge, there are no studies
regarding human colorectal adenocarcinoma that identify the
biological activity of colon cancer cells or the mechanism of
FBXL20 upregulation in the colon cell lines. The aim of the present
study was to determine whether colon cancer cells, which
overexpressed FBXL20, showed signs of an abnormal Wnt signaling
pathway by measurement of the β-catenin, E-cadherin, SET, p53,
caspase 3, PP2A, c-Myc and Axin expression levels, in addition,
cell proliferation and migration ability were observed.
Materials and methods
Cell culture
Colorectal adenocarcinoma cell lines (Lovo, SW480,
SW620, Ls174T, HCT116 and HT29) were purchased from the American
Type Culture Collection (Manassas, VA, USA). The colorectal cancer
(CRC) cell lines were cultured in Dulbecco’s modified Eagle’s
medium supplemented with 10% heat-inactivated fetal bovine
serum.
Plasmid construction and cell
transfection
The oligonucleotides containing the whole coding DNA
sequences of the FBXL20 gene were synthesized through a chemical
method (Sangon Biotech, Shanghai, China). The annealed
complementary oligonucleotides were subsequently inserted into the
BamH1/BbsI site of the pReceiver-M03 expression
plasmid. The engineered pReceiver-M03-FBL20 expression plasmid was
verified by restriction digestion and sequenced by the Beijing
Genomic Institute (Beijing, China). The recombinant
pReceiver-M03-negative control (NC) vector was obtained as
described above, however, the inserted sequence was not homologous
with any human gene. The Lovo cells were transfected with 1 μg
pReceiver-M03-FBL20 expression plasmid according to the
manufacturer’s instructions (Lipofectamine 2000; Invitrogen Life
Technologies, Carlsbad, CA, USA).
Total RNA and protein extraction
Total RNA was extracted from the colorectal
adenocarcinoma cells by the TRIzol reagent (Invitrogen Life
Technologies) and purified with RNeasy columns (Tiangen Biotech
Co., Ltd,, Beijing, China). An RNase-Free DNase kit (Takara Bio.,
Inc., Otsu, Shiga, Japan) was used to digest DNA. Cells were lysed
with radioimmunoprecipitation assay buffer (Beyotime Co., Shanghai,
China) containing protease inhibitors (Sigma-Aldrich, St. Louis,
MO, USA) and protein quantification was conducted using a
bicinchoninic acid protein assay kit (Beyotime Co.).
Proliferation assays
The proliferation assay was performed 24 h after
transfecting the pReceiver-M03-FBL20 expression plasmid into a
96-well plate by the addition of 10 μl cell counting kit 8 (CCK8;
Sigma-Aldrich) into the medium, followed by incubation for 30 min
under normal cell culture conditions. Cell viability was measured
by absorbance at 450 nm using a microplate reader (Bio-Rad,
Hercules, CA, USA).
Cell invasion and wound healing
assay
Cell invasion assays were performed with the 24-well
cell invasion assay kit (Sigma-Aldrich) according to the
manufacturer’s instructions. Cells (n=900) were resuspended in
culture medium without FBS and placed in the upper Transwell
chamber in triplicate. After a 24-h incubation at 37°C (5%
CO2 atmosphere), the cells in the upper surface of the
membrane were removed using a cotton swab and the cells that had
migrated to the lower surface of the membrane were fixed and
stained. The migrated cells on the lower surface of the membrane
filter were scored from six random fields under a CKX31 microscope
(magnification, ×400; Olympus, Tokyo, Japan). The results are shown
as percentage migrating rate (%): % = (migrated cells/total cells)
× 100. The wound healing assay was performed according to the
manufacturer’s instructions. To generate a wound field, cells were
cultured until they formed a monolayer around the insert.
Subsequent to removing the insert, an open wound field was
generated and cells were able to migrate from either side of the
gap.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed in triplicate with a
Mastercycler® ep realplex system (Eppendorf, Hamburg,
Germany) using the SYBR Premix Ex Taq mix (Takara) according to the
manufacturer’s instructions. The primers were purchased from Sangon
Biotech. GAPDH served as an endogenous control.
Western blot analysis
The total cell protein (50 μg) was used for western
blotting. The samples were resolved in 10% SDS-PAGE gels and
transferred to polyvinylidene fluoride membranes. The membranes
were immersed in western blocking buffer (Beyotime Co.) for 1 h and
probed with the primary polyclonal antibody against FBL20,
E-cadherin, SET, Axin, PP2A, p53, caspase 3 and β-actin (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C. The
blots were washed in tris-buffered saline containing 0.1% Tween-20
and labeled with horseradish peroxidase-conjugated secondary
anti-rabbit antibody (Santa Cruz Biotechnology, Inc.). Proteins
were enhanced by BeyoECL Plus (Beyotime Co.) for visualization. The
protein expression levels were expressed relative to β-actin
levels.
