Introduction
Osteosarcoma (OS) is the most common type of primary
malignancy of bone. Despite intensive chemotherapy and adequate
surgical resection, ~30–50% of patients succumb to OS, mainly due
to distant metastasis to the lung (1,2).
Snail-1 is a zinc-finger transcription factor expressed in
migratory processes during embryonic development that has been
implicated in cancer (3,4). A previous study showed that Snail-1 is
highly expressed in OS cells, and is associated with the migratory
and invasive properties of OS cells (5).
Studies have shown that Snail-1 upregulation in
epithelial cells induces the expression of E-cadherin. Snail-1
contributes to the maintenance of the adhesive and polarized
phenotype of epithelial cells where it is mainly expressed
(6). If E-cadherin expression is
downregulated, the epithelial cells acquire a fibroblastoid
morphotype accompanied by the acquisition of invasive and migratory
properties (7–9). This event is critical for the invasion
and metastasis of carcinoma cells (10,1).
Therefore, the loss of E-cadherin expression may be considered as
an indicator of poor clinical prognosis and it is important to
identify the molecular mechanism that regulates the expression of
E-cadherin. A study has identified that E-cadherin is regulated by
transcriptional factors such as Snail-1 (12). In the present study, whether Snail-1
regulates E-cadherin expression in OS cells was investigated and
the association between E-cadherin expression and the migratory and
invasive properties of the cells was explored
Materials and methods
Cell line and culture
The human SaOS2 OS cell line was
purchased from the China Center for Type Culture Collection (Wuhan,
China). The cells were cultured in McCoy’s 5A medium (Hyclone,
Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS;
Hangzhou Sijiqing Biological Engineering Materials Co. Ltd.,
Hangzhou, China) and antibiotics (100 U/ml penicillin and 100 μg/ml
streptomycin) in humidified air with a 5% CO2 atmosphere
at 37°C (Thermo Direct Heat CO2 incubator; Thermo Fisher
Scientific, Waltham, MA, USA). The study was approved by the ethics
committee of the Affiliated Jangyin People’s Hospital of Southeast
University Medical College (Jiangyin, Jiangsu).
Transfection
The SaOS2 cells were plated in 100-mm
dishes and transfected at 50–80% confluence with an expression
vector for short hairpin RNA (shRNA) targeting Snail-1 or with a
control vector, using the liposome-mediated transfection method
(5). To establish cells in which
Snail-1 expression was stably suppressed and mock-transfected
cells, the SaOS2 cells were transfected with a plasmid
(pcDNA3.1/shSnail-1 or pcDNA3.1/GAPDH, respectively; Introgen
Therapeutics Inc., Austin, TX, USA) for two days. The cells were
then trypsinized and plated at a low density. The stable clones
were selected by maintaining the cells in medium containing the
antibiotic G418 (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China).
Animal
BALB/c nude mice (between six and seven weeks old)
were obtained from the Shanghai SLRC Laboratory Animal Co., Ltd.
(license no. SCXK [hu] 2007–0005; Shanghai, China). The mice were
bred and housed in a pathogen free, temperature-controlled and
air-conditioned environment with a 10/14 h light/dark cycle. Two
groups of these immunodeficient mice were subcutaneously injected
with either mock- or Snail-1 shRNA-transfected cells
[1×106 cells/200 μl phosphate-buffered saline (PBS)].
Tumor growth was measured with a caliper two or three times every
two days. Tumor volume was calculated using the following formula:
Volume = (L × W2)/2, where L is the tumor length and W
is the tumor width (both in millimeters) and L>W. After 17 days
the mice were sacrificed in accordance with the guidelines for the
welfare of animals in experimental neoplasia and the tissues were
stored at −80°C.
Cell viability assay
The viability of the cells was determined by the
Cell Counting kit-8 (CCK-8; Nanjing KeyGen Biotech. Co. Ltd.,
Nanjing, China) assay. The cells (1×104/well) were
plated in 96-well plates in 200 μl medium per well. At different
time points, the CCK-8 solution was added and the cells were
cultured for 4 h. The absorbance at 570 nm was measured with a
microplate reader (Sunrise; Tecan, Männedorf, Switzerland), using
wells without cells as blanks and using untreated cells as the
negative control. Cell death was calculated as a percentage of
inhibition using the following formula: inhibition (%) = (1 - mean
experimental absorbance/mean control absorbance) × 100.
