Article
Evaluation of the mechanism of epithelial‑mesenchymal transition in human ovarian cancer stem cells transfected with a WW domain‑containing oxidoreductase gene
- Authors:
-
View Affiliations / Copyright
Affiliations:
Department of Oncology, Shandong University School Of Medicine, Jinan, Shandong 250012, P.R. China, Department of Medical Oncology, Jinan Central Hospital Affiliated to Shandong University, Jinan, Shandong 250013, P.R. China
-
Pages:
426-430
|
Published online on:
April 14, 2014
https://doi.org/10.3892/ol.2014.2063
- Expand metrics +
Metrics:
Total
Views: 0
(Spandidos Publications: | PMC Statistics:
)
Metrics:
Total PDF Downloads: 0
(Spandidos Publications: | PMC Statistics:
)
This article is mentioned in:
Abstract
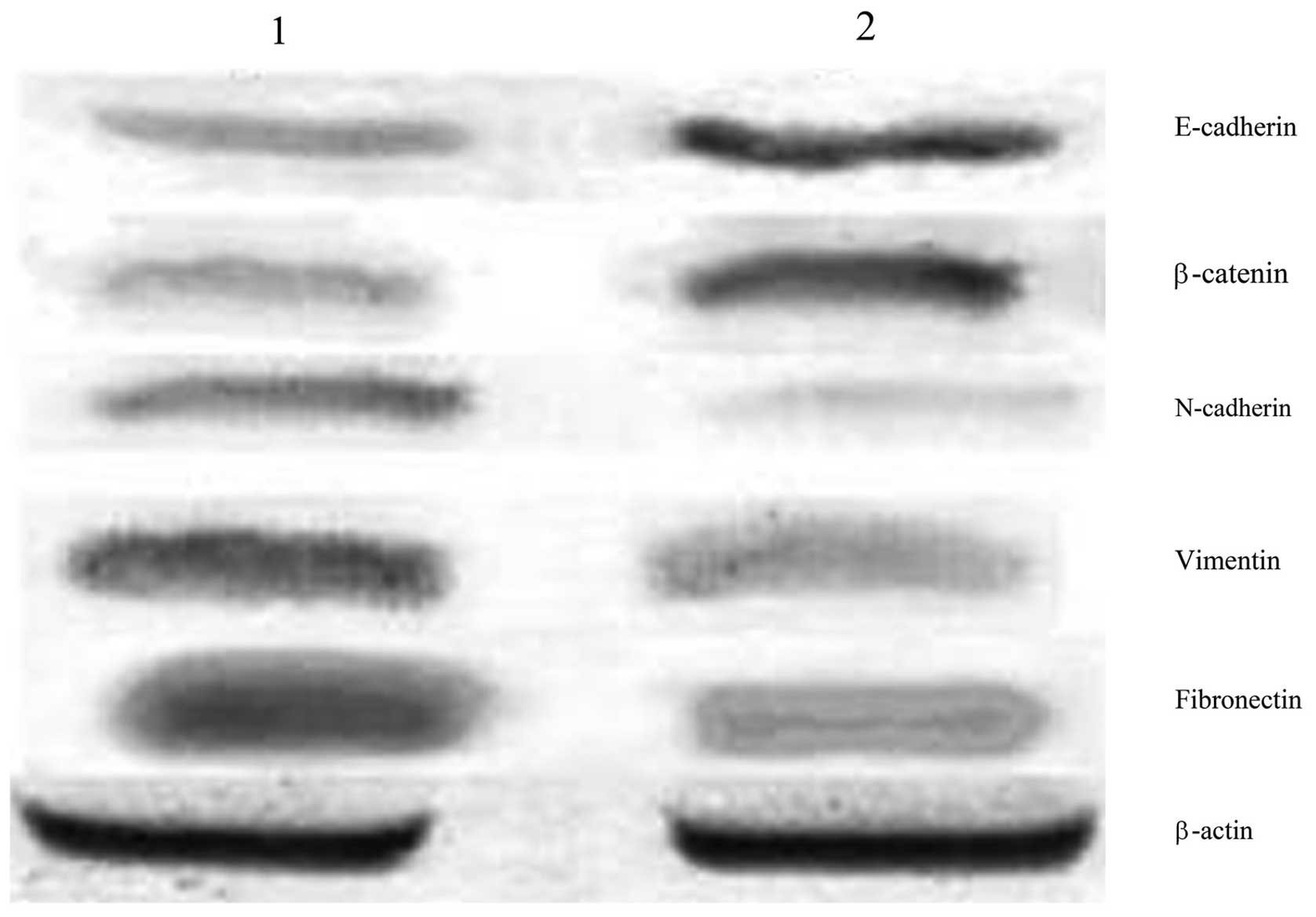
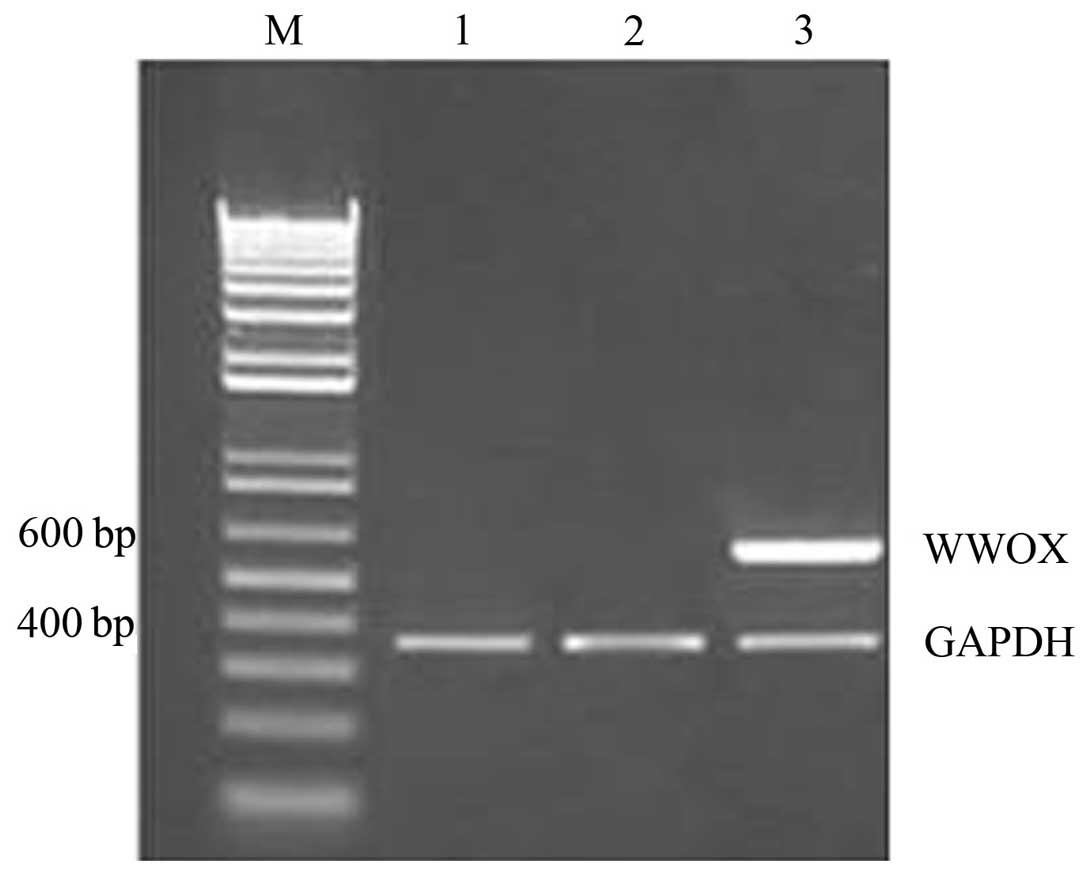
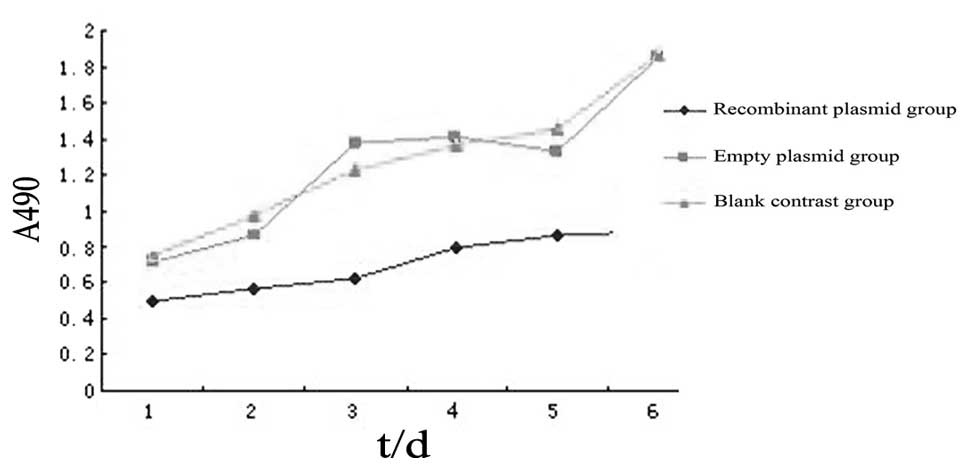
