Introduction
The generation of hybrid cells by cell fusion plays
a significant role in biotechnology and biomedicine. It has been
used for various purposes (1–8), among
which the most successful has been the production of hybridomas to
generate monoclonal antibodies (9,10).
Recently, the technique has found its novel application in fusing
dendritic cells (DCs) with tumor cells for cancer immunotherapy
(11–13). Cell fusion can be induced in three
main ways; virus-mediated cell fusion (14), polyethylene glycol (PEG)-induced
cell fusion (15,16) and electric-pulse-induced cell fusion
or electrofusion (17). Although
virus envelope-mediated cell fusion often generates a higher
cell-fusion rate (18,19), its use in therapeutic applications
is limited due to the viral proteins. PEG-mediated fusion is widely
used due to the simplicity of its procedure. However, the method
often generates less hybrid cells, even when chemical conjugates
have been introduced (20,21). Electrofusion has also been widely
used recently to fuse cells, and methods to increase the
heterologous cell fusion have been proposed (22,23),
however, its use in large-scale clinical applications is
limited.
We hypothesized that a biotin-streptavidin
(SA)-biotin (BSB) bridge built between two to-be-fused cells will
physically pull the two cells together and dramatically increase
heterologous cell fusions induced by PEG or electroporation. The
idea was tested in various types of cells.
Materials and methods
Mice and cells
Female C57BL/6J mice at 6–8 weeks of age were
purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The
mice were housed in a pathogen-free animal facility. The animal
experiments were carried out in accordance with the Guidelines for
the Care and Use of Laboratory Animals (NIH Publication number
85-23) and the institutional guidelines of Clemson University
(Clemson, SC, USA). The study was approved by the ethics committee
of Clemson University. Two mouse tumor cell lines, B16F0 melanoma
cells [CRL-6322; American Type Culture Collection (ATCC), Manassas,
VA, USA] and S180 sarcoma cells (TIB-66; ATCC) were used. The cells
were cultured in Dulbecco’s modified Eagle’s medium (Life
Technologies, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (FBS; Life Technologies). All cell culture media
contained 100 μg/ml gentamicin (Life Technologies) and cells were
cultured at 37°C with 5% CO2. Bone marrow-derived DCs
were cultured as previously described (24). Briefly, bone marrow cells were
flushed from the femur and tibia bones of female C57BL/6J mice with
RPMI 1640 and passed through a 40 μm cell strainer. Following the
removal of the red blood cells by lysis, using
ammonium-chloride-potassium lysing solution [0.15 M
NH4Cl, 1 mM KHCO3 and 0.1 mM
Na2EDTA (pH 7.3)] at room temperature for 5 min, the
bone marrow cells were suspended in DC medium containing RPMI-1640
(Gibco-BRL, Carlsbad, CA, USA), 10% FBS, 50 mg/ml gentamicin and 20
ng/ml recombinant murine granulocyte-macrophage colony-stimulating
factor (rmGM-CSF) (Sigma Aldrich, St. Louis, MO, USA) and seeded
into 100 mm bacterial culture petri dishes at a concentration of
2×106 cells/10 ml/100 mm dish. At day 3, 10 ml of fresh
DC medium was added into each dish. At day 6, half of the medium
was removed and replaced with fresh DC medium containing 10 ng/ml
rmGM-CSF. At day 8, the cells were centrifuged at 500 × g for 5 min
and resuspended in fresh DC medium with 10 ng/ml rmGM-CSF, 100
ng/ml murine tumor necrosis factor-α (Sigma Aldrich) and 1 mM
prostaglandin E2 (Sigma Alrdich). At day 10, the non-adherent cells
(>70% mature DCs) were collected and were ready for further
studies.
Biotin labeling and dye staining
Prior to labeling, 10 million tumor cells in a T75
flask or 10 million DC cells in a 100-mm petri dish were washed
twice with PBS. The cells were then labeled with biotin by adding 2
μl of N-hydroxysuccinimide-dPEG24-biotin (25 mg/ml; Quanta
Biodesign, Ltd., Powell, OH, USA) into 10 ml PBS and incubating at
4°C for 40 min. Subsequent to biotinylation, the cells were washed
twice with PBS. The biotinylated tumor cells were stained red with
PKH26 dye or green with PKH67 dye (Sigma Aldrich), and DCs were
stained green with PKH67 dye, according to the manufacturer’s
instructions. Following the dye labeling and washing, the DCs were
resuspended in PBS. The dye labeled-B16F0 cells were irradiated at
100 Gy, washed once with PBS and resuspended in 5 ml of PBS. For
certain experiments, the cells were only stained with the
fluorescent dyes without biotinylation.
