Introduction
Malignant fibrous histiocytoma (MFH) is one of the
most common soft-tissue sarcomas in adults (1) and has the aggressive clinical feature
of a high rate of local recurrence, and a short survival time. MFH
accounts for ~10% of all head and neck sarcomas (2), and the tumor size and depth, and the
presence of regional lymph node metastases are significant risk
factors affecting the survival rate (3).
Radiotherapy is an integral part of cancer
management, with >50% of all patients undergoing radiation
treatment (4), particularly in the
head and neck region. However, it is well known that radiation can
induce major side-effects, including radionecrosis and oncogenesis.
A recent multicenter study showed that radiation-induced
soft-tissue sarcomas accounted for 0.9% of 5,046 patients with
soft-tissue sarcomas, and that the most common diagnosis is MFH
(36.4%) (5).
To date, there have been numerous reported cases of
secondary sarcoma subsequent to radiation therapy for
nasopharyngeal carcinoma, but only one case of MFH of the sphenoid
bone due to radiotherapy for nasopharyngeal carcinoma has been
reported (6). In the present study,
a case of MFH of the upper chest wall that appeared four years
after initial radiotherapy for squamous cell carcinoma of the
nasopharynx is described. In addition, the successful two-step
surgical procedure is highlighted and the literature concerning MFH
is reviewed. The patient provided written informed consent.
Case report
A 28-year-old female was admitted to the Department
of Thoracic Surgery of Tangdu Hospital (Xi’an, China) complaining
of a fast-growing mass in the upper chest wall, which had been
diagnosed as MFH by biopsy in another hospital. Subsequent to
obtaining the medical history of the patient, the following
information was established. In August 2007, the patient was
diagnosed with poorly-differentiated squamous cell carcinoma of the
nasopharynx due to durative nasal hemorrhage and diminishing
hearing in the left ear. In view of the metastases of the cervical
and supraclavicular lymph nodes, the patient did not undergo
radical surgery. Therefore, wide irradiation (from the nasal tip to
subclavicular region) was delivered in a total dose of 70 Gy in 35
fractions, followed by three courses of chemotherapy based on
platinum (20 mg/m2 cisplatin combined with 500
mg/m2 5-fluorouracil on days one to five). The patient
then received regular medical examinations and was confirmed to be
disease-free. In December 2011, a small mass with a diameter of 1
cm was detected in the subclavicular region and a tumor biopsy was
carried out. According to the histological characteristics and
immunohistochemisty results the patient was diagnosed with MFH.
Staining for AE1/AE3, S-100, smooth muscle actin (SMA), desmin,
cluster of differentiation 31 (CD31), CD34 and anaplastic lymphoma
receptor tyrosine kinase (ALK) was negative, while the staining for
vimentin was strongly positive (+++) with >50% positive tumor
cells. A Ki-67 of >30% was detected. In fear of possible
amputation of the left upper extremity, the patient refused
surgical treatment and underwent targeted cryoablation therapy
using argon and helium. The tumor grew more slowly until January
2013. Within the next month, the tumor mass was enlarged to 15×15×5
cm, and the tumor surface began to ulcerate and bleed and became
infected. In less than a three-month period prior to being admitted
to the Department of Thoracic Surgery of Tangdu Hospital (Xi’an,
China), the patient experienced two emergency treatments due to
hemorrhagic shock and an unmanageable infection.
