Introduction
Taxanes and platinum-containing agents are key drugs
that are used in the chemotherapy for gynecological cancers.
However, in Japanese patients who received optimal debulking
surgery to treat stage II–IV clear-cell carcinoma of the ovary, an
adjuvant chemotherapy regimen combining irinotecan (CPT-11) with
cisplatin (CDDP) has been shown to prolong the progression-free
survival time more than a regimen combining paclitaxel with
platinum (1). In addition, a
combination of CPT-11 and mitomycin C (MMC) has been reported to be
effective in elderly Japanese patients with gynecological cancers
who did not respond to the combination regimen of taxanes and
platinum (2). Thus, although not
currently used as the standard chemotherapy for gynecological
conditions, regimens that include CPT-11 may have a useful
role.
However, CPT-11 occasionally causes severe
neutropenia. The inherited factors associated with the occurrence
of this side-effect include genetic variants of uridine diphosphate
glucuronosyltransferase 1A1 (UGT1A1), such as the *6
and *28 alleles (3–14). The risk of severe neutropenia is
increased in individuals who are homozygous for the *28
allele (3–5,7).
However, this association is not observed when CPT-11 is
administered at a low dose for lung or colorectal cancer (9,15,16).
Low-dose CPT-11 regimens, including CPT-11 + CDDP or CPT-11 + MMC,
are used in certain gynecological cancers. However, associations
between the occurrence of severe neutropenia and the
UGT1A1*28 variant in these cases is not well known. By
contrast, UGT1A1*6 is a variant found in the Asian
population at a frequency higher than that of *28 (17–19).
In Japanese patients with colorectal and lung cancer, the
*6/*28 and *6/*6 genotypes are significantly
correlated with the occurrence of severe neutropenia (7). This may indicate that these genotypes
may be risk factors for CPT-11-induced severe neutropenia in
Japanese patients with gynecological cancers. Furthermore, a study
of the CPT-11 + CDDP regimen in gynecological conditions
demonstrated that the risk of severe neutropenia is higher in
patients with the *1/*6 genotype than in patients with the
*1/*1 genotype (13).
However, this study did not clarify the affect of the *6/*28
and *6/*6 genotypes on the risk of developing
neutropenia.
In addition, the 421C>A (Q141K) variant of
ATP-binding cassette sub-family G member 2 (ABCG2), which
encodes the breast cancer resistance protein (BCRP), a transporter
known to target various anticancer drugs, including CPT-11, has
been reported to reduce the expression of ABCG2 and cause
resistance to CPT-11 in vitro (20). However, the 421C>A variant is not
associated with CPT-11-induced severe neutropenia in patients with
lung and colorectal cancer (8,21).
Whether this single-nucleotide polymorphism (SNP) has any
association with CPT-11-induced neutropenia in gynecological cancer
is not known.
Thus, investigations of the associations between the
occurrence of severe neutropenia during treatment with a low-dose
CPT-11 regimen and these genetic variants in gynecological
malignancies are, at best, incomplete. The present study was
designed to clarify the role of these genetic factors in the
occurrence of grade 4 neutropenia in patients treated with CPT-11 +
CDDP or CPT-11 + MMC chemotherapy for gynecological cancer.
Materials and methods
Patients
The Institutional Review Board of the National
Hospital Organization Hokkaido Cancer Center (Sapporo, Japan)
approved the present study, and informed consent was obtained from
all patients. Subjects were Japanese patients who received
CPT-11-based chemotherapy in the Department of Gynecology, National
Hospital Organization Hokkaido Cancer Center. The chemotherapy
regimens used were CPT-11 + CDDP and CPT-11 + MMC. All the patients
were evaluated to ensure they exhibited sufficient organ function,
including bone marrow function, prior to beginning the regimens
involving CPT-11. No patients were receiving drugs known to
interact with CPT-11. The following were the exclusion criteria of
this study: Previous CPT-11 administration, an Eastern Cooperative
Oncology Group performance status of ≥3 and an age of <18 or
>80 years old.
The clinical data, including the neutrophil count,
of these patients were retrospectively investigated using
information obtained from medical records. The absolute neutrophil
count (ANC) nadir value was assessed during the first cycle of the
regimen containing CPT-11. Severe neutropenia (grade 4) was
determined using the Common Terminology Criteria for Adverse
Events, version 3.0 (22).
Genotyping
Genomic DNA was isolated from peripheral blood that
was anticoagulated with K2-EDTA using a Puregene DNA
Isolation kit (Qiagen, Hilden, Germany), according to the
manufacturer’s instructions. The genotypes of the UGT1A1
gene, including *6 and *28, were determined according
to a previously described method (23). The presence of the 421C>A
mutation in exon 5 of the ABCG2 gene was determined by PCR,
followed by sequencing. The primers used in the PCR and sequencing
of this variant were synthesized by Sigma-Genosys Japan, Inc.
(Ishikari, Japan). The sequences of the forward and reverse primers
were 5′-GGTTCATCATTAGCTAGAACTTTAC-3′ and
5′-TGGAAAGCAACCATTTTTGA-3′, respectively. The PCR amplification was
conducted using a PTC-200 pelitier thermal cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and AmpliTaq Gold® 360
Master Mix (Life Technologies, Inc., Carlsbad, CA, USA). The
cycling conditions used were as follows: Initial denaturation at
95°C for 10 min, subsequent denaturation at 95°C for 30 sec,
annealing at 58°C for 30 sec and primer extension at 72°C for 30
sec, repeated for 30 cycles, followed by a final extension at 72°C
for 7 min. The ABCG2 genotypes (421C>A) were determined
by direct sequencing of the purified PCR products.