Apoptosis detection
Apoptosis detection of the cells was performed using
a TRITC Staining Apoptosis Detection kit (Nanjing KeyGen Biotech.
Co. Ltd.) and flow cytometry (BD Biosciences, San Jose, CA, USA).
Briefly, the cells were trypsinized, washed with PBS, centrifuged
and fixed for 20 min at 15–25°C (fixation solution: 4%
paraformaldehyde in PBS buffer, pH 7.4, freshly prepared). The
cells were washed for 30 min with PBS, and then were incubated with
blocking solution for 10 min at 15–25°C (the blocking solution
contained 3% H2O2 in methanol). Subsequently,
the cells were incubated in permeabilization solution for 2 min on
ice (2–8°C; the permeabilization solution contained 0.1% Triton
X-100 and 0.1% sodium citrate, freshly prepared). Terminal
deoxynucleotidyl transferase (TdT) dUTP nick end labeling (TUNEL)
reaction mixture (50 μl; Nanjing KeyGen Biotech Co., Ltd.) was
added to the samples and the samples were incubated for 60 min at
37°C in a wet and dark atmosphere (TUNEL reaction mixture contained
45 μl equilibration buffer, 1 μl TRITC-5-dUTP and 4 μl TdT, freshly
prepared), then resuspended in 500 μl 4′,6-diamidino-2-phenylindole
(DAPI)/RNase buffer (Nanjing KeyGen Biotech Co., Ltd.) and
incubated for 30 min at 37°C. The samples were assayed by
fluorescence microscopy using an excitation wavelength of 543 nm
and emission wavelength of 571 nm (green) within 1 h.
Matrigel invasion assay
A modified Boyden chamber (Neuro Probe Inc.,
Gaithersburg, MD, USA) was used. The pore size of the polycarbonate
filters was 8.0 mm. The bottom chamber of the Transwell chamber was
filled with 30 ml McCoy’s 5A medium containing 10% FBS. The cells
were then suspended at a density of 1×105 cells/ml in
500 ml McCoy’s 5A medium supplemented with 0.5% FBS, and
1,25(OH)2-D3 with a concentration of
10−6 M was added to the 8-mm porous BD BioCoat Matrigel
chamber inserts (BD Biosciences, San Jose, CA, USA). Subsequently,
the inserts were placed in the wells which were filled with 0.7 ml
medium supplemented with 10% fetal calf serum as a chemoattractant.
After two days of incubation, the upper side of the filter was
scraped with a cotton tip to eliminate cells that had not migrated
through it. The invasive ability of the cells was determined by
counting the cells that had migrated to the lower side of the
filter with a microscope. The experiments were performed in
triplicate and ≥10 fields were counted in each experiment.
Western blot analysis
The proteins were extracted from the
SaOS2 cells in lysis buffer and then separated by
SDS-PAGE and electrophoretically transferred onto polyvinylidene
difluoride membranes. The membranes were probed with primary
antibodies overnight at 4°C, and then incubated with the secondary
antibodies. The images of the western blot products were collected
and analyzed with Quantity One V4.31 software (Bio-Rad, Hercules,
CA, USA). The primary Snail-1 and E-cadherin polyclonal antibodies
were purchased from Abnova (Taiwan, China). Goat anti-mouse
HRP-conjugated secondary antibody was obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA) and goat anti-rabbit
HRP-conjugated secondary antibody was purchased from Amersham
Pharmacia Biotech (Piscataway, NJ, USA).
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated from the SaOS2
cells using TRIzol reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. The reverse
transcription reactions were conducted with a Transcriptor First
Strand cDNA Synthesis kit (Roche, Indianapolis, IN, USA). The PCR
primers were designed by Premier Primer software, version 5.0
(Premier Biosoft, Palo Alto, CA, USA). qPCR with SYBR Green PCR
Master mix (Applied Biosystems, Foster City, CA, USA) was performed
using an ABI Prism 7500 Sequence Detection system (Applied
Biosystems). The fluorescent signals were collected during the
extension phase, the Ct values of the sample were calculated and
the transcript levels were analyzed by the 2−ΔΔCt
method.
Data analysis
Statistical comparisons were performed with the
software package SPSS, version 13.0 (SPSS, Inc., Chicago, IL, USA)
using Student’s t-test for paired observations or one-way analysis
of variance with Student-Newman-Keuls, least significant difference
and Dunnett’s methods. All data are presented as the mean ±
standard deviation (SD). P<0.05 was considered to indicate a
statistically significant difference. The mean values and SD were
calculated for the experiments conducted in triplicate.