Following hospitalization, an integral clinical
examination was performed within our institution. Upon physical
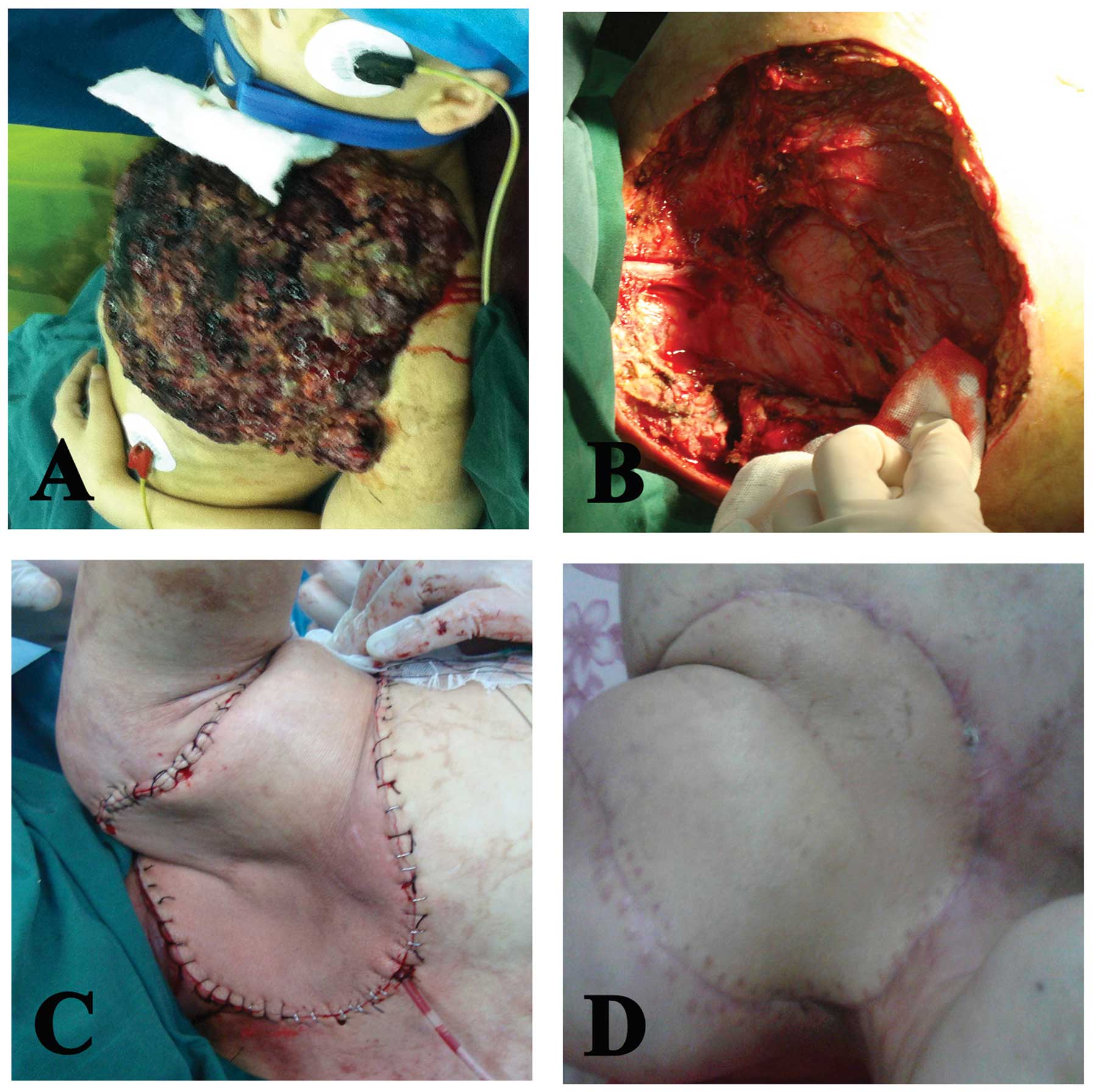
examination, a mushroom-shaped mass (20×20×6 cm) that had the odor
of necrotic tissue was found in the left anterior-superior chest
wall (Fig. 1A). The laboratory
examination indicated an exceptional white blood cell count
(65.13×109/l) and a slightly decreased hemoglobin
concentration (106 g/l, post-transfusion with 10.0 units red blood