Statistical analysis
The results presented in the current study are the
means ± standard error of the mean. Statistical analysis was
performed by Student’s t-test, Fisher’s exact probability test and
analysis of variance with SPSS 17.0 software (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
FBXL20 expression is significantly
decreased in the Lovo cell line
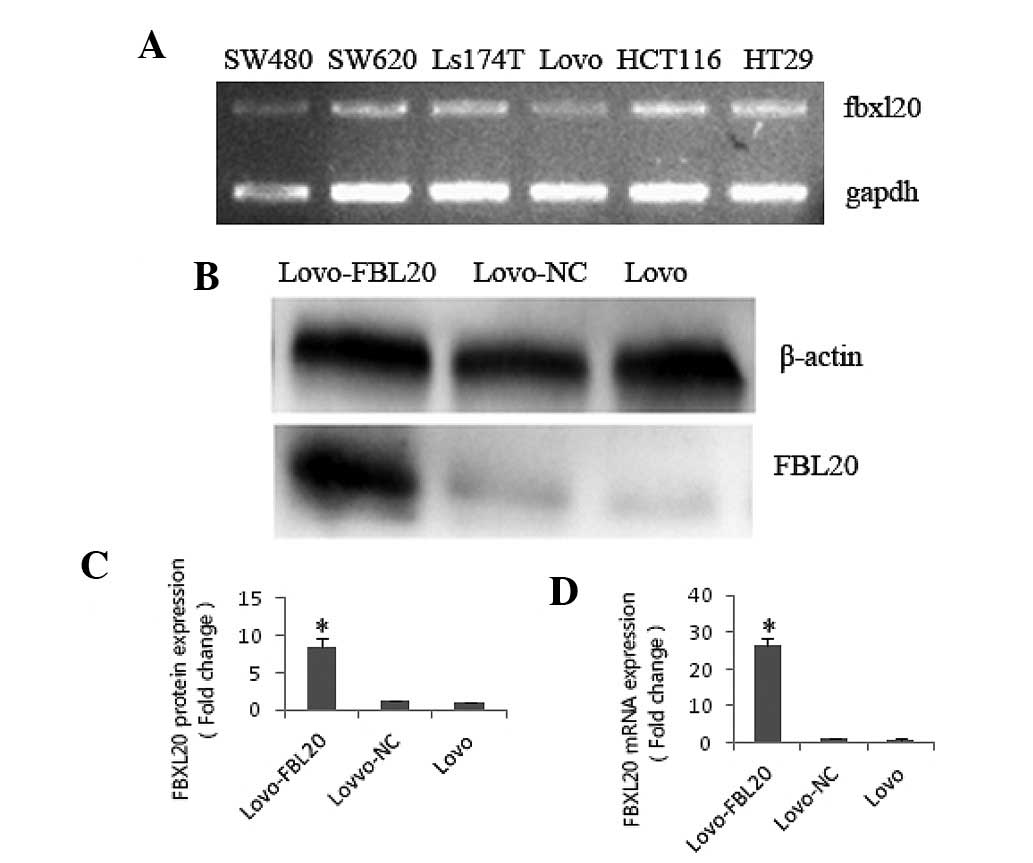
FBXL20 expression levels in the CRC cell lines (five
types) were identified by qPCR. It was shown that the FBXL20
expression level was significantly lower in the Lovo cell line
compared with the SW480, SW620, Ls174T, HT29 and HCT116 cell lines
(Fig. 1A).
FBXL20 expression level in the Lovo-FBL20
cell line
To investigate the function of FBXL20, the
pReceiver-M03-FBL20 expression plasmid, which overexpressed the
FBXL20 gene, was transfected into the Lovo cell line. At 48 h after
transfecting the pReceiver-M03-FBL20 expression plasmid or
pReceiver-M03-NC into the Lovo cells, the FBXL20 expression level
was verified by qPCR (fold change, 27.392) and western blotting
(fold change, 8.43), compared with that in the Lovo cells (Fig. 1B–D).