Results
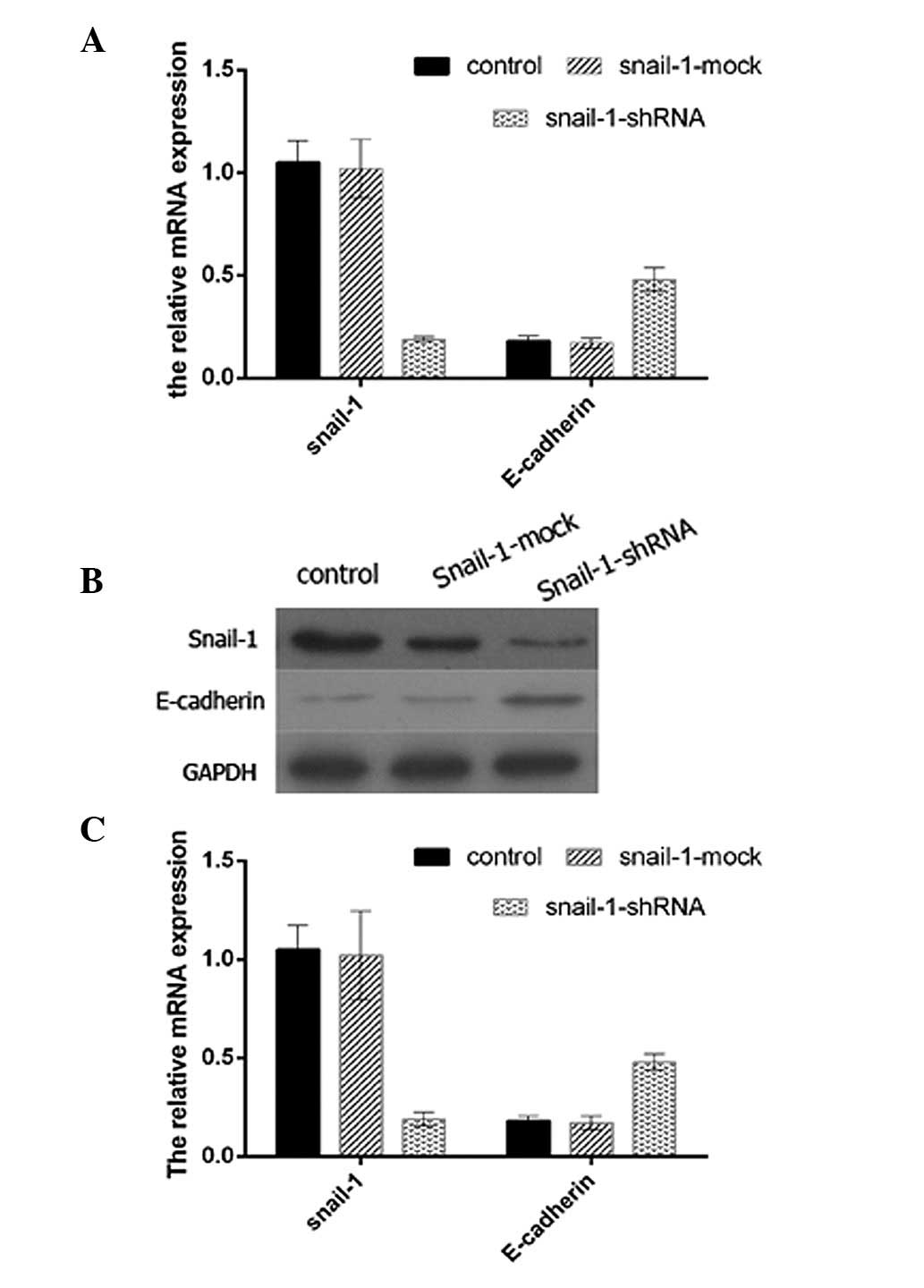
Inhibition of Snail-1 increases the
expression levels of E-cadherin in SaOS2 cells
To determine the association between Snail-1 and
E-cadherin, shRNA targeting Snail-1 was successfully transfected
into SaOS2 cells and the expression levels of Snail-1
and E-cadherin in the cells were detected by western blot analysis.