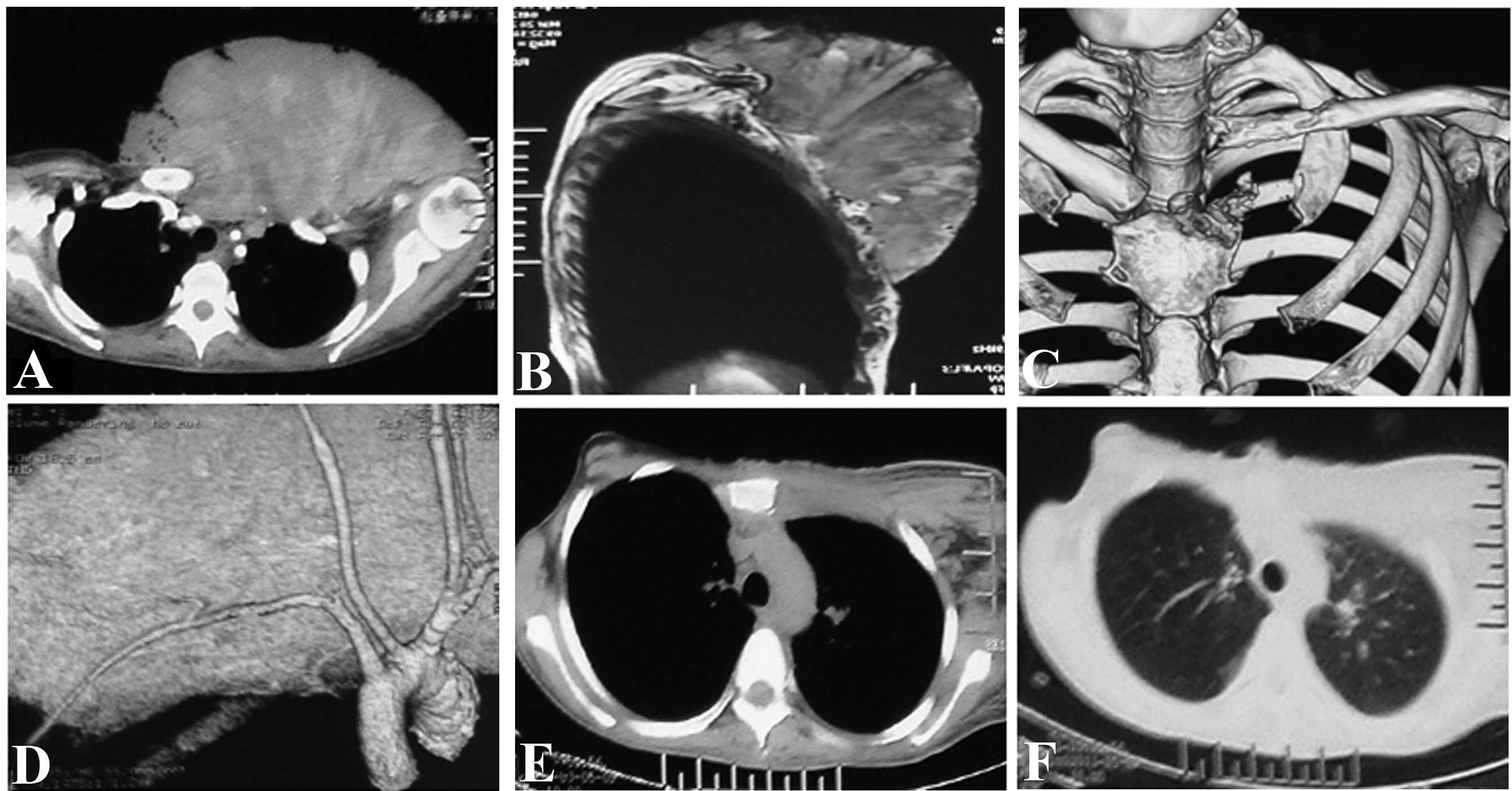
cells one week earlier). The magnetic resonance imaging (MRI) and
computed tomography (CT) plus three-dimensional imaging of the
thoracic cage showed an expansive mass in the left
anterior-superior chest wall, with an irregular margin invading the
left clavicle, the first rib, the superior segment of the sternum
and the sternoclavicular joint. However, there was no evidence of
invasion of the pleural cavity (Fig.