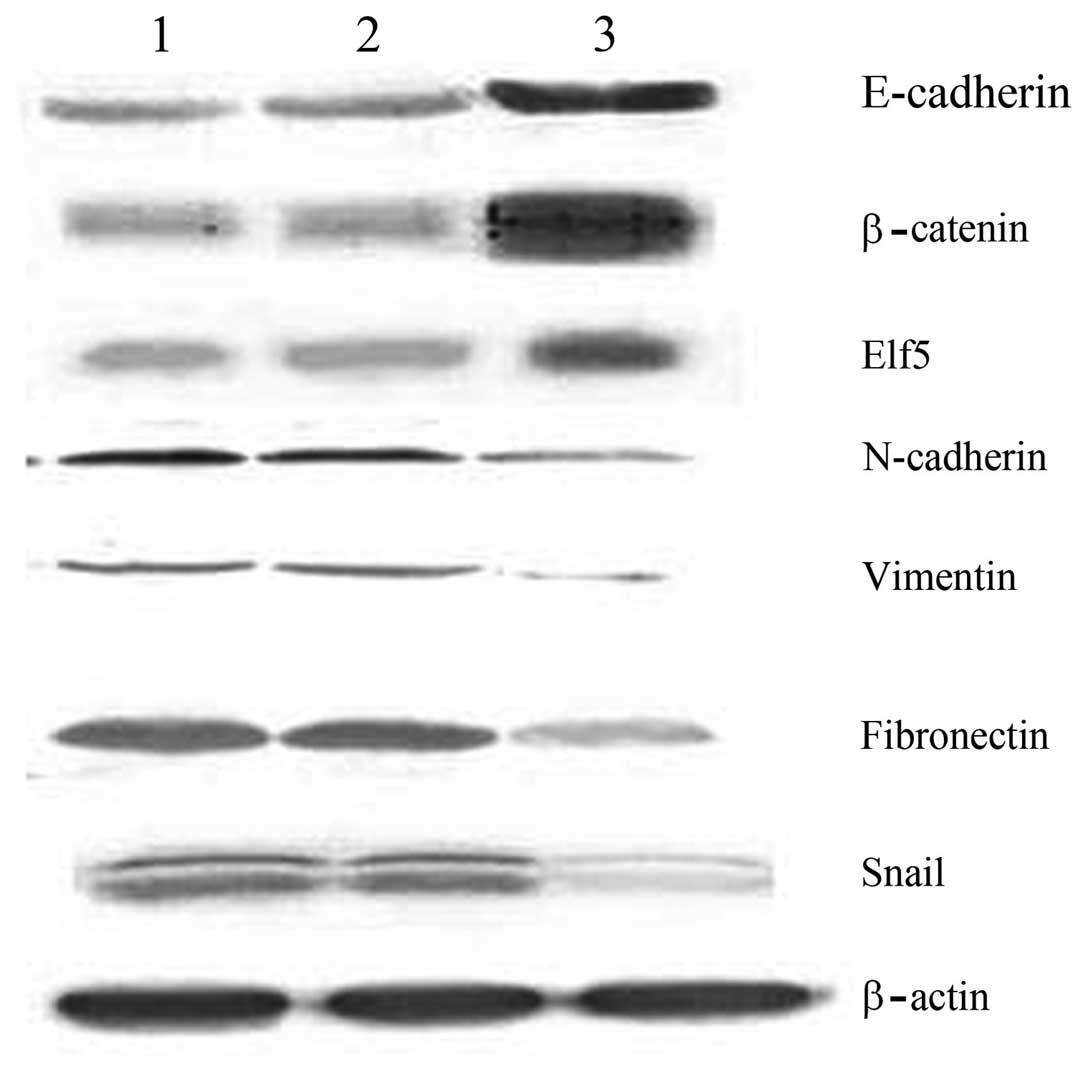
The aim of the present study was to investigate the impact of the WW domain‑containing oxidoreductase (WWOX) gene on the mechanisms underlying epithelial‑mesenchymal transition (EMT) in human ovarian cancer stem cells. Western blot analysis was performed to detect the differences in the expression of the EMT markers, E‑cadherin, β‑catenin, N‑cadherin, vimentin and fibronectin, between human ovarian cancer stem cells and the human epithelial ovarian carcinoma cell line, HO‑8910. A pcDNA3.1‑WWOX eukaryotic expression vector was subsequently transfected into the ovarian cancer stem cells (recombinant plasmid group) or an empty plasmid (empty plasmid group) and non‑transfected ovarian cancer stem cells (blank control group) served as the controls. Following the transfection of the WWOX gene, methyl