To confirm the efficacy of Snail-1 shRNA, qPCR and western blot
analysis were performed (data not shown). The expression levels of
E-cadherin in the SaOS2 cells transfected with the
Snail-1-shRNA vector were significantly higher than those in the
SaOS2 cells with no treatment (control group) or
infected with the negative control shRNA (shRNA-Mock) (Fig. 1). Therefore, inhibition of Snail-1
expression in SaOS2 cells resulted in higher levels of
E-cadherin protein expression.
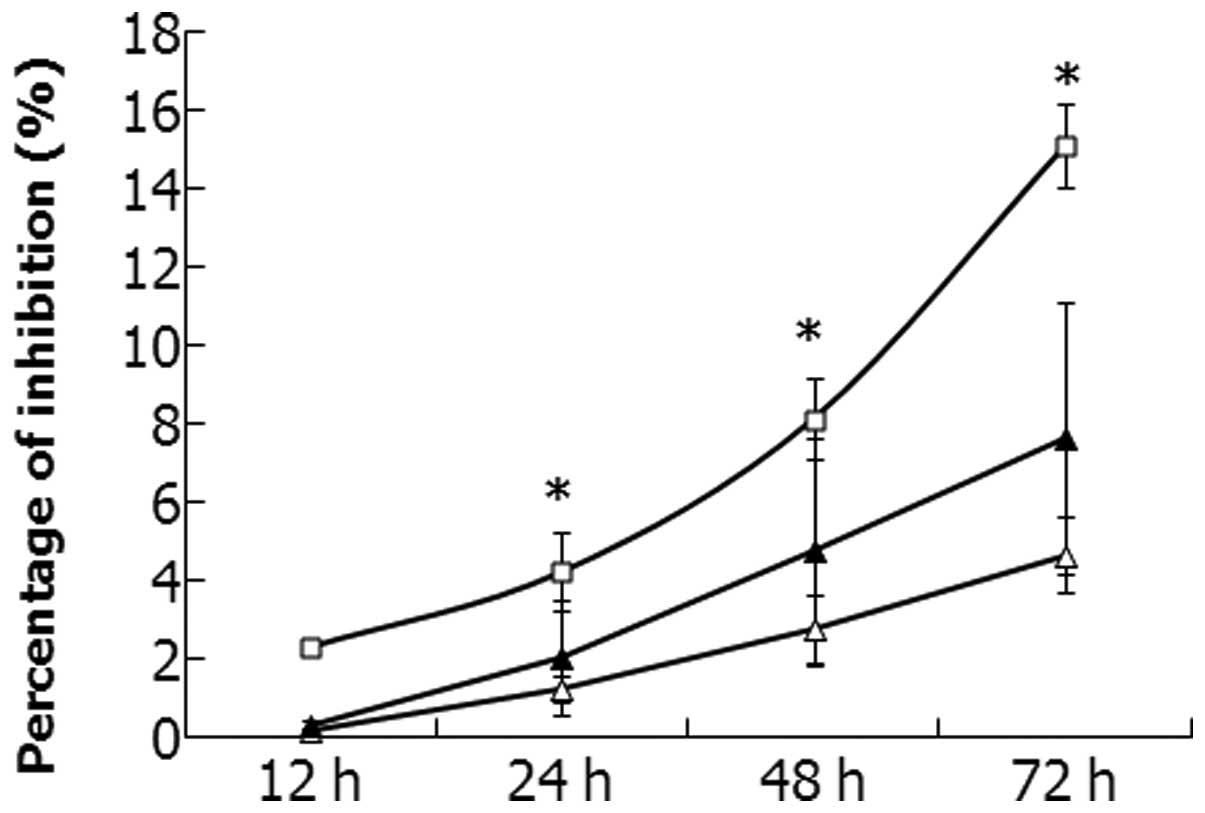
Proliferation of SaOS2 cells
and Snail-1-shRNA-transfected SaOS2 cells
CCK-8 assays were performed to investigate the
proliferation of the SaOS2 cells transfected with
shRNA-Mock and shRNA-Snail-1. The growth inhibition of all cells
increased in a time-dependent manner (Fig. 2). The results suggest that the
growth of the SaOS2 cells was inhibited significantly
when Snail-1 expression was inhibited (P<0.05).