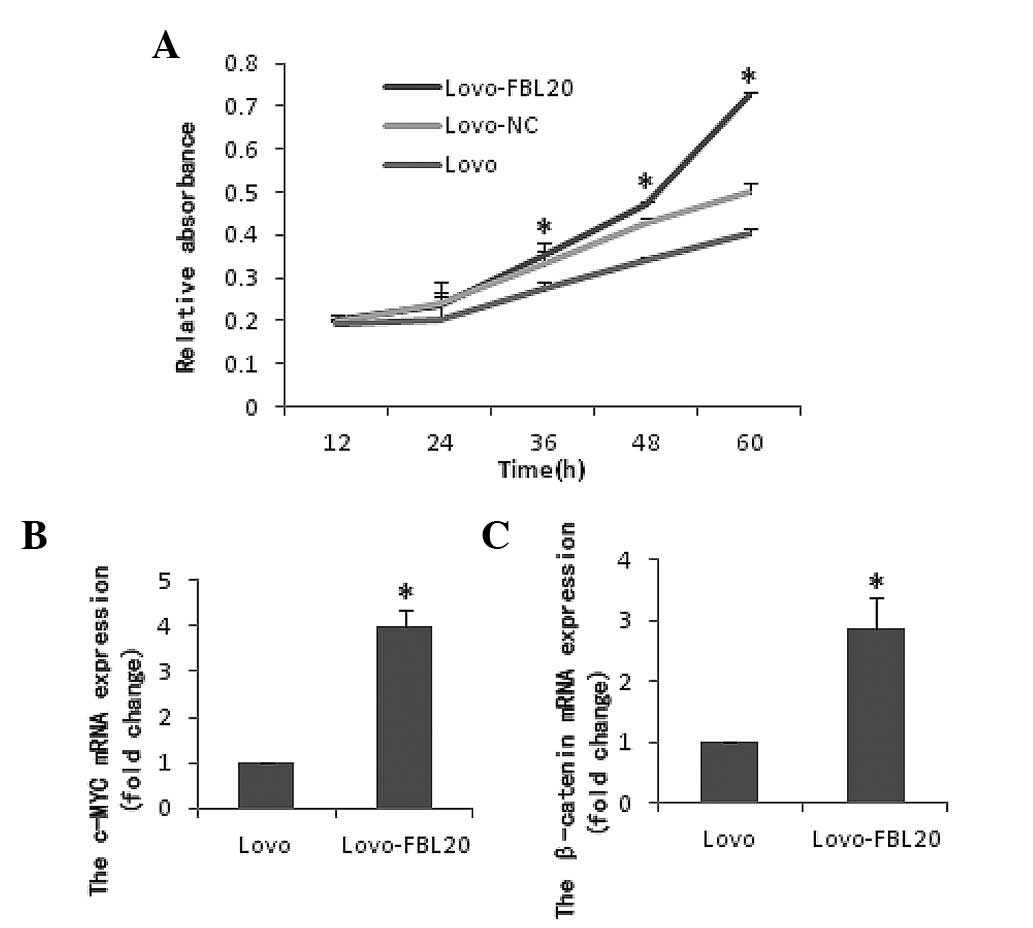
Effect of FBXL20 overexpression on cell
viability and migration in the Lovo-FBL20 cell line
Cell viability (CCK8 assay) and cell invasion
(Transwell chamber and wound healing assays) were performed using
the Lovo cells that were transiently transfected with the
pReceiver-M03-FBL20 expression plasmid. It was observed that the
cell proliferation and migration activity was significantly
increased in the Lovo-FBL20 cells compared with that of the Lovo
cells. The percentages for the migrating rates in Lovo-FBL20 and
Lovo cells were 42.18% (37.9±85.28%) and 14.05% (12.6±16.05%),
respectively (data not shown). The difference between the
Lovo-FBL20 and Lovo cells was identified to be statistically
significant (P=0.007; Figs. 2 and
3).