SA connection
Specific biotinylated cells were further labeled
with SA. In order to be certain that all the biotin molecules on
the cell were occupied by SA, an excess amount of SA was used. A
total of 1 mg purified SA was added to 10 million cells in 10 ml
PBS and incubated at 4°C for 20 min with occasional gentle mixing
by shaking. The cells were then washed twice with PBS to remove the
unbounded SA and resuspended in PBS. In order to prevent cell
pairing or clustering at this step, an excess amount of SA was
added to relatively diluted cell suspensions (~10 ml PBS was used
for 10 million cells).
Cell fusion
Biotin or biotin-SA labeled, green or red
dye-stained cells were mixed at a ratio of 1:1 in PBS and incubated
for 30 min at room temperature for biotin-SA binding on the cells
to occur. The cell fusion was induced by the standard PEG method or
electroporation without the alignment step. For electroporation,
0.7 ml of cell mixture was aliquoted into 4 mm gap BTX cuvettes and
subjected to electroporation (450V, 60 μs, twice with 200 ms
intervals) using a BTX model ECM830 electroporator (BTX Harvard
Apparatus, Holliston, MA, USA). The fused cells were collected and
placed in T75 flasks with appropriate culture media for later use.
Virus envelope-mediated cell fusions were performed using the HVJ
Envelope Cell Fusion kit, GenomOne-CF EX, according to the
manufacturer’s instructions (Cosmo Bio Co., Ltd., Tokyo, Japan).
The dye labeling and cell-fusion rates were evaluated under a
fluorescent microscope or by fluorescence-activated cell sorting
(FACS) analysis.
Statistical analysis
One way analysis of variance with Bonferroni
post-test were performed using the built-in software provided with
GraphPad Prism® 4 (GraphPad, La Jolla, CA, USA).
Results and Discussion
The induction of cell fusion is critically important
in biomedical research and clinical practice. Its applications are,
however, hindered by the limitation of large scale production and
relatively low fusion rate. Although efforts have been made to
improve the fusion rate by using chemical conjugates (20,21) or
increasing the heterologous cell fusion (22,23),
optimal conditions for cell fusion remain to be established. In
this study, a BSB bridge enhanced cell fusion rates induced with
PEG-mediated fusion or electrofusion by 10–30% depending on cell
types when compared with cell fusion without the bridge. The
procedure increased heterologous cell pairing and eliminated the
alignment step required for current electrofusion. More
importantly, it can be used to make large scale cell fusions for
clinical applications.
BSB bridge enhances cell fusion
To evaluate the idea of using the BSB bridge to
enhance the cell-fusion rate, mouse melanoma B16F0 tumor cells were
coated with biotin first and half of the cells were stained with a
green fluorescent dye, PKH67, and the other half with a red
fluorescent dye, PKH26. The red cells were then treated with SA in
an excess amount to ensure that all the biotins on a cell were
occupied. This step is significant as it first prevents the bridge
formation between red cells and more importantly, there will be
unoccupied biotin binding sites on SA available for binding biotins
on the other cell type as each SA has four biotin binding sites.