thiazolyl tetrazolium cell viability and Transwell® invasion assays, and western blot analysis were performed to detect changes in the proliferative capability and invasive capacity of ovarian cancer stem cells, as well as the expression of EMT markers and regulatory factors, Elf5 and Snail. The expression levels of E‑cadherin and β‑catenin in the ovarian cancer stem cells were identified to be significantly lower than those in the HO‑8910 cells, whereas the expression levels of N‑cadherin, vimentin and fibronectin in the ovarian cancer stem cells were found to be significantly higher than those in the HO‑8910 cells. At each time point, the cellular proliferative capacity of the recombinant plasmid group was observed to be significantly lower than that of the empty plasmid or blank control groups (P<0.05 vs. the controls). The number of penetrating cells in the recombinant plasmid, empty plasmid and the blank control groups were 105.5±3.1, 199.7±3.4 and 191.4±4.1, respectively (mean ± standard error of the mean; P<0.05 vs. the controls). In addition, the protein expression of E‑cadherin, β‑catenin and Elf5 in the recombinant plasmid group was found to be significantly higher than that in the other two groups, whereas the protein expression of N‑cadherin, vimentin, fibronectin and Snail in the recombinant plasmid group was significantly lower than that in the other two groups. An EMT exists in ovarian cancer stem cells, and the WWOX gene inhibits the cellular proliferation of ovarian cancer stem cells and reduces their invasive capability. Therefore, the WWOX gene may reverse the EMT in ovarian cancer stem cells by regulating the expression of the EMT regulatory factors, Elf5 and Snail.