Statistics
The Hardy-Weinberg equilibrium (HWE) test of the
genotype frequency of UGT1A1 and ABCG2 in the
subjects was conducted using Fisher’s exact test. Spearman’s rank
correlation test was used to analyze the correlation between the
total dose of CPT-11 and the ANC nadir values. Mann-Whitney’s U
test with Bonferroni’s correction was applied for the comparison of
the association of the genotypes of UGT1A1 and ABCG2
with the ANC nadir values. In the univariate analysis of the
characteristics of the patients prior to chemotherapy,
Mann-Whitney’s U test was applied to compare the values between
grade 0–3 (G0-3) and grade 4 (G4) neutropenia groups. For the
univariate analysis of the genotypes, previous treatments,
regimens, type of cancer and performance status, Fisher’s exact
test was applied to compare the values between the two groups.
Variables with P<0.1 in these univariate analyses were then
adopted as explanatory variables when conducting the multivariate
logistic regression analysis, in which the incidence of G4
neutropenia was a dependent variable. The SPSS Statistics 21
software (IBM Japan Inc., Tokyo, Japan) and GraphPad Prism 5.0
(GraphPad Prism Software, San Diego, California, USA) were used for
statistical analyses. A two-tailed value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 44 patients (24 with ovarian cancer, 10
with endometrial cancer, 9 with cervical cancer and 1 with a tumor
of the lower abdominal wall) were enrolled and evaluated during the
period between July 2007 and September 2011. The patients received
the following chemotherapy: Either 40–60 mg/m2 CPT-11
(on days 1, 8 and 15) and 40–60 mg/m2 CDDP (on day 1;
n=22) or 70–150 mg/m2 CPT-11 (on days 1 and 15 or on
days 1, 8 and 15) and 4–10 mg/m2 MMC (on day 1 or on
days 1 and 15; n=22). In total, 10 patients developed G4
neutropenia (22.7%). The patient characteristics prior to
chemotherapy are shown in Tables I
and II.
 | Table IAssociations between patient
characteristics prior to CPT-11-based chemotherapy and the toxicity
outcome of neutropenia. |
Table I
Associations between patient
characteristics prior to CPT-11-based chemotherapy and the toxicity
outcome of neutropenia.
| G0-3 neutropenia
(n=34) | G4 neutropenia
(n=10) | |
|---|
|
|
| |
|---|
|
Characteristics | Median | Range | Median | Range | P-valuea |
|---|
| Age, years | 55 | 18–79 | 59.5 | 52–72 | 0.098 |
| Height, cm | 153.5 | 147.0–167.7 | 151.4 | 138.5–161.0 | 0.202 |
| Weight, kg | 54.7 | 38.7–95.7 | 51.0 | 42.0–65.5 | 0.481 |
| BSA,
m2 | 1.49 | 1.27–1.95 | 1.46 | 1.24–1.67 | 0.300 |
| BMI,
kg/m2 | 21.8 | 16.0–40.6 | 22.4 | 19.3–28.4 | 0.933 |
| WBC,
mm3 | 4465 | 2590–8940 | 4290 | 3070–7230 | 0.911 |
| Neutrophils,
mm3 | 2590 | 970–7108 | 2652 | 1627–6116 | 0.737 |
| Total bilirubin,
mg/dl | 0.52 | 0.20–1.18 | 0.48 | 0.22–1.04 | 0.889 |
| Albumin, mg/dl | 3.9 | 2.9–4.5 | 3.9 | 2.3–4.3 | 0.966 |
| AST, IU/l | 20 | 12–87 | 15.5 | 11–23 | 0.018 |
| ALT, IU/l | 17 | 5–121 | 10.5 | 4–24 | 0.001 |
| γ-GTP, IU/l | 24.2 | 11–89 | 15.5 | 7–35 | 0.059 |
| ALP, IU/l | 245 | 138–696 | 238.5 | 169–376 | 0.600 |
| SCr, mg/dl | 0.67 | 0.37–1.33 | 0.645 | 0.48–0.92 | 0.793 |
 | Table IICorrelations between the development
of grade 4 neutropenia and genotypes, previous treatments,
regimens, cancer types and performance status. |
Table II
Correlations between the development
of grade 4 neutropenia and genotypes, previous treatments,
regimens, cancer types and performance status.