The green cells with biotin alone and the red cells with biotin-SA
were mixed together at a ratio of 1:1. While there was no pairing
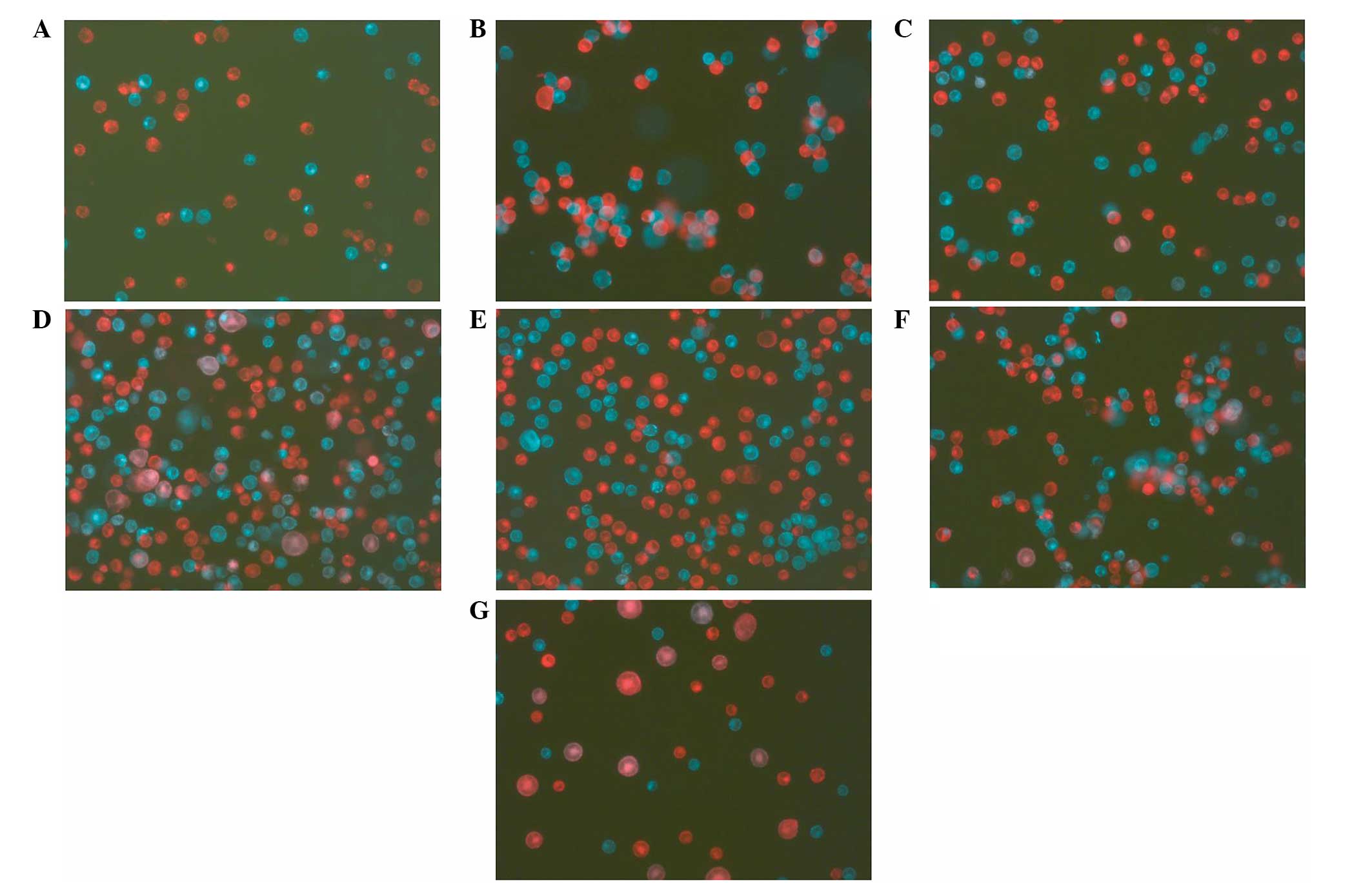
in cell mixtures without a BSB bridge (Fig. 1A), within 5 min, the cells with a
BSB bridge were paired (Fig. 1B)
between green and red cells and there were no green-green or
red-red pairings. The possibility of cell-cluster formation (more
than two cells clustered together) can be decreased by adjusting
the cell concentration in the mixture. Cell mixtures were then
treated with the two most common cell-fusion methods; PEG and
electroporation without alignment. Compared with the fusions
without BSB bridges (Fig. 1C and
1E), the bridge significantly increased the fusion rate by PEG
(Fig. 1D) or by electroporation
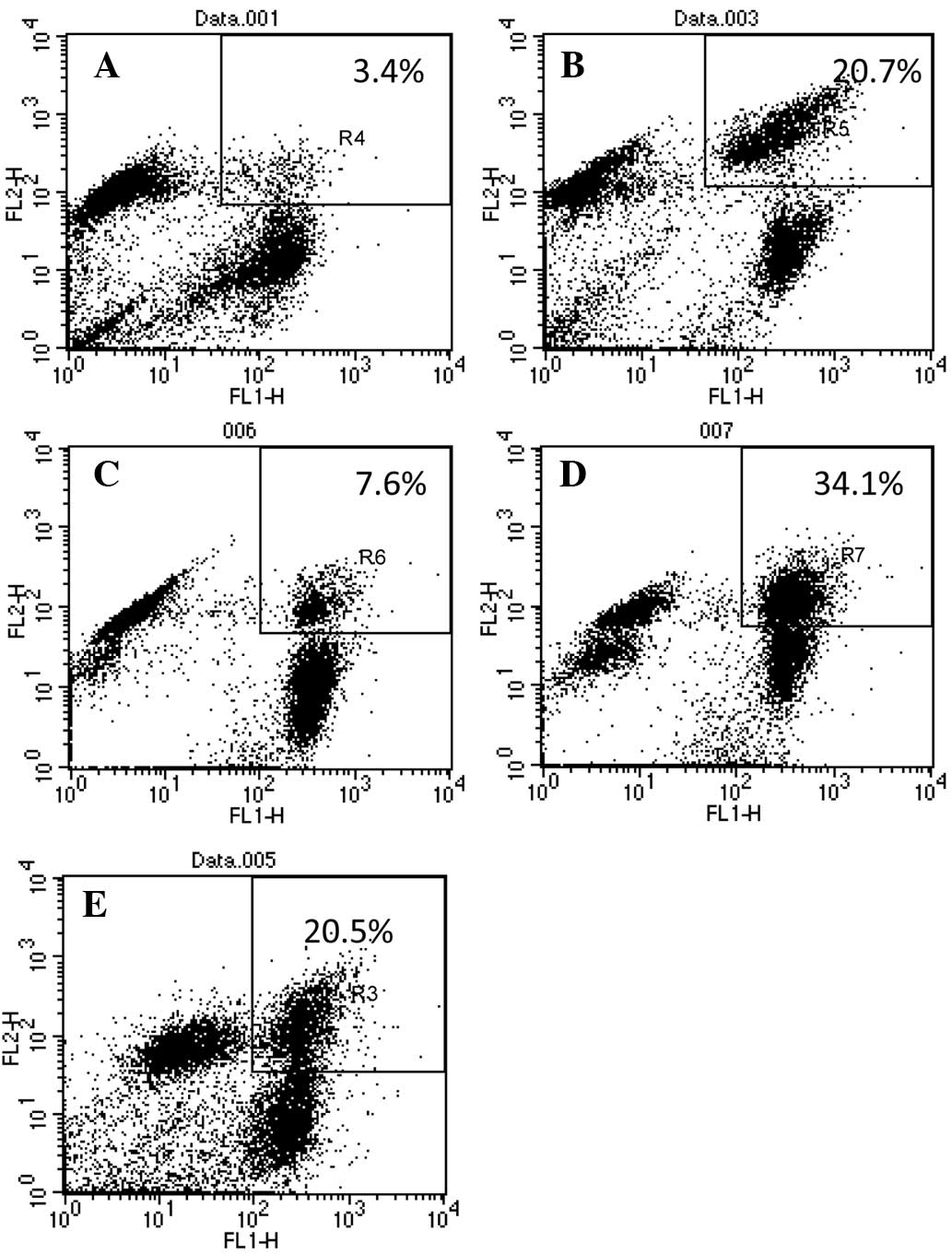
(Fig. 1F). FACS analysis
quantitatively confirmed the observation (Fig. 2) and showed that the fusion rate
increased from 3.58±0.73 to 20.62±0.72% for PEG fusion, and from
5.98±0.57 to 31.44±1.69% for electroporation (Table I). It is significant to point out
that the electroporation was performed using a simple
electroporator (BTX model ECM830). Current electrofusions not only
use more expensive machines, including BTX ECM 2001, but also
require a cell-alignment step, which dramatically hinders its use
in large-scale clinical applications. It is widely accepted that
the Hemagglutinating virus envelope-mediated cell fusion generates
the highest cell-fusion rate (14).
As a control in the present study, green and red cells without the
BSB bridge were fused using the HVJ Envelope Cell Fusion kit,
GenomOne-CF EX and the fusion rate was 20.43±1.0% (Table I, Fig.