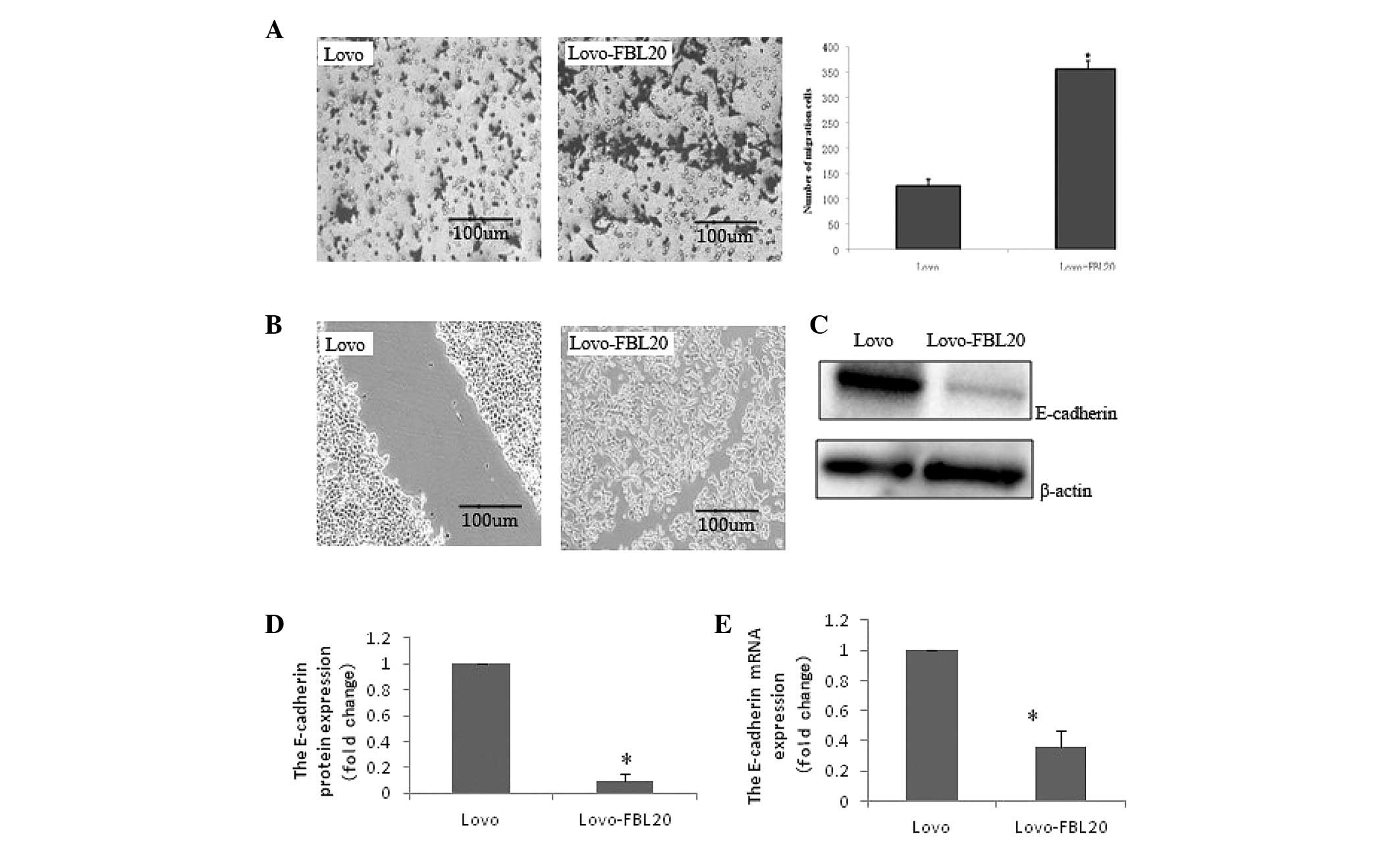
Effect of FBXL20 overexpression on the
expression of E-cadherin, SET, pp2a, Axin, β-catenin, c-Myc, p53
and caspase 3 in the Lovo-FBL20 cells
To investigate the function of FBXL20, specific
molecules were detected in the Lovo-FBL20 cells by qPCR and western
blotting. qPCR and western blotting demonstrated that the mRNA
expression and protein expression level of E-cadherin in the
Lovo-FBL20 cells was significantly reduced compared with that in
the Lovo cells, and the reduction rates were 63.50 and 90.20%, for
mRNA and protein expression, respectively, which was considered to
be statistically significant (P=0.001 and P<0.001; Fig. 3)
The mRNA levels of β-catenin and c-Myc in the
Lovo-FBL20 cells were significantly upregulated compared with the
Lovo cells. The β-catenin and c-Myc expression levels in the
Lovo-FBL20 cells were 2.870 and 3.997-fold, respectively, compared
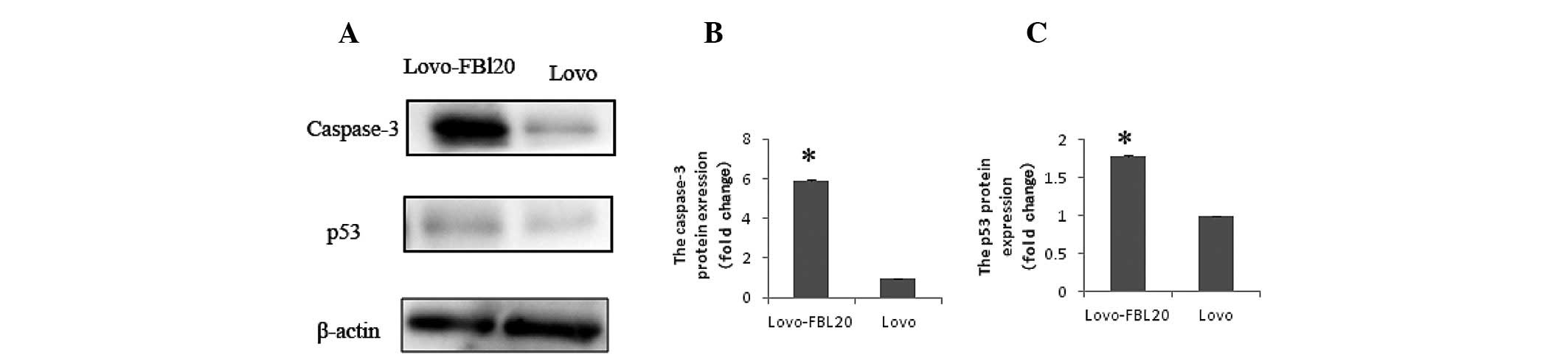
with that in the Lovo cells (P<0.001 and P<0.001; Fig. 2). Additionally, it was found that
the p53 and caspase 3 expression levels were significantly
increased in the Lovo-FBL20 cell line. The p53 and caspase 3
expression levels in Lovo-FBL20 were 1.780 and 5.892-fold,
respectively, compared with that in the Lovo cells, which was
identified to be a statistically significant difference (P=0.007
and P<0.001; Fig. 4).