2A–C) and no signs of systematic metastasis. The CT angiography
and interventional angiography indicated that the main blood supply
of the tumor came from the proximal end of the left subclavian
artery, particularly the internal thoracic artery (Fig. 2D).
To improve the systematic condition for improved
tolerance of the following surgery, the patient was administered
specific necessary supportive care, including albumin prepared from
human plasma, antibiotics, hemostatics, a blood transfusion and
enteral nutrition. Nonetheless, the patient remained so weak that
the development of a two-step surgical strategy was required. At
first, tumor-reductive surgery, in which a tumor mass weighing
>4 kg was excised, was performed. Following this, the systematic
condition was further favored through the aforementioned methods.
Nine days later, the secondary limb-sparing surgery was performed
that included resection of the whole tumor mass, the left clavicle,
part of the first rib, the affected sternum and the
sternoclavicular joint, with a wide margin beyond 2 cm from the
tumor (Fig. 1B). The surgical
defect in the chest wall was then reconstructed using a left
latissimus dorsi myocutaneous flap (Fig. 1C and D), and dermatoplasty in the
left back was conducted using autologous skin at a thickness of 0.2
mm, obtained from the homolateral thigh of the patient.
The mass was fixed in formalin, embedded in paraffin
and underwent histological and immunohistochemical staining
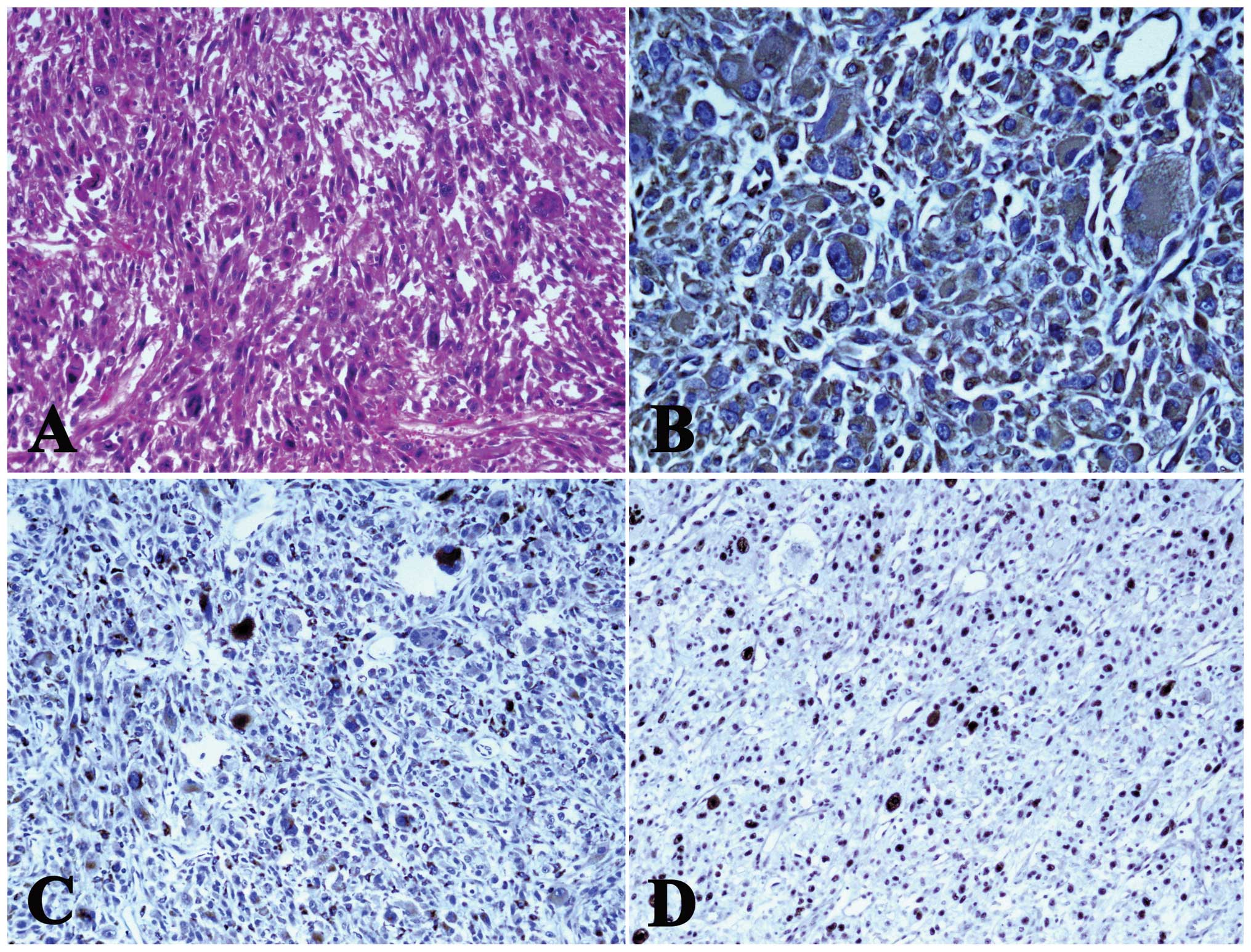
according to the standard methods. Hematoxylin and eosin-stained
sections showed the presence of a malignant neoplasm composed of
spindle-shaped cells, with an eosinophilic cytoplasm and scattered,