1G and 2E). Although, with the
BSB bridge, PEG induced a similar fusion rate as the viral envelope
(20.62±0.72 and 20.43±1.0%; Table
I), and electroporation induced a significantly higher fusion
rate (31.44±1.69%) compared with the viral envelope-induced fusion
rate (20.43±1.0%). Therefore, the BSB bridge-mediated cell fusion
is equivalent or superior to the virus envelope-mediated cell
fusion.
 | Table ISummary of cell fusions. |
Table I
Summary of cell fusions.
| Fusion rate without
BSB bridge (%) | Fusion rate with BSB
bridge (%) |
|---|
|
|
|
|---|
| Cells | PEG | Electroporation | Virus | PEG | Electroporation |
|---|
| B16F0/B16F0a | 3.4 | 7.6 | 20.0 | 20.7 | 34.1 |
| 4.6 | 5.4 | 18.9 | 19.6 | 30.0 |
| 2.1 | 5.8 | 22.4 | 21.9 | 29.8 |
| 5.8 | 6.8 | - | 18.5 | 36.4 |
| 2.0 | 4.3 | - | 22.4 | 26.9 |
| 3.58±0.73 | 5.98±0.57 | 20.43±1.0 |
20.62b±0.72 |
31.44c,h±1.69 |
| DC/B16F0 | 3.5 | 5.5 | 22.0 | 10.0 | 16.8 |
| 2.2 | 2.6 | 23.7 | 12.3 | 15.0 |
| 4.1 | 3.3 | 19.4 | 9.8 | 17.5 |
| 1.7 | 4.2 | - | 15.9 | 17.5 |
| 0.0 | 4.8 | - | 0.0 | 16.4 |
| 2.88±0.56 | 4.08±0.52 | 21.7±1.25 |
12d±1.42 |
16.64e,i±0.46 |
| Lymphocyte/B16F0 | 1.6 | 1.9 | 18.9 | 43.3 | 22.3 |
| 2.2 | 2.6 | 22.6 | 37.8 | 19.8 |
| 3.1 | 2.5 | 17.8 | 29.6 | 25.7 |
| 0.9 | 1.7 | - | 36.4 | 17.9 |
| 1.95±0.46 | 2.18±0.22 | 19.77±1.45 |
36.78f±2.82 |
21.38g,j±1.70 |
BSB bridge enhances DC/tumor cell
fusion
To determine if the BSB bridge can increase the
fusion rate of cells that are significant in biomedical
applications, DCs were cultured from mouse bone marrow, coated with
biotin and stained with green fluorescent dye PKH67, and mouse
B16F0 tumor cells were coated with biotin, stained with red
fluorescent dye PHK26 and treated with SA. The two cells were mixed
at a ratio of 1:1 and fused using PEG or electroporation. In the
control groups, DCs were stained green and tumor cells were stained
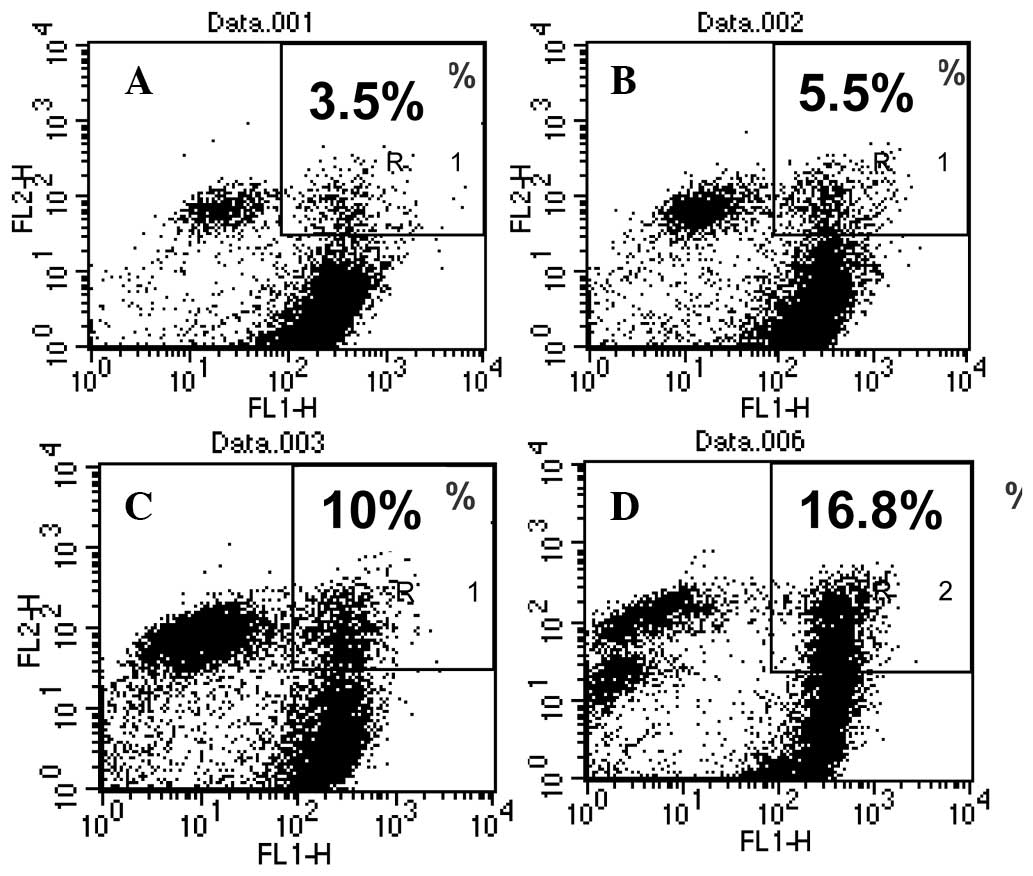
red without the bridge. The results showed that the BSB bridge
significantly increased the DC/tumor cell fusion rate from
2.88±0.56% to 12±1.42% for PEG fusion and from 4.08±0.5% to
16.64±0.46% for electroporation (Table
I, Fig. 3). To evaluate the
technology in other cells, lymphocytes were isolated from mouse
spleen, coated with biotin and stained with red fluorescent dye