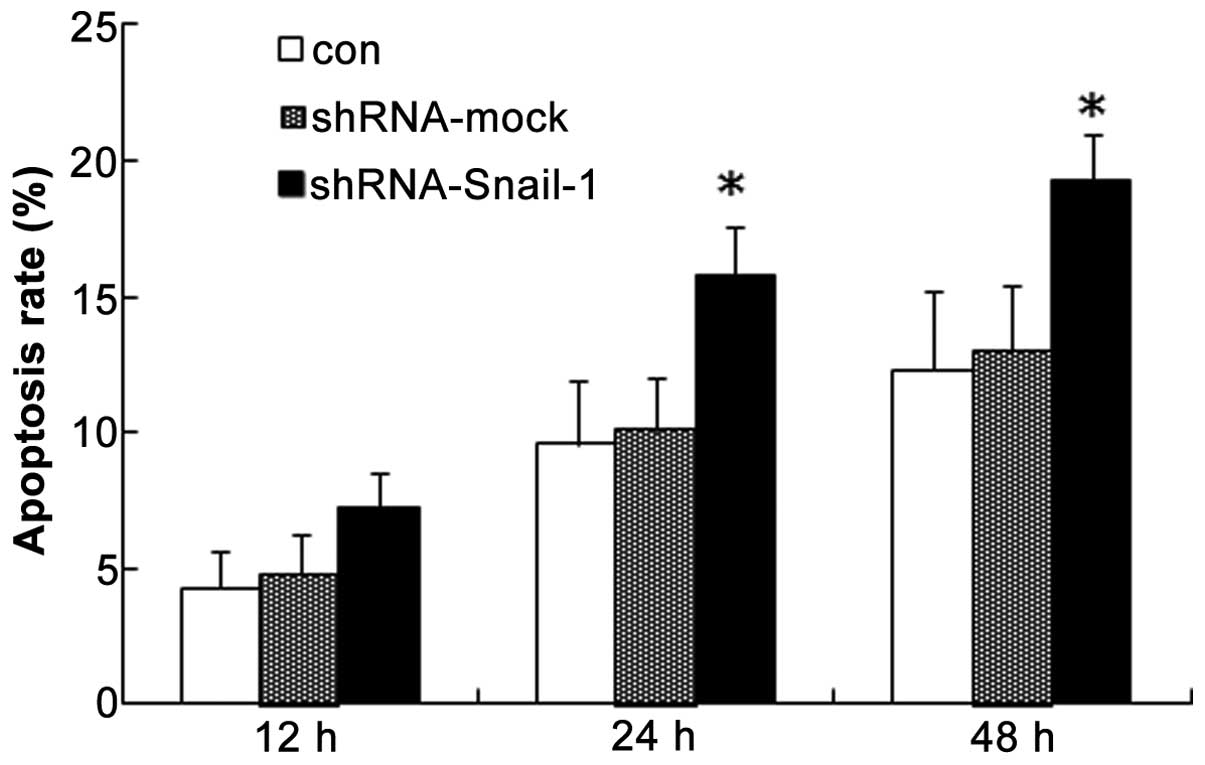
Apoptosis differs between normal and
sh-Snail-1-transfected SaOS2 cells
A quantitative analysis of the fluorescent signals
of the cells was performed by fluorescence-activated cell sorting.
As shown in Fig. 3, the percentage
of TRITC-positive SaOS2 cells was significantly
increased from 12.3% in the control group to 20.3% in the
SaOS2 cells transfected with shRNA-Snail-1 after
treatment for 48 h (P<0.05). These data indicate that the rate
of apoptosis of the SaOS2 cells increased when Snail-1
expression was inhibited.