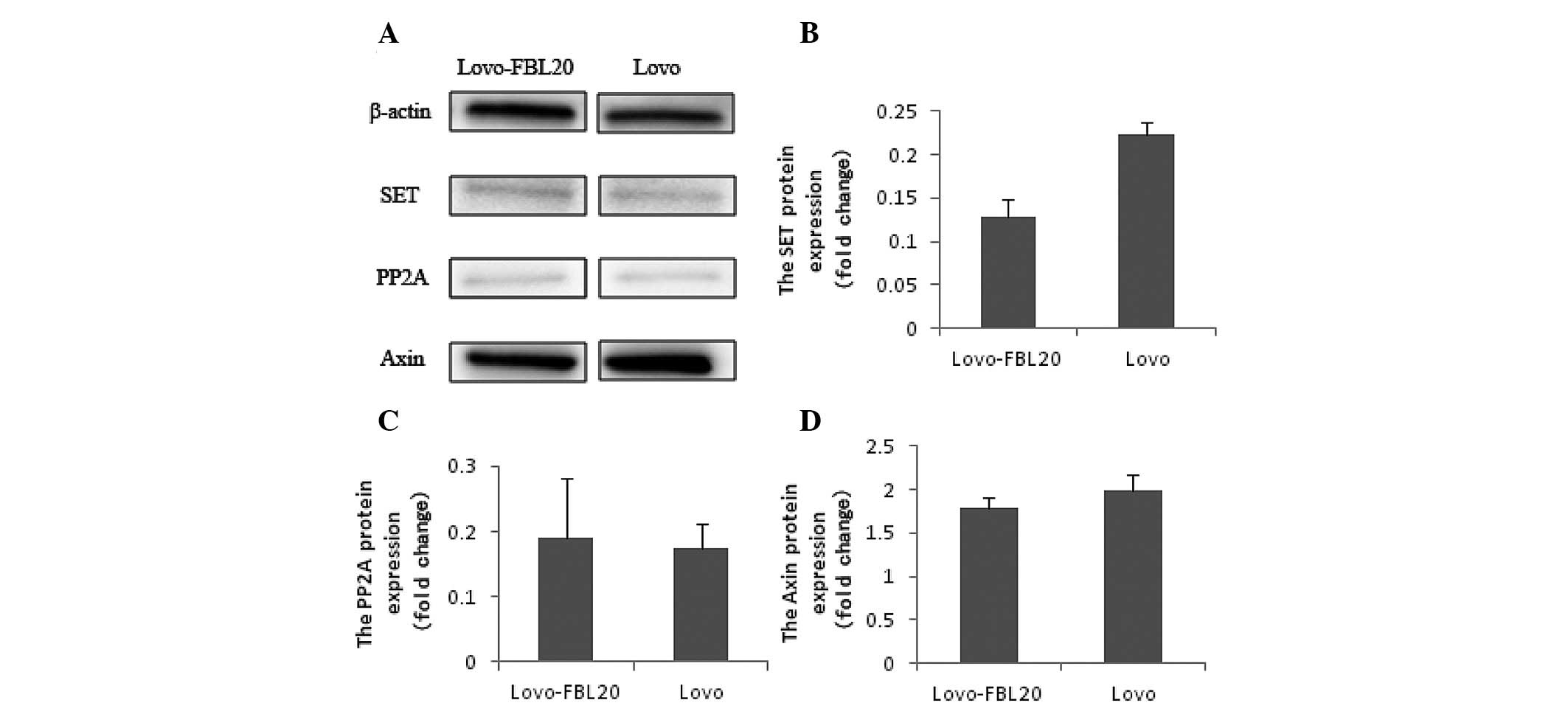
However, the protein expression levels of SET, PP2A
and Axin were not found to be significantly different between the
Lovo-FBL20 and Lovo cells by western blot analysis (Fig. 5).