PKH26. Following treatment with an excess amount of SA, the red
lymphocytes were mixed with green B16F0 cells coated with biotin
and fused with PEG or electroporation. The results (Table I) indicated that the BSB bridge
significantly increased the fusion rate induced by PEG or
electroporation. Notably, with the BSB bridge, electroporation
induced more DC/B16F0 fusions compared with PEG (16.64±0.46% vs.
12±1.42%; p<0.001), while PEG induced more lymphocyte/B16F0
fusions compared with electroporation (36.78±2.82% vs. 21.38±1.70%;
p<0.001) (Table I).
In conclusion, the coating of to-be-fused cells with
biotin or biotin-SA and the formation of a BSB bridge significantly
increases cell-fusion rates induced by PEG or electroporation in
various types of therapeutically significant cells. Furthermore,
the BSB bridge decreases self-self fusions and eliminates the
cell-alignment step required for current electrofusion. Therefore,
this simple improvement in technology will encourage more
applications of cell fusion in therapeutic development.
Acknowledgements
The authors would like to thank Mr Eric Holle and
his staff for the professional care of the mice used in the present
study, and Ms Ashlee Tietje for proofreading the manuscript. The
study was partially supported by the Greenville Health System
Oncology Endowment (Y.W. and T.E.W) and the William K. and Frances
J. Bryan New Hope Fund for Cancer Research (Y.W. and T.E.W).
References
|
1
|
Okada Y and Tadokoro J: The distribution
of cell fusion capacity among several cell strains or cells caused
by HVJ. Exp Cell Res. 32:417–430. 1963.
|
|
2
|
Okada Y, Yamada K and Tadokoro J: Effect
of antiserum on the cell fusion reaction caused by HVJ. Virology.
22:397–409. 1964.
|
|
3
|
Okada Y, Kim J, Maeda Y and Koseki I:
Specific movement of cell membranes fused with HVJ (Sendai virus).
Proc Natl Acad Sci. 71:2043–2047. 1974.
|
|
4
|
Furusawa M, Nishimura T, Yamaizumi M and
Okada Y: Injection of foreign substances into single cells by cell
fusion. Nature. 249:449–450. 1974.
|
|
5
|
Frye LD and Edidin M: The rapid
intermixing of cell surface antigens after formation of mouse-human
heterokaryons. J Cell Sci. 7:319–335. 1970.
|
|
6
|
Singer SJ and Nicolson GL: The fluid
mosaic model of the structure of cell membranes. Science.