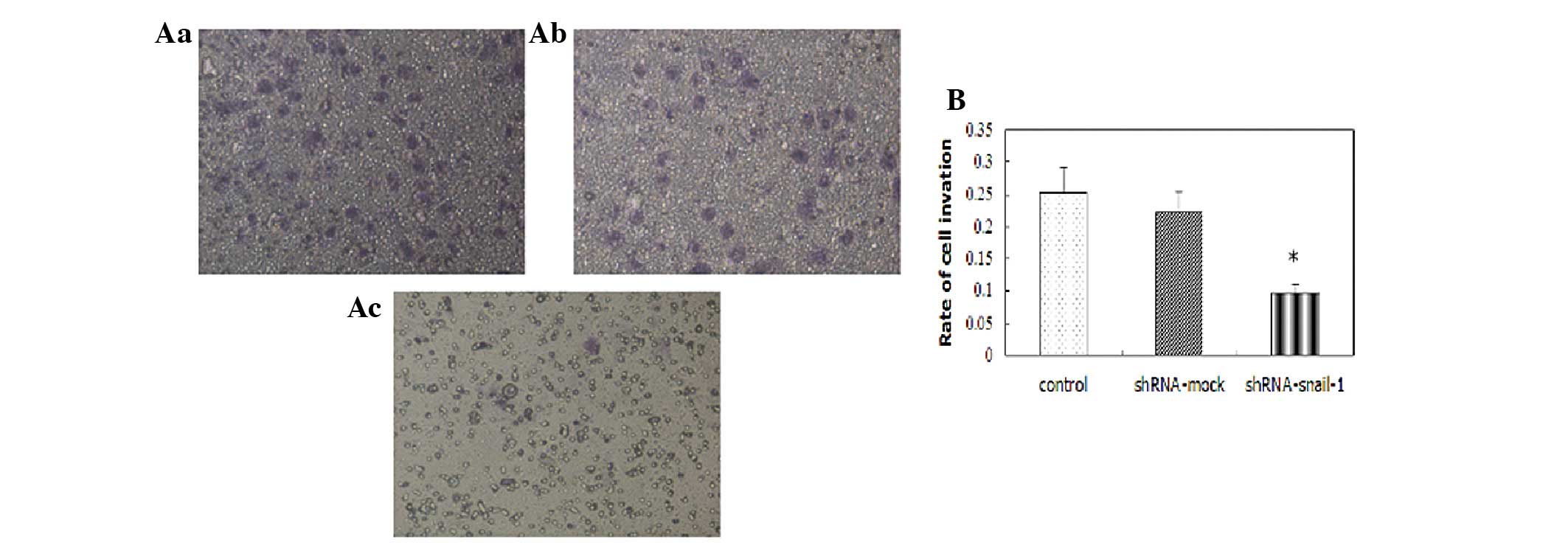
Change of the invasive ability of
SaOS2 cells following transfection with
shRNA-Snail-1
A significant difference in the number of invading
cells was observed between the SaOS2 cells transfected
with shRNA-mock and those transfected with shRNA-Snail-1 in the
migration assay. Furthermore, the invasion rate of the
SaOS2 cells transfected with shRNA-Snail-1 was
significantly lower than that of the control SaOS2 cells
(P<0.05) in the Matrigel invasion assay (Fig. 4).
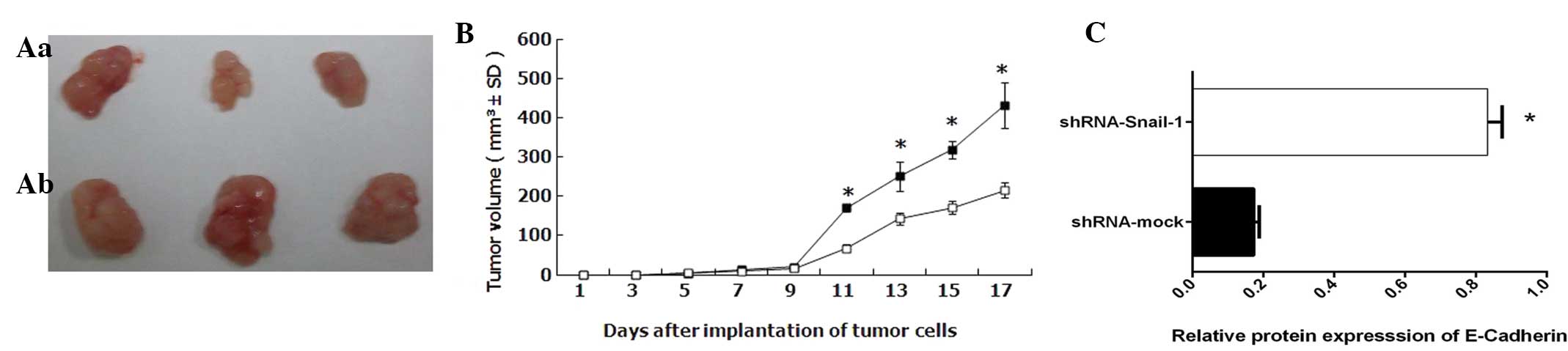
Tumor growth in vivo
Tumor growth was attenuated in the animals injected
with cells transfected with shRNA-mock cells than in those injected
with cells transfected with shRNA-Snail-1 (Fig. 5). The tumors generated by the cells
transfected with shRNA-Snail-1 were less differentiated with much
lower E-cadherin expression levels than those observed in
shRNA-mock-transfected cells.