Discussion
CRC remains a predominant cause of cancer mortality
worldwide. Numerous studies have documented the significant roles
of the F-Box family in the initiation of CRC. Grim et al
(11) identified that the
simultaneous deletion of Fbw7 and p53 resulted in highly penetrant,
aggressive, metastatic adenocarcinoma, and allografts that
originated from these tumors became highly malignant
adenocarcinoma. In another study, FWD1, an F-box/WD40-repeat
protein, was shown to form a specific multimolecular complex with
β-catenin, Axin, GSK-3β and APC. The level of β-catenin in the
cytoplasm was decreased by FWD1-facilitated ubiquitination and
degradation of β-catenin (12). In
addition, the E-cadherin and SET expression levels were markedly
upregulated when the FBXL20 gene was knocked out in colorectal
adenocarcinoma. However, no studies were found concerning the
association between FBXL20, E-cadherin and SET.
In the present study, cell proliferation activity
was significantly increased in the Lovo-FBL20 cells, compared with
that of the corresponding control cells. The selective
ubiquitination of proteins by ubiquitin E3 ligases exhibits a
significant regulatory role in the control of cell differentiation,
growth and transformation, and the dysregulation of ubiquitin E3
ligases is often associated with pathological outcomes, including
tumorigenesis (13). Cell
proliferation was notably reduced following inhibition of RNF5 (a
ubiquitin E3 ligase) expression and resulted in reorganization of
the actin cytoskeleton in reaction to the stress in MCF-7; however,
this was not observed in p53 mutant breast cancer cells, indicating
a p53-dependent function (14).
Glycoprotein 78 (gp78) is a cell surface receptor that also
functions as an E3 ubiquitin ligase in the endoplasmic
reticulum-associated degradation pathway. A study by Tsai et
al (15) reported that the
expression of gp78 exhibits a causal role in the metastasis of an
aggressive human sarcoma. Furthermore, gp78 is associated with, and
targets, the transmembrane metastasis suppressor, KIA1, for
degradation. Suppression of gp78 increases the abundance of KIA1
and reduces the metastatic potential of tumor cells (15). Based on the observation of the
present study, it is speculated that the increased proliferation
and migration abilities of the Lovo-FBL20 cells resulted from the
upregulated expression level of FBXL20.
The mechanism by which the upregulated FBXL20
expression level leads to the increased proliferation of the
colorectal adenocarcinoma cells remains unknown. The c-Myc and
β-catenin expression levels in the present study were significantly
upregulated in the Lovo-FBL20 cells compared with those in the
corresponding control cells. Numerous studies have confirmed that
the development of CRC was closely correlated with the activation
of the Wnt signaling pathway (9,16,17).
Furthermore, the activated Wnt signaling pathway was determined by
the expression level of β-catenin in the cytoplasm as well as
c-Myc. In addition, the c-Myc expression level was correlated with
the proliferation capacity of neoplastic cells (18). Stimulation of the Wnt/β-catenin and
Ras oncogene by P21-activated kinase 1 increases the progression of
CRC and enhances survival by the stimulation of hypoxia-inducible
factor 1α and β-catenin (19). In
another study, Wu et al (20) showed that β-catenin increased T-cell
factor 4 (TCF4) transcription activity and expression of its target
genes, including cyclin D1 and c-Myc, in CRC cells. Correlation
analysis showed that β-catenin expression in CRC tissues was
positively associated with c-Myc expression. These findings raised
the possibility that the increased proliferation capacity of the
Lovo-FBL20 cells was due to the upregulation of c-Myc expression,
as a consequence of the activation of the Wnt signaling pathway
caused by accumulation of β-catenin in the cytoplasm.