| Neutropenia, n
(%) | |
|---|
|
| |
|---|
|
Characteristics | G0-3 | G4 | P-valuea |
|---|
| Total patients | 34 (77.3) | 10 (22.7) | |
| Genotype |
| UGT1A1 |
| Dominant
model | | | 0.287 |
| −/−b | 21 (84.0) | 4 (16.0) | |
| −/+c, +/+d | 13 (68.4) | 6 (31.6) | |
| Recessive
model | | | 0.037 |
| −/−b, −/+c | 31 (83.8) | 6 (16.2) | |
| +/+d | 3 (42.9) | 4 (57.1) | |
| ABCG2
421C>A |
| Dominant
model | | | 0.456 |
| C/C | 23 (82.1) | 5 (17.9) | |
| C/A, A/A | 11 (68.8) | 5 (31.2) | |
| Recessive
model | | | 0.120 |
| C/C, C/A | 31 (81.6) | 7 (18.4) | |
| A/A | 3 (50.0) | 3 (50.0) | |
| Previous
treatment | | | 1.000 |
| No | 5 (83.3) | 1 (16.7) | |
| Yes | 29 (76.3) | 9 (23.7) | |
| Regimen | | | 0.069 |
| CPT-11 + CDDP | 20 (90.9) | 2 (9.1) | |
| CPT-11 + MMC | 14 (63.6) | 8 (36.4) | |
| Type of cancer | | | 0.147 |
| Ovarian | 21 (87.5) | 3 (12.5) | |
| Other | 13 (65.0) | 7 (35.0) | |
| Performance
status | | | 1.000 |
| 0 | 22 (78.6) | 6 (21.4) | |
| 1, 2 | 12 (75.0) | 4 (25.0) | |
UGT1A1 and ABCG2 genotypes and allele
frequencies
The number of patients with each genotype of
UGT1A1 was: *1/*1, n=25; *1/*6, n=3;
*1/*28, n=9; *6/*28, n= 3; *6/*6, n=4; and
*28/*28, n= 0. For the ABCG2 421C>A variant, there
were 28 patients with the C/C genotype, 10 with C/A and six with
the homozygous variant (A/A). No deviation from HWE was observed in
the distribution of the genotypes of UGT1A1 and ABCG2
(P=0.204 and P=0.285, respectively). The allele frequencies of the
polymorphisms were as follows: 0.159 for UGT1A1*6, 0.136 for
UGT1A1*28 and 0.250 for 421A of ABCG2, which are
similar to those previously reported in the Asian population
(17–19).
Association between CPT-11-induced
neutropenia and the genotypes of UGT1A1 or ABCG2
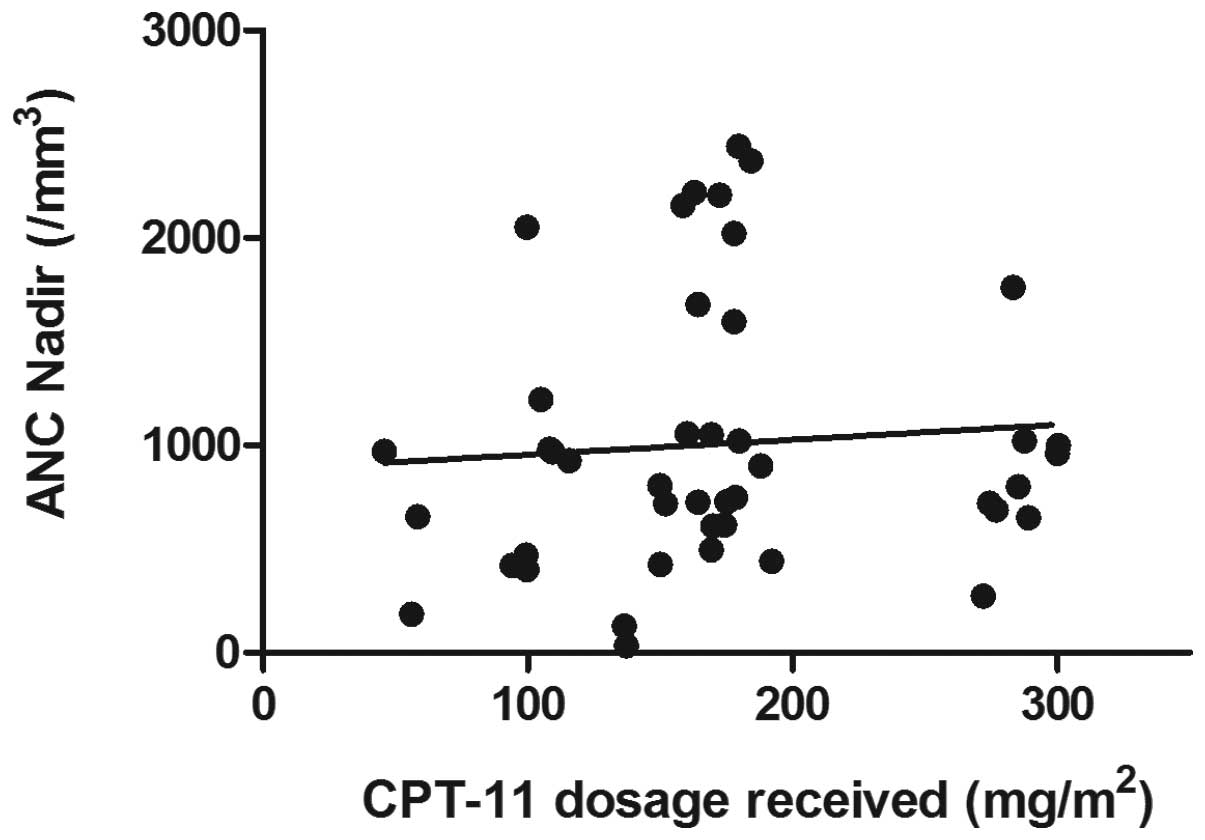
As shown in Fig. 1,
no correlation was found between the total dose of CPT-11 in the
first cycle and the ANC nadir values (R2=0.006,
P=0.185). By contrast, comparison of the ANC nadir values among
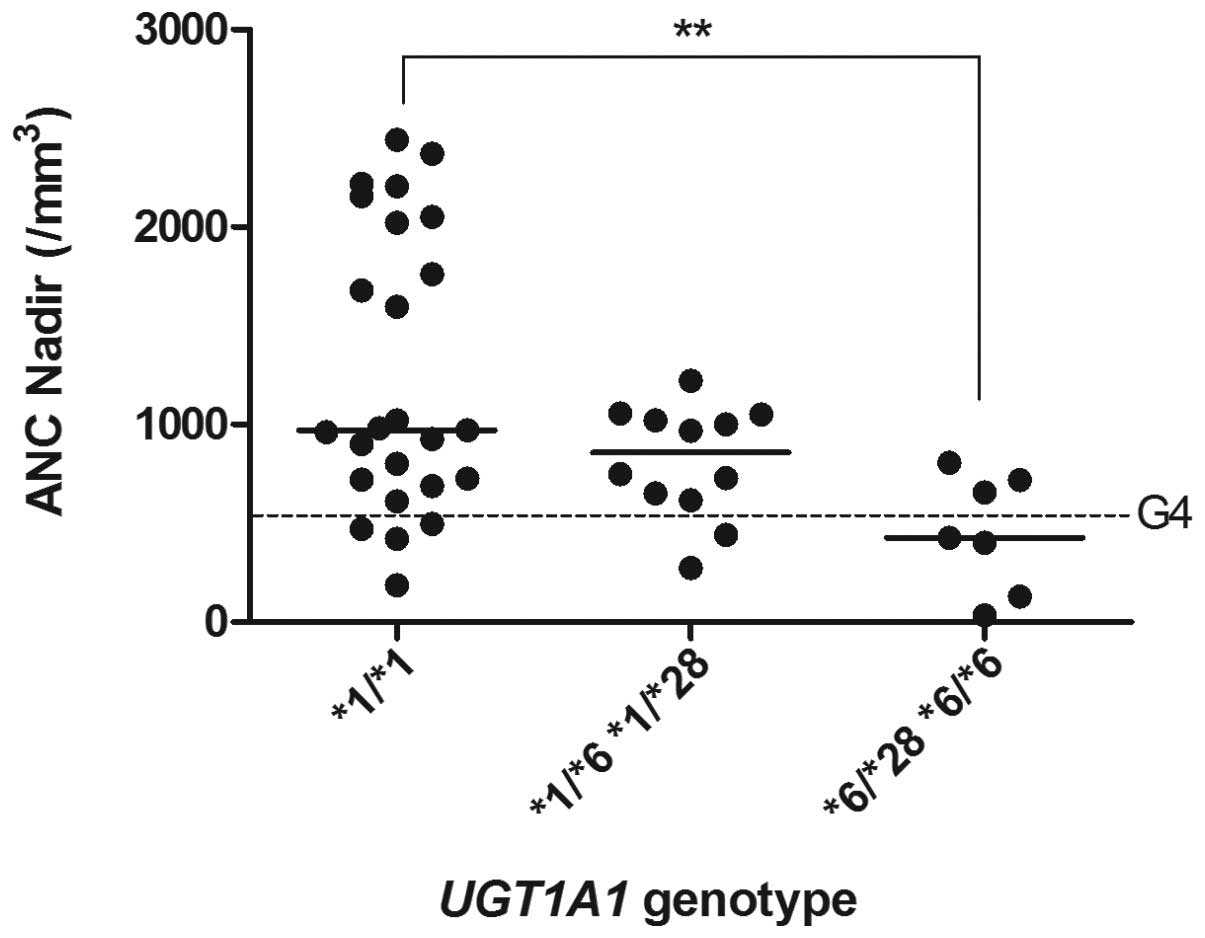
patients with each UGT1A1 genotype revealed statistically
significant differences between the *1/*1 group and the