175:720–731. 1972.
|
|
7
|
Rodriguez-Tomé P and Lijnzaad P: The
radiation hybrid database. Nucleic Acids Res. 27:115–118. 1999.
|
|
8
|
Islam MQ, da Meirelles LS, Nardi NB,
Magnusson P and Islam K: Polyethylene glycol-mediated fusion
between primary mouse mesenchymal stem cells and mouse fibroblasts
generates hybrid cells with increased proliferation and altered
differentiation. Stem Cells Dev. 15:905–919. 2006.
|
|
9
|
Galfre G, Howe SC, Milstein C, Butcher GW
and Howard JC: Antibodies to major histocompatibility antigens
produced by hybrid cell lines. Nature. 266:550–552. 1977.
|
|
10
|
Margulies DH: Monoclonal antibodies:
producing magic bullets by somatic cell hybridization. J Immunol.
174:2451–2452. 2005.
|
|
11
|
Wei Y, Li J and Wagner TE: Dendritoma
vaccine for cancer. Curr Can Ther Rev. 5:134–141. 2009.
|
|
12
|
Avigan D, Rosenblatt J and Kufe D:
Dendritic/tumor fusion cells as cancer vaccines. Semin Oncol.
39:287–295. 2012.
|
|
13
|
Browning MJ: Antigen presenting cell/
tumor cell fusion vaccines for cancer immunotherapy. Hum Vaccin
Immunother. 9:1545–1548. 2013.
|
|
14
|
Köhler G and Milstein C: Continuous
cultures of fused cells secreting antibody of predefined
specificity. Nature. 256:495–497. 1975.
|
|
15
|
Anné J and Peberdy JF: Conditions for
induced fusion of fungal protoplasts in polyethylene glycol
solutions. Arch Microbiol. 105:201–205. 1975.
|
|
16
|
Pontecorvo G: Production of mammalian
somatic cell hybrids by means of polyethylene glycol treatment.
Somatic Cell Genet. 1:397–400. 1975.
|
|
17
|
Zimmermann U, Vienken J, Halfmann J and
Emeis CC: Electrofusion: a novel hybridization technique. Adv
Biotechnol Processes. 4:79–150. 1985.
|
|
18
|
Hiraoka K, Yamamota S, Otsuru S, Nakai S,
Tamai K, Morishita R, Ogihara T and Kaneda Y: Enhanced
tumor-specific long-term immunity of hemaggluttinating virus of
Japan-mediated dendritic cell-tumor fused cell vaccination by
coadministration with CpG oligodeoxynucleotides. J Immunol.
173:4297–4307. 2004.
|
|
19
|
Uekawa N, Nishioka T, Terauchi K, Ohta S,
Sugimoto M, Shimada J and Maruyama M: Generation and
characterization of novel monoclonal antibodies against murine and
human TARSH proteins. Hybridoma (Larchmt). 26:381–385. 2007.
|
|
20
|
Bakker Schut TC, Kraan YM, Barlag W, de
Leij L, de Grooth BG and Greve J: Selective electrofusion of
conjugated cells in flow. Biophys J. 65:568–572. 1993.
|
|
21
|
Tomita M and Tsong TY: Selective
production of hybridoma cells: antigenic-based pre-selection of B
lymphocytes for electrofusion with myeloma cells. Biochim Biophys
Acta. 1055:199–206. 1990.
|
|
22
|
Kimura Y, Gel M, Techaumnat B, Oana H,
Kotera H and Washizu M: Dielectrophoresis-assisted massively
parallel cell pairing and fusion based on field constriction
created by a micro-orifice array sheet. Electrophoresis.
32:2496–2501. 2011.
|
|
23
|
Skelley AM, Kirak O, Suh H, Jaenisch R and
Voldman J: Microfluidic control of cell pairing and fusion. Nat
Methods. 6:147–152. 2009.
|
|
24
|
Lutz MB, Kukutsch N, Ogilvie AL, Rössner
S, Koch F, Romani N and Schuler G: An advanced culture method for
generating large quantities of highly pure dendritic cells from
mouse bone marrow. J Immunol Methods. 223:77–92. 1999.
|