A notable finding of the present study is that the
E-cadherin expression level was significantly reduced and the
migration ability of the Lovo-FFBL20 cells was significantly
increased, which indicated that the migration ability was
correlated with the E-cadherin expression level. These data also
support the recent observation that downregulation of E-cadherin in
pancreatic adenocarcinoma may promote invasion and metastasis of
cancer cells (21). In addition,
another study showed that induced expression of NFATc1CA
downregulated E-cadherin expression and increased the invasive
activity in tumor xenografts in vivo (22). Recent studies have shown that
numerous E3 ubiquitin ligases, including Cb1, smurf1, BCA2 and
SCF(β-TRCP), are critical in cell adhesion and migration (21). Cell adhesion is negatively regulated
by Cb1 via ubiquitination of α integrin and Rap1, and inhibits
actin polymerization by ubiquitination of mDab1 and WAVE2, while
SCF(β-TRCP) ubiquitinates Snail (a transcriptional repressor of
E-cadherin) to inhibit cell migration. All of the aforementioned
studies are consistent with the results of the current study which
identified that the FBXL20 mRNA and protein expression was markedly
higher in the Lovo-FBL20 cells than the control Lovo cells, and the
migration rates of the Lovo-FBL20 cells were significantly higher
than those of the Lovo cells. Therefore, we hypothesize that the
higher migration rates are associated with the higher FBXL20 RNA
and protein expression levels. It is well known that E-cadherin
expression negatively correlates with migration activity, and
FBXL20 is a type of E3 ubiquitin ligase, which functions via
ubiquitin in specific cells. Therefore, we suspect that FBXL20 may
be involved in the ubiquitination of E-cadherin (23).
P53 is a well-known tumor suppressor gene whose loss
of function emphasizes the most common genetic alteration in human
malignancy. It is involved in DNA damage repair, cell cycle
regulation, apoptosis, ageing and cellular senescence (18). In the present study, the p53 and
caspase 3 expression levels were significantly increased in the
Lovo-FBL20 cells. Li et al (24) reported that the Wnt signaling
pathway regulator, Axin, regulates p53-dependent apoptosis in
response to DNA damage. However, in another study, it was shown
that Axin downregulates TCF4 transcription via β-catenin,
independently of p53, and that Axin may inhibit the proliferation
and invasion of lung cancer cells via β-catenin (25). In addition, in the present study,
there was no statistical difference identified between the
Lovo-FBL20 and Lovo cells with regard to the Axin level. Thus, Axin
may upregulate caspase 3 transcription independent of p53.
In the present study, SET expression was not found
to be correlated with an increased FBXL20 expression in the
Lovo-FBL20 cells. Although, it was shown that the SET expression
level was markedly increased following silencing of the FBXL20 gene
in the colorectal adenocarcinoma cell lines, SW480 and SW620. These
findings support the results that the Axin level did not increase
with the upregulation of p53 in the Lovo-FBL20 cells. PP2A is a
multi-subunit serine/threonine phosphatase that is integral
for intracellular signaling, cell cycle progression and gene
regulation. Ikeda et al (26) reported that the heterodimeric form
of PP2A directly binds to Axin, and subsequently Axin is
dephosphorylated and induced into the ubiquitin degradation
process. In another study, SET has been shown to be a natural
inhibitor of the PP2A protein (27). Notably, the marked difference in
PP2A expression level between the Lovo-FBL20 and Lovo cells was not
found in the present study. These novel findings highlight that
FBXL20 does not participate directly in the ubiquitin process of
SET.
In conclusion, the present data on the roles of
FBXL20 in colorectal adenocarcinoma cells are comparable with other
F-Box family members, which mediate the specific substrate protein
into the ubiquitin proteasome pathway. The present study indicated
that FBXL20 may mediate the ubiquitin degradation of E-cadherin
resulting in an increased invasive ability of malignant cells.
However, further studies are required to identify all of the
factors that are involved in the FBXL20-mediated ubiquitin
proteasome process in colorectal adenocarcinoma.
Acknowledgements
The present study was supported by the Central
Finance to Support Local University Development Foundation of China
(grant no. SCKBMI-13-003) and research funds from the Science and
Technology Agency of Sichuan Province (grant no. 2012ZZ008).
References
|
1
|
Vonlaufen A, Troillet FX and Armenian B:
Screening for colorectal cancer: recommendations. Rev Med Suisse.
9:754–757. 2013.(In French).
|
|
2
|
Du XL, Cai Y and Symanski E: Association
between chemotherapy and cognitive impairments in a large cohort of
patients with colorectal cancer. Int J Oncol. 42:2123–2133.
2013.PubMed/NCBI
|
|
3
|
George B and Kopetz S: Predictive and
prognostic markers in colorectal cancer. Curr Oncol Rep.
13:206–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ren F, Sheng WQ and Du X: CD33: a cancer
stem cells marker, is used in colorectal cancers. World J
Gastroentrol. 19:2603–2611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith JC, Brooks L, Hoff PM, et al: KRAS
mutations are associated with inferior clinical outcome in patients
with metastatic colorectal cancer, but are not predictive for
benefit with cediranib. Eur J Cancer. 49:2424–2432. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haugen MH, Johansen HT, Pettersen SJ, et
al: Nuclear legumain activity in colorectal cancer. PLoS One.