homozygous variant (*6/*28 or *6/*6) group (Fig. 2). No significant differences were
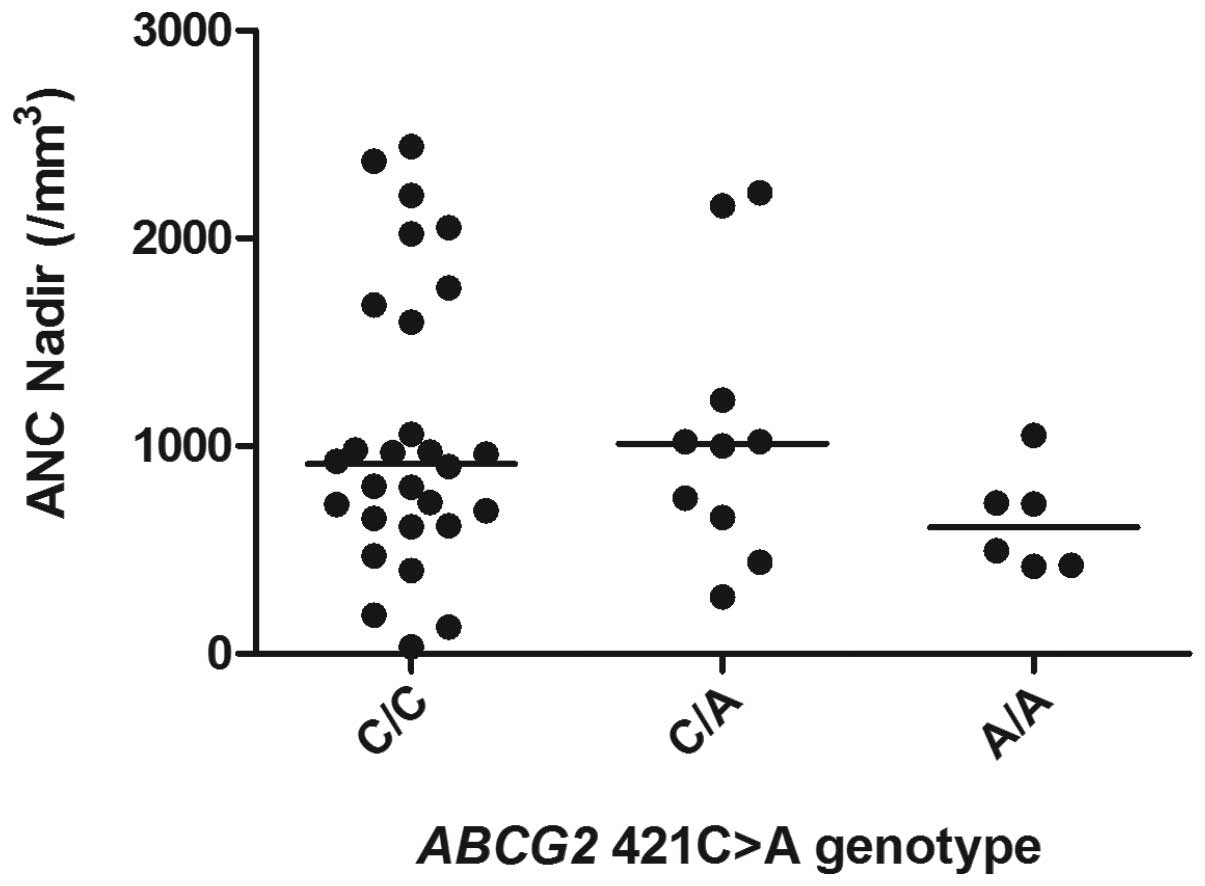
observed for the other genotype pairs (Fig. 2). In addition, there were no
significant differences in the ANC nadir values for any of the
ABCG2 421C>A genotypes (Fig.
3).
Associations of G4 neutropenia with
patient characteristics and the genotypes of UGT1A1 and ABCG2
Investigation of the association between the
incidence of G4 neutropenia and patient characteristics revealed a
significant difference in pre-treatment liver function values for
AST and ALT (P=0.018 and P=0.001, respectively; Table I), between patients with and without
severe neutropenia. Although no significant difference in the
incidence of G4 neutropenia was observed with age (P=0.098),
patients developing this symptom appeared to be older.
A recessive model of inheritance best explained the
significant difference in incidence of G4 neutropenia with respect
to the UGT1A1 gene (P=0.037; Table II). By contrast, there was no
significant difference in either the dominant or recessive models
of inheritance of the ABCG2 421C>A mutation and the risk
of neutropenia.
There were no significant correlations between the
incidence of G4 neutropenia with previous treatment, regimen, type
of cancer or performance status. However, the CPT-11 and MMC
combination regimen appeared to increase the incidence of G4
neutropenia compared with the combination of CPT-11 and CDDP
(P=0.069).
Although liver enzyme function (AST, ALT and γ-GTP)
was significantly different in patients with and without G4
neutropenia (P<0.1) in the univariate analysis, these factors
were not used as explanatory variables in the logistic regression
analysis, as all patients developing G4 neutropenia demonstrated
values of these parameters that were within the normal range.
Multivariate logistic regression analysis was then
used to confirm the significant association between the presence of
the homozygous variant UGT1A1 genotype (*6/*28 or
*6/*6) and the risk of G4 neutropenia (P=0.029; odds ratio,
6.90; 95% confidence interval, 1.22–38.99). No significant
differences were observed in the associations between the incidence