abnormal mitotic figures. The tumor cells showed marked cellular
pleomorphism, with unusually large and irregularly-shaped nuclei
(Fig. 3A). The tumor cells were
strongly and diffusely positive for vimentin (Fig. 3B) and CD68 (Fig. 3C). These spindle cells were weakly
and focally positive for Ki-67 (MIB-1). Additionally, the MIB-1
labeling index was 30% (Fig. 3D).
However, the tumor cells were negatively stained for myoglobin,
SMA, desmin and S-100. The final pathological diagnosis was of
MFH.
The post-operative course was uneventful. The
thoracic CT was rechecked and no signs of a remaining tumor were
detected. The patient was discharged from the hospital following a
49-day stay.
Discussion
MFH is the most common type of mesenchymal tissue
sarcoma in middle-aged or elderly adults, frequently arising from
the proximal end of the extremities and in the retroperitoneum
(1). MFH originating from the
thoracic wall is the fifth most uncommon sarcoma (7), and post-irradiation MFH, which is
mainly followed by radiotherapy for breast cancer, is even more
infrequent than primary MFH. To the best of our knowledge, there
has only been one reported case of MFH of the sphenoid bone due to
radiotherapy for nasopharyngeal carcinoma (6). In the present study, a case of MFH of
the upper chest wall that appeared more than four years after
initial radiotherapy for squamous cell carcinoma of the nasopharynx
is described. In addition, subsequent to reviewing the medical
history of the patient, a relatively steady period was found
following targeted cryoablation therapy and rapid progression
without distant metastasis, which improved our knowledge with
regard to the clinical features of the irradiation-induced MFH.