8:e529802013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Welcker M, Larimore EA, Frappier L and
Clurman BE: Nucleolar targeting of the fbw7 ubiquitin ligase by a
pseudosubstrate and glycogen synthase kinase 3. Mol Cell Biol.
31:1214–1224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miranda-Carboni GA, Krum SA, Yee K, et al:
A functional link between Wnt signaling and SKP2-independent p27
turnover in mammary tumors. Genes Dev. 22:3121–3134. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu JJ, Li K, Dong L and Chen Y: Role of
FBXL20 in human colorectal adenocarcinoma. Oncol Rep. 28:2290–2298.
2012.PubMed/NCBI
|
|
10
|
Amold HK, Zhang X, Daniel CJ, et al: The
Axin1 scaffold protein formation of a degradation complex for
c-Myc. EMBO J. 28:500–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grim JE, Knoblaugh SE, Guthrie KA, et al:
Fbw7 and p53 cooperatively suppress advanced and chromosomally
unstable intestinal cancer. Mol Cell Biol. 32:2160–2167. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kiatagawa M, Hatakeyama A, Shirane M, et
al: An F-box protein, FWD1, mediates ubiquitin-dependent
proteolysis of beta-catenin. EMBO J. 18:2401–2410. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Micel LN, Tentler JJ, Smith PG and
Eckhardt GS: Role of ubiquitin ligases and the protesasome in
oncogenesis: novel targets for anticancer therapies. J Clin Oncol.
31:1231–1238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bromberg KD, Kluger HM, Delaunay A, et al:
Increased expression of the E3 ubiquitin ligase RNF5 is associated
with decreased survival in breast cancer. Cancer Res. 67:8172–8179.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsai YC, Mendoza A, Mariano JM, et al: The
ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1
for degradation. Nat Med. 13:1504–1509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kioras K, Konstantara A, Kotoula V, et al:
The prognostic significance of WNT pathway in surgically-treated
colorectal cancer: β-catenin expression predicts for disease-free
survival. Anticancer Res. 33:4573–4584. 2013.PubMed/NCBI
|
|
17
|
Freeman TJ, Smith JJ, Chen X, et al:
Smad4-mediated signaling inhibits intestinal neoplasia by
inhibiting expression of β-catenin. Gastroenterology. 142:562–571.
2012.PubMed/NCBI
|
|
18
|
Kanwar SS, Yu Y, Nautiyal J, et al: The
Wnt/beta-catenin pathway regulates growth and maintenance of
colonospheres. Mol Cancer. 9:2122010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu KH, Huynh N, Patel O, et al:
P21-activated kinase 1 promotes colorectal cancer survival by
up-regulation by hypoxia-inducible factor-1α. Cancer Lett.
340:22–29. 2013.
|
|
20
|
Wu J, Xie N, Xie K, Zeng J, et al: GPR48,
a poor prognostic factor, promotes tumor metastatis and activates
β-catenin/TCF signaling in colorectal cancer. Carcinogenesis.
34:2861–2869. 2013.PubMed/NCBI
|
|
21
|
Guo S, Xu J, Xue R, et al: Overexpression
of A1BI correlates inversely with E-cadherin expression in
pancreatic adenocarcinoma and may promote lymph node metastasis.
Int J Clin Oncol. March 30–2013.(Epub ahead of print).
|
|
22
|
Oikawa T, Nakamura A, Onishi N, et al:
Acquired expression of NFATc1 downregulates E-cadherin and promotes
cancer cell invasion. Cancer Res. 73:5100–5109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang C: Roles of E3 ubiquitin ligases in
cell adhesion and migration. Cell Adh Migr. 4:10–18. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Q, He Y, Wei L, et al: AXIN is an
essential co-activator for the promyelocytic leukemia protein in
p53 activation. Oncogene. 30:1194–1204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang LH, Xu HT, Han Y, et al: Axin
downregulates TCF-4 transcription via beta-catenin, but not p53,
and inhibits the proliferation and invasion of lung cancer cells.
Mol Cancer. 9:252010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ikeda S, Kishida M, Matsuura Y, et al:
GSK-3beta-dependent phosphorylation of adenomatous polyposis coli
gene product can be modulated by beta-catenin and protein
phosphatase 2A complexed with Axin. Oncogene. 19:537–545. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Irie A, Harada K, Araki N and Nishimura Y:
Phosphorylation of SET protein at Ser171 by protein kinase D2
diminishes its inhibitory effect on protein phosphatase 2A. PloS
One. 7:e512422012. View Article : Google Scholar : PubMed/NCBI
|