of G4 neutropenia and the heterozygous variant genotype
(*1/*6 or *1/*28), the type of regimen or the age of
the patient.
Discussion
The principal objective of the present study was to
clarify the cause of severe neutropenia that occurred in the first
cycle of a low-dose CPT-11 regimen in patients with gynecological
cancer. A complicating factor was that the total dose of CPT-11
used in the present study varied from 40 to 150 mg/m2
among the patients. Since the variation in the total dose received
may be associated with the occurrence of adverse reactions, it was
first determined that there was no correlation between the total
dose and the ANC nadir values. This indicates that the total dose
of CPT-11 does not necessarily affect the ANC nadir values. To
determine if the UGT1A1 polymorphism is a factor, the
association between the UGT1A1*6 and *28 genotype and
the ANC nadir values in patients with gynecological cancers was
investigated. This revealed that the patients with the homozygous
variant (*6/*28 or *6/*6) had significantly decreased
ANC nadir values and also that all the patients with these variants
developed G3/4 neutropenia (i.e., a neutrophil count of
<1000/mm3). This demonstrates the role of deficient
UGT1A1 activity due to the presence of the homozygous variant
genotype (UGT1A1*6/*28 or *6/*6) in the occurrence of
severe neutropenia caused by the treatment of gynecological
conditions with a low-dose CPT-11 regimen. However, the association
of the UGT1A1*28/*28 genotype could not be investigated, as
this genotype was not detected in the 44 patients studied. It has
been previously reported that during high-dose CPT-11 chemotherapy,
the ANC nadir values in the first cycle were significantly
decreased in patients with the *28/*28 genotype (4). The UGT1A1*6 allele was not
detected in this study of mainly Caucasian cancer patients. An
investigation into the *28/*28 genotype and any decrease in
the ANC nadir values in Japanese patients following low-dose CPT-11
treatment would require a large sample size due to the low-allele
frequency of the *28 variant in the Japanese population.