Historically, MFH tumors consist of spindle-shaped
fibroblastic cells and bizarre mononuclear histiocytic cells
arranged in a storiform pattern (8). In other words, MFH has a potential for
bilateral differentiation. MFH has been classified into five
subtypes: Inflammatory, myxoid, giant cell, storiform-pleomorphic
and angiomatoid MFH (8). These
categories are rarely used nowadays, which is largely due to their
proper classification as entirely different types of sarcoma, which
was generated by utilizing advances in IHC. Furthermore, all the
aforementioned categories are positive for vimentin (9). Immunopositivity for vimentin,
α1-antichymotrypsin and Ki-67 has been demonstrated in MFH and is
helpful for diagnosis (6). By
contrast, the tumor tissue should be immunonegative for S-100
protein and cytokeratins. In addition, histiocytic markers (CD68,
α1-antichymotrypsin and factor XIII) no longer play a definitive
role in the diagnosis of MFH, as immunoreactivity to these markers
has been found to be non-specific (10). In the present case, the tumor cells
were positive for vimentin, CD68 and Ki-67, and negative for S-100,
SMA, desmin, CD31, CD34, ALK and AE1/AE3. Therefore, the patient
was unconditionally diagnosed with MFH.
Although the combination of histological
characteristics and immunohistochemical results was useful in the
diagnosis of MFH in the present case, a suspected diagnosis of
secondary sarcoma remains. The criteria published by Cahan et
al (11) formed the basis of
the diagnosis of radiation-induced soft-tissue sarcoma. Criteria
found in the present patient included previous radiation treatment
for various types of tumors (benign or malignant), development of a
sarcoma in the area that was irradiated, the presence of a sarcoma
that was histologically different from the primary cancer and a
minimum dormant period of five years between the initial radiation
and the development of the sarcoma. A recent modification of this
definition indicated that a dormant period of three years was
adequate for the diagnosis of post-irradiation sarcoma (12). Accordingly, we believe that the MFH
tumor was secondary to radiotherapy for primary nasopharyngeal
squamous cell carcinoma in the present case.
Oncogenesis is a well-known multi-step process that
involves multiple genetic changes and results in the transformation
of normal cells into malignant cells. Ionizing radiation has long
been recognized to have carcinogenic potential. The long-term
follow-up of the A-bomb survivors in Hiroshima and Nagasaki
provided certain conclusive evidence for radiation-induced
secondary malignant neoplasm due to the increase in the incidence
of leukemia and solid tumors (13).
Several mechanisms have been proposed for the pathogenesis of
radiation-induced secondary malignancies. First, gene mutations can
occur following exposure to radiation. These include base damage
and single- and double-stranded DNA breaks, which can then cause a
malignant transformation of the irradiated cell (14). Secondly, impairment in the DNA
repair proteins could lead to an increased susceptibility to
radiation-induced carcinogenesis. For example, it has been observed
that radiation doses of >0.2 Gy fail to activate the
G2/M cell cycle check-point, which could result in
failure to repair DNA damage and eventually carcinogenesis
(15). Finally, another mechanism
(16) is the radiation-induced
tissue inflammation and bystander effect that involves
intercellular communication through gap junctions and systemic
cytokine signaling, which have significant roles in the process of
carcinogenesis.
Following the diagnosis of MFH in the present study,
further medical imaging investigations and physical examinations
were performed to delineate the extent of the local invasion and to
assess for distant metastasis. Due to the poor general condition of
the patient, a two-step surgical proposal was offered. As expected,
the patient successfully survived surgery and was rehabilitated
well.
Surgery remains the preferential choice for the
treatment of primary or secondary MFH, although it has a high rate
of local recurrence (17). A recent
study (5) documented that following
surgical resection for radiation-induced soft-tissue sarcoma, the
occurence rate of positive margins ranged from 17–46%, and the rate
of local recurrence ranged from 26–65% among 42 patients. Wide
local excision with a 2–3 cm margin has been the most commonly
employed approach. However, obtaining negative margins always
ensures that surgeons face extensive reconstructive challenges.
Those patients with post-irradiation sarcomas often have
soft-tissue fibrosis, which not only make it difficult to identify
normal tissue planes and to determine the true extent of tumor
intraoperatively, but also can affect wound healing following the
surgery (18).