View References
|
1
|
Ishii H, Iwatsuki M, Ieta K, et al: Cancer
stem cells and chemoradiation resistance. Cancer Sci. 99:1871–1877.
2008.
|
|
2
|
Cioce M and Ciliberto G: On the
connections between cancer stem cells and EMT. Cell Cycle.
11:4301–4302. 2012.
|
|
3
|
Yan HC, Yu N and Tong JY: Isolation of
cancer stem cells from ovarian cancer cell line HO9810 and
identification of their biological characteristics. Jiang Su Yi
Yao. 38:431–435. 2012.(In Chinese).
|
|
4
|
Yan HC, Xue JQ, Lu XY, et al: Effects of
WWOX gene transfection on cell growth of epithelial ovarian cancer.
Zhonghua Fu Chan Ke Za Zhi. 5:361–365. 2008.(In Chinese).
|
|
5
|
Jordan NV, Johnson GL and Abell AN:
Tracking the intermediate stages of epithelial-mesenchymal
transition in epithelial stem cells and cancer. Cell Cycle.
10:2865–2873. 2011.
|
|
6
|
Jing Y, Han Z, Zhang S, et al:
Epithelial-mesenchymal transition in tumor microenvironment. Cell
Biosci. 1:292011.
|
|
7
|
Jiang J, Tang YL and Liang XH: EMT: a new
vision of hypoxia promoting cancer progression. Cancer Biol Ther.
11:714–723. 2011.
|
|
8
|
Scheel C and Weinberg RA: Cancer stem
cells and epithelial-mesenchymal transition: concepts and molecular
links. Semin Cancer Biol. 22:396–403. 2012.
|
|
9
|
Lee HJ and Ormandy CJ: Elf5, hormones and
cell fate. Trends Endocrinol Metab. 23:292–298. 2012.
|
|
10
|
Chakrabarti R, Hwang J, Andres Blanco M,
et al: Elf5 inhibits the epithelial-mesenchymal transition in
mammary gland development and breast cancer metastasis by
transcriptionally repressing Snail2. Nat Cell Biol. 14:1212–1222.
2012.
|
|
11
|
Bednarek AK, Laflin KJ, Daniel RL, et al:
WWOX, a novel WW domain-containing protein mapping to human
chromosome 16q23.3–24.1, a region frequently affected in breast
cancer. Cancer Res. 60:2140–2145. 2000.
|
|
12
|
Yan HC, Lu XY and Han QY: WWOX mRNA
expression in epithelial ovarian cancer and its clinical
significance. Acta Academiae Medicinae Xuzhou. 2:126–128. 2007.(In
Chinese).
|
|
13
|
Zuo HC, Lu XZ, Han QY and Jin XS:
Construction and Identification of WWOX gene eukaryotic expression
vector. Jiang Su Yi Yao. 3:287–288. 2008.(In Chinese).
|
|
14
|
Zuo HC, Zhang ZZ, Wu XY, et al: Effects of
wwox gene transfection on control mechanism of cell cycle of
epithelial ovarian cancer. Zhonghua Fu Chan Ke Za Zhi. 5:383–385.
2010.
|
|
15
|
Yan H, Yu N and Tong J: Effects of
5-Aza-2′ deoxycytidine on the methylation state and function of the
WWOX gene in the HO-8910 ovarian cancer cell line. Oncol Lett.
6:845–849. 2013.
|
|
16
|
Yan HC and Zhang J: Effects of sodium
valproate on the growth of human ovarian cancer cell line HO8910.
Asian Pac J Cancer Prev. 13:6429–6433. 2012.
|
|
17
|
Yan HC and Sun J: Methylation status of
WWOX gene promoter CpG islands in epithelial ovarian cancer and its
clinical significance. Biomedical Reports. 1:375–378. 2013.
|