It has been reported in Chinese patients with
colorectal cancer that the incidence of CPT-11-induced G3/4
neutropenia is significantly higher in females than in males
(24). Therefore, not only the
differences in race, dose and regimen, but also the differences in
gender should be considered when investigating associations between
the ANC nadir values and the UGT1A1 genotypes. In the
present study, four patients who did not have variant alleles
(*1/*1 genotype) developed G4 neutropenia. This indicates
that factors other than UGT1A1 genetic variation may be
involved in the occurrence of severe neutropenia.
Multiple studies have indicated that high-dose
CPT-11 regimens can be safely used in patients of ≥70 years of age
(25–27). Although, it has also been
demonstrated that the incidence of G3/4 neutropenia increases at
≥65 years of age (28). This
indicates that the affect of aging on the risk of CPT-11-induced
severe neutropenia requires scrutinization.
Additionally, in Japanese patients with colon and
stomach cancer, the incidence of G3/4 neutropenia has been reported
to be ~15% higher with CPT-11 + MMC compared with CPT-11 + CDDP
(10). Thus, CPT-11 + MMC may
increase the risk of severe neutropenia compared with CPT-11 +
CDDP, but this is controversial.
In the univariate analysis of the present study,
which was conducted as the initial investigation of these factors,
age and regimen did indeed demonstrate a tendency to exert an
effect on the risk of G4 neutropenia. However, the multivariate
logistic regression analysis did not reveal a statistically
significant association with age and/or regimen. Multivariate
analysis demonstrated the involvement of only UGT1A1*6/*28
and *6/*6 as a risk factor for the occurrence of G4
neutropenia in patients with gynecological cancers who received
low-dose CPT-11.
A previous study of Japanese patients with mainly
lung and colorectal cancers reported that the risk of G3/4
neutropenia was significantly higher in patients with
UGT1A1*6/*6 (10,12). In addition, the *6/*6
genotype is also reportedly involved in the occurrence of G4
neutropenia in Korean patients with non-small cell lung cancer
(6,11). The data from the present study of
patients with gynecological cancer also indicates a role for the
UGT1A1*6/*6 genotype in neutropenia, similar to these
previous studies of other types of cancers. In addition, the
UGT1A1*6/*28 genotype has also been reported to increase the
risk of CPT-11-induced G3/4 neutropenia in Japanese patients with
colorectal or lung cancer (7,29),
which agrees with the data from the present study on this genotype.
By contrast, Gao et al (24)
reported that there was no association between *6/*28 and
G3/4 neutropenia in Chinese patients with colorectal cancer who
received CPT-11. Such inconsistent associations indicate a
necessity for further investigation of the UGT1A1*6/*28
genotype.
In a previous study that assessed the role of the
heterozygous variant genotype, it was reported that
UGT1A1*1/*6 and *1/*28 were not involved in the
occurrence of G3/4 neutropenia in Japanese patients with colorectal
cancer who had been treated with CPT-11 combined with
5-fluorouracil and leucovorin (30), which is similar to the results of
the present study. However, in another study of Japanese patients
with mainly lung or colorectal cancer (12), and also in a previous study of
Japanese patients with gynecological cancers (13), the risk of G3/4 neutropenia in
*1/*6 patients was demonstrated to be higher than that in
*1/*1 patients. Thus, the associations between the
heterozygous genotypes and the risk of neutropenia in Japanese
patients are not consistent and require clarification.
The present study indicated that the ABCG2
421C>A mutation exerted no affect on the occurrence of
CPT-11-induced G4 neutropenia. This correlates with a previous
study in Korean patients with non-small cell lung cancer who
received CPT-11 + CDDP chemotherapy (21). However, PA317 cells transfected with
the ABCG2 421C>A mutation show a lower expression of BCRP
protein and less drug resistance than wild-type cells, indicating
that this mutation changes the phenotype in vitro (20). Notably, a case-controlled study of
Japanese cancer patients indicated that rs2622604, an SNP in an
intron in ABCG2, increased the risk of severe
myelosuppression due to CPT-11 treatment (31). Therefore, it is important to
continue to assess the significance of the variations in
ABCG2 and CPT-11-induced neutropenia.
In the present study, an association was
demonstrated between the incidence of G4 neutropenia and the
UGT1A1*6/*28 or *6/*6 genotype in Japanese patients
with gynecological cancers who received low-dose CPT-11 therapy. As
the study was retrospective and used a small number of specimens,
the additional effect of the UGT1A1*28/*28 genotype could
not be investigated. The present study was limited to an
investigation of treatment-induced neutropenia and other
side-effects, including diarrhea and thrombocytopenia, caused by
CPT-11.