For those tumors in which clear margins cannot be
obtained, radiotherapy is used as an adjuvant treatment in certain
settings. In a large study (19),
patients with MFH of the extremities who underwent limb sparing
surgery followed by radiation experienced a 10-year relapse-free
survival rate of 62% and an overall survival rate of 80%, which was
higher than that in earlier studies. Traditionally, chemotherapy is
employed only for widespread disease, but large trials have not
shown a significant benefit (20).
The potential use of the multiple tyrosine kinase inhibitor,
sunitinib, for MFH is currently undergoing a phase II trial
(21). Todoroki et al
(22) reported the case of a
long-term survivor of relapsed MFH of the thigh treated with an
autologous tumor vaccine combined with limb-sparing surgery and
radiotherapy. Although promising, the widespread use of
molecular-targeted therapy for MFH appears to remain distant.
Recently, the National Comprehensive Cancer Network guidelines
stated that adjuvant therapy should be considered on an individual
case basis (23).
Peiper et al (3) demonstrated that the tumor size and
depth, and the presence of regional lymph node metastases and
positive resection margins are significant risk factors affecting
the survival rates of patients with MFH. Due to a low incidence
rate of radiation-induced MFH, there has been no large study
performing a survival analysis for this malignancy. Certain studies
have indicated that following treatment, patients should be
followed up closely, with palpation of the regional lymph node beds
and strict detection within the radiation field by CT or MRI
approximately every 3–6 months for two years, followed by at least
annual visits subsequently (23,24).
Clearly, the patient of the present study will have to undergo
extensive follow-up examinations.
In summary, the present study reports a case of MFH
of the upper chest wall that appeared four years after initial
radiotherapy for squamous cell carcinoma of the nasopharynx.
Additionally, the successful two-step surgery procedure was
revealed, which highlighted the prior choice of surgical treatment
for this malignant disease. Even so, the importance of the
prevention of radiation-induced MFH is emphasized, including the
use of proper radiotherapy doses or regions, more advanced
radiation techniques and regular follow-up examinations subsequent
to irradiation. Additionally, studies on the diagnostic and
prognostic characteristics of the malignancy should be pursued.
Further studies are required with regard to the assessment of
adjuvant therapies as the our knowledge of this disease and its
treatment requires enhancement.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81000938).
References
|
1
|
Stadler FJ, Scott GA and Brown MD:
Malignant fibrous tumors. Semin Cutan Med Surg. 17:141–152.
1998.
|
|
2
|
Bentz BG, Singh B, Woodruff J, Brennan M,
Shah JP and Kraus D: Head and neck soft tissue sarcomas: a
multivariate analysis of outcomes. Ann Surg Oncol. 11:619–628.
2004.
|
|
3
|
Peiper M, Zurakowski D, Knoefel WT and
Izbicki JR: Malignant fibrous histiocytoma of the extremities and
trunk: an institutional review. Surgery. 135:59–66. 2004.
|
|
4
|
Ron E: Ionizing radiation and cancer risk:
evidence from epidemiology. Radiat Res. 150(5 Suppl): S30–S41.
1998.
|
|
5
|
Riad S, Biau D, Holt GE, Werier J,
Turcotte RE, Ferguson PC, Griffin AM, Dickie CI, Chung PW, Catton
CN, O’Sullivan B and Wunder JS: The clinical and functional outcome
for patients with radiation-induced soft tissue sarcoma. Cancer.
118:2682–2692. 2012.
|
|
6
|
Teo WY, Tan HK, Goh BC and Putti TC:
Postirradiation sarcoma of the sphenoid bone - a case report. Ann
Acad Med Singapore. 35:104–107. 2006.
|
|
7
|
Foran P, Colleran G, Madewell J and
O’Sullivan PJ: Imaging of thoracic sarcomas of the chest wall,
pleura, and lung. Semin Ultrasound CT MR. 32:365–376. 2011.
|
|
8
|
Hartel PH, Bratthauer G, Hartel JV and
Fanburg-Smith JC: Primary malignant fibrous histiocytoma
(myxofibrosarcoma/pleomorphic sarcoma not otherwise specified) of
the breast: clinicopathologic study of 19 cases. Ann Diagn Pathol.