Since variants of not only UGT1A1, but also
other genes, including UGT1A7, UGT1A9, ABCB1
and ABCC2, have been reported to be involved in the
occurrence of CPT-11-induced severe neutropenia (6,11,21,32–35),
rare variants of these genes should be investigated in the future.
An investigation of the physiological and environmental factors and
the risk of severe neutropenia is also required. In addition to
age, gender and smoking may also be factors associated with the
occurrence of CPT-11-induced severe neutropenia (24,36).
In conclusion, the present study revealed that the
UGT1A1*6/*28 and *6/*6 genotypes are associated with
the occurrence of severe neutropenia in Japanese patients with
gynecological cancer treated with low-dose CPT-11. This finding
indicates that the diagnosis of UGT1A1 variants is as useful
for chemotherapy using CPT-11 in gynecological conditions as it is
in colorectal and lung cancer patients.
Acknowledgements
The authors would like to thank the patients who
participated in this study.
References
|
1
|
Takano M, Kikuchi Y, Yaegashi N, et al:
Adjuvant chemotherapy with irinotecan hydrochloride and cisplatin
for clear cell carcinoma of the ovary. Oncol Rep. 16:1301–1306.
2006.PubMed/NCBI
|
|
2
|
Tanaka H, Kihira T, Nomura Y and Ishihara
A: Salvage chemotherapy with a combination of irinotecan
hydrochloride and mitomycin C in elderly Japanese patients with
gynecological malignancies: a pilot study. J Infect Chemother.
12:220–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iyer L, Das S, Janisch L, et al: UGT1A1*28
polymorphism as a determinant of irinotecan disposition and
toxicity. Pharmacogenomics J. 2:43–47. 2002.
|
|
4
|
Innocenti F, Undevia SD, Iyer L, et al:
Genetic variants in the UDP-glucuronosyltransferase 1A1 gene
predict the risk of severe neutropenia of irinotecan. J Clin Oncol.
15:1382–1388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rouits E, Boisdron-Celle M, Dumont A,
Guérin O, Morel A and Gamelin E: Relevance of different UGT1A1
polymorphisms in irinotecan-induced toxicity: a molecular and
clinical study of 75 patients. Clin Cancer Res. 10:5151–5159. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han JY, Lim HS, Shin ES, et al:
Comprehensive analysis of UGT1A polymorphisms predictive for
pharmacokinetics and treatment outcome in patients with
non-small-cell lung cancer treated with irinotecan and cisplatin. J
Clin Oncol. 24:2237–2244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minami H, Sai K, Saeki M, et al:
Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic
polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet
Genomics. 17:497–504. 2007.PubMed/NCBI
|
|
8
|
Jada SR, Lim R, Wong CI, et al: Role of
UGT1A1*6, UGT1A1*28 and ABCG2 c.421C>A polymorphisms in
irinotecan-induced neutropenia in Asian cancer patients. Cancer
Sci. 98:1461–1467. 2007.
|
|
9
|
Hoskins JM, Goldberg RM, Qu P, Ibrahim JG
and McLeod HL: UGT1A1*28 genotype and irinotecan-induced
neutropenia: dose matters. J Natl Cancer Inst. 99:1290–1295.
2007.
|
|
10
|
Sai K, Saito Y, Sakamoto H, et al:
Importance of UDP-glucuronosyltransferase 1A1*6 for irinotecan
toxicities in Japanese cancer patients. Cancer Lett. 261:165–171.
2008.
|
|
11
|
Han JY, Lim HS, Park YH, Lee SY and Lee
JS: Integrated pharmacogenetic prediction of irinotecan
pharmacokinetics and toxicity in patients with advanced non-small
cell lung cancer. Lung Cancer. 63:115–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Onoue M, Terada T, Kobayashi M, et al:
UGT1A1*6 polymorphism is most predictive of severe neutropenia
induced by irinotecan in Japanese cancer patients. Int J Clin
Oncol. 14:136–142. 2009.
|
|
13
|
Takano M, Kato M, Yoshikawa T, et al:
Clinical significance of UDP-glucuronosyltransferase 1A1*6 for
toxicities of combination chemotherapy with irinotecan and
cisplatin in gynecologic cancers: a prospective multi-institutional
study. Oncology. 76:315–321. 2009.
|
|
14
|
Takahara N, Nakai Y, Isayama H, et al:
Uridine diphosphate glucuronosyl transferase 1 family polypeptide
A1 gene (UGT1A1) polymorphisms are associated with toxicity and
efficacy in irinotecan monotherapy for refractory pancreatic
cancer. Cancer Chemother Pharmacol. 71:85–92. 2013. View Article : Google Scholar
|
|
15
|
Stewart CF, Panetta JC, O’Shaughnessy MA,
et al: UGT1A1 promoter genotype correlates with SN-38
pharmacokinetics, but not severe toxicity in patients receiving
low-dose irinotecan. J Clin Oncol. 25:2594–2600. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sugiyama T, Hirose T, Kusumoto S, et al:
The UGT1A1*28 genotype and the toxicity of low-dose irinotecan in
patients with advanced lung cancer. Oncol Res. 18:337–342.
2010.
|
|
17
|
Guillemette C: Pharmacogenomics of human
UDP-glucuronosyltransferase enzymes. Pharmacogenomics J. 3:136–158.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaniwa N, Kurose K, Jinno H, et al: Racial
variability in haplotype frequencies of UGT1A1 and glucuronidation
activity of a novel single nucleotide polymorphism 686C> T
(P229L) found in an African-American. Drug Metab Dispos.
33:458–465. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurose K, Sugiyama E and Saito Y:
Population differences in major functional polymorphisms of
pharmacokinetics/pharmacodynamics-related genes in Eastern Asians
and Europeans: implications in the clinical trials for novel drug
development. Drug Metab Pharmacokinet. 27:9–54. 2012. View Article : Google Scholar
|
|
20
|
Imai Y, Nakane M, Kage K, et al: C421A
polymorphism in the human breast cancer resistance protein gene is
associated with low expression of Q141K protein and low-level drug
resistance. Mol Cancer Ther. 1:611–616. 2002.PubMed/NCBI
|
|
21
|
Han JY, Lim HS, Yoo YK, et al:
Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with
irinotecan-pharmacokinetics and clinical outcome in patients with
advanced non-small cell lung cancer. Cancer. 110:138–147. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Cancer Institute. Common
Terminology Criteria for Adverse Events v 3.0 (CTCAE). Cancer
Therapy Evaluation Program. 2006.
|
|
23
|
Moriya H, Saito K, Helsby N, et al: The
association between heterozygosity for UGT1A1*6, UGT1A1*28, and
variation in the serum total-bilirubin level in healthy young
Japanese adults. Genet Test Mol Biomarkers. 17:464–469. 2013.
|
|
24
|
Gao J, Zhou J, Li Y, Lu M, Jia R and Shen
L: UGT1A1*6/*28 polymorphisms could predict irinotecan-induced
severe neutropenia not diarrhea in Chinese colorectal cancer
patients. Med Oncol. 30:6042013.
|
|
25
|
Comella P, Farris A, Lorusso V, et al:
Irinotecan plus leucovorin-modulated 5-fluorouracil I.V. bolus
every other week may be a suitable therapeutic option also for
elderly patients with metastatic colorectal carcinoma. Br J Cancer.
89:992–996. 2003. View Article : Google Scholar
|
|
26
|
Chau I, Norman AR, Cunningham D, et al:
Elderly patients with fluoropyrimidine and thymidylate synthase
inhibitor-resistant advanced colorectal cancer derive similar
benefit without excessive toxicity when treated with irinotecan
monotherapy. Br J Cancer. 91:1453–1458. 2004. View Article : Google Scholar
|
|
27
|
Souglakos J, Pallis A, Kakolyris S, et al:
Combination of irinotecan (CPT-11) plus 5-fluorouracil and
leucovorin (FOLFIRI regimen) as first line treatment for elderly
patients with metastatic colorectal cancer: a phase II trial.
Oncology. 69:384–390. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rougier P, Bugat R, Douillard JY, et al:
Phase II study of irinotecan in the treatment of advanced
colorectal cancer in chemotherapy-naive patients and patients
pretreated with fluorouracil-based chemotherapy. J Clin Oncol.
15:251–260. 1997.PubMed/NCBI
|
|
29
|
Okuyama Y, Hazama S, Nozawa H, Kobayashi
M, Takahashi K, Fujikawa K, Kato T, Nagata N, Kimura H, Oba K,
Sakamoto J and Mishima H: Prospective phase II study of FOLFIRI for
mCRC in Japan, including the analysis of UGT1A1 *28/*6
polymorphisms. Jpn J Clin Oncol. 41:477–482. 2011.
|
|
30
|
Sunakawa Y, Ichikawa W, Fujita K, et al:
UGT1A1*1/*28 and *1/*6 genotypes have no effects on the efficacy
and toxicity of FOLFIRI in Japanese patients with advanced
colorectal cancer. Cancer Chemother Pharmacol. 68:279–284.
2011.
|
|
31
|
Cha PC, Mushiroda T, Zembutsu H, et al:
Single nucleotide polymorphism in ABCG2 is associated with
irinotecan-induced severe myelosuppression. J Hum Genet.
54:572–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sai K, Saito Y, Maekawa K, et al: Additive
effects of drug transporter genetic polymorphisms on irinotecan
pharmacokinetics/pharmacodynamics in Japanese cancer patients.
Cancer Chemother Pharmacol. 66:95–105. 2010. View Article : Google Scholar
|
|
33
|
Cecchin E, Innocenti F, D’Andrea M, et al:
Predictive role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants
and their haplotypes on the outcome of metastatic colorectal cancer
patients treated with fluorouracil, leucovorin, and irinotecan. J
Clin Oncol. 27:2457–2465. 2009. View Article : Google Scholar
|
|
34
|
Lévesque E, Bélanger AS, Harvey M, et al:
Refining the UGT1A haplotype associated with irinotecan-induced
hematological toxicity in metastatic colorectal cancer patients
treated with 5-fluorouracil/irinotecan-based regimens. J Pharmacol
Exp Ther. 345:95–101. 2013.
|
|
35
|
Glimelius B, Garmo H, Berglund A, et al:
Prediction of irinotecan and 5-fluorouracil toxicity and response
in patients with advanced colorectal cancer. Pharmacogenomics J.
11:61–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van der Bol JM, Mathijssen RH, Loos WJ, et
al: Cigarette smoking and irinotecan treatment: pharmacokinetic
interaction and effects on neutropenia. J Clin Oncol. 25:2719–2726.
2007.PubMed/NCBI
|