15:407–413. 2011.
|
|
9
|
Al-Agha OM and Igbokwe AA: Malignant
fibrous histiocytoma. between the past and present. Arch Pathol Lab
Med. 132:1030–1035. 2008.
|
|
10
|
Szollosi Z, Nemeth T, Egervari K and Nemes
Z: Histiocyte-like cells expressing factor XIIIa do not belong to
the neoplastic cell population in malignant fibrous histiocytoma.
Pathol Res Pract. 201:369–377. 2005.
|
|
11
|
Cahan WG, Woodward HQ, Higinbotham NL,
Stewart FW and Coley BL: Sarcoma arising in irradiated bone: report
of eleven cases. 1948. Cancer. 1:8–34. 1948.
|
|
12
|
Arlen M, Higinbotham NL, Huvos AG, Marcove
RC, Miller T and Shah IC: Radiation-induced sarcoma of bone.
Cancer. 28:1087–1099. 1971.
|
|
13
|
Preston D, Shimizu Y, Pierce DA, Suyama A
and Mabuchi K: Studies of mortality of atomic bomb survivors.
Report 13: Solid cancer and noncancer disease mortality: 1950–1997.
Radiat Res. 160:381–407. 2003.
|
|
14
|
Mullenders L, Atkinson M, Paretzke H,
Sabatier L and Bouffler S: Assessing cancer risks of low-dose
radiation. Nat Rev Cancer. 9:596–604. 2009.
|
|
15
|
Krueger SA, Joiner MC, Weinfeld M,
Piasentin E and Marples B: Role of apoptosis in low-dose
hyper-radiosensitivity. Radiat Res. 167:260–267. 1997.
|
|
16
|
Prise KM and O’Sullivan JM:
Radiation-induced bystander signalling in cancer therapy. Nat Rev
Cancer. 9:351–360. 2009.
|
|
17
|
Matsumoto S, Ahmed AR, Kawaguchi N, Manabe
J and Matsushita Y: Results of surgery for malignant fibrous
histiocytomas of soft tissue. Int J Clin Oncol. 8:104–109.
2003.
|
|
18
|
Hoy E, Granick M, Benevenia J, Patterson
F, Datiashvili R and Bille B: Reconstruction of musculoskeletal
defects following oncologic resection in 76 patients. Ann Plast
Surg. 57:190–194. 2003.
|
|
19
|
Issakov J, Kollender Y, Soyfer V, Bickels
J, Flusser G, Meller I and Merimsky O: A single-team experience of
limb sparing approach in adults with high-grade malignant fibrous
histiocytoma. Oncol Rep. 14:1071–1076. 2005.
|
|
20
|
Marchese R, Bufo P, Carrieri G and Bove G:
Malignant fibrous histiocytoma of the kidney treated with
nephrectomy and adjuvant radiotherapy: a case report. Case Rep Med.
4:8020262010.
|
|
21
|
Clinical Trial Registry. http://clinicaltrials.gov/ct2/show/NCT00400569?term=sunitinib+malignant+fibrous&rank=2.
Accessed December 29, 2011
|
|
22
|
Todoroki T, Kondo T, Sugahara S, Morishita
Y, Mori K and Ohno T: Long-term survivor of relapsed MFH on the
thigh treated with autologous formalin-fixed tumor vaccine (AFTV)
combined with limb-sparing surgery and radiotherapy. World J Surg
Oncol. 9:962011.
|
|
23
|
NCCN. NCCN Clinical Practice Guidelines in
Oncology. Soft Tissue Sarcoma V.2.2007. http://www.globalgist.org/docs/NCCN_guidelines.pdf.
Accessed January 23, 2012
|
|
24
|
Henderson MT and Hollmig ST: Malignant
fibrous histiocytoma: changing perceptions and management
challenges. J Am Acad Dermatol. 67:1335–1341. 2012.
|