Discussion
OS is the most common type of primary malignant
tumor of bone in children and young adolescents (13,14).
With the use of chemotherapeutics in combination with aggressive
surgery, the long-term survival rate of patients has improved
(15). However, a number of
patients have metastases at initial diagnosis, which is common to
the lung (16). Therefore, to more
effectively control this disease and improve the patient survival
rate, it is important to elucidate the molecular mechanism of human
sarcomagenesis and to develop novel treatment options for OS.
Epithelial-mesenchymal transition (EMT) has been
widely studied for its role in early development and cancer
metastasis. EMT results in the transformation of a differentiated
epithelial cell to a mesenchymal cell with stem-like properties and
is characterized by loss of cell-to-cell adhesion, specifically
through the dismantling of adherens, tight and gap junctions, as
well as loss of cell polarity and increased motility (17,18).
In embryogenesis, EMT functions by promoting the migration of
mesenchymal cells during gastrulation and neural crest cell
migration, then later during tissue remodeling and organogenesis,
ultimately contributing to the development of differentiated
tissues with specific phenotypes (3,19,20).
In cancer progression, EMT appears to be at least partially
responsible for the invasive nature of tumor cells and it
facilitates metastasis by converting a non-motile cancerous
epithelial cell into a motile mesenchymal cell capable of
disseminating from the tumor mass and entering the circulatory or
lymphatic system (21). In a number
of studies, EMT has been associated with the progression of
numerous types of cancer (22–24),
but few of these studies concerned OS. The loss of E-cadherin is
the hallmark of EMT in cancer development (25,26).
The present study showed that with decreased E-cadherin expression
levels, the malignant biology behavior of cells was repressed,
indicating that EMT was also associated with the progress of OS
(Figs. 2–4).
Snail is the first identified and most important
transcriptional repressor of E-cadherin (27,28).
It functions as a suppressor of the transcription of shotgun (an
E-cadherin homolog) to control embryogenesis in Drosophila
(3,29). Snail also plays a fundamental role
in EMT by suppressing E-cadherin expression in mammalian cells
(30,31). Overexpression of Snail has been
identified in epithelial and endothelial cells of invasive breast
cancer but the overexpression of Snail is not detected in normal
breast cells. The expression of Snail in breast carcinomas is
associated with metastasis, tumor recurrence and poor prognosis
(32–34). In a previous study, it was shown
that Snail-1 was overexpressed in SaOS2 cells (5). Snail also downregulates the expression
levels of other epithelial molecules, including claudins, occludins
and Muc1, and induces the expression of genes associated with a
mesenchymal and invasive phenotype, such as fibronectin and matrix
metallopeptidase-9. The Snail family of zinc-finger transcription
factors consists of Snail-1 (Snail), Snail-2 (Slug) and Snail-3
(Smuc) (3). In the present study
Snail-1 was focused on and it was hypothesized that Snail-1 is able
to regulate EMT in OS through E-cadherin.
To test this hypothesis, SaOS2 cells were
treated with shRNA-Snail-1. As a result, following inhibition of
Snail-1, the expression levels of E-cadherin decreased and the
cells were less able to grow and invade and easily underwent
apoptosis. E-cadherin is involved in EMT associated with
carcinogenesis (25,27,28).
Loss of E-cadherin has been causally associated with the transition
of adenoma to carcinoma and the acquisition of migration capacity.
Therefore, according data in the present study (Fig. 1), we considered that inhibition of
the expression of Snail-1 prevents EMT and induces E-cadherin
expression in OS. The results of the present study predict that
patients with OS with high levels of Snail-1 and low levels of
E-cadherin have a poor prognosis for the progression of OS. The
expression levels of Snail-1 and E-cadherin may be used as
indicators of the progression of OS. The monitoring of tumor
overexpression of Snail-1 and E-cadherin by reverse
transcription-PCR analysis of the RNA present in the serum/plasma
may be useful as a non-invasive method for selecting suitable
patients for therapy.
In conclusion, the present study indicates that
Snail-1 is a regulator of E-cadherin and inhibition of Snail-1
represses EMT in OS, therefore Snail-1 has potential as a target of
OS therapy.
References
|
1
|
Ferguson WS and Goorin AM: Current
treatment of osteosarcoma. Cancer Invest. 19:292–315. 2001.
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009.
|
|
3
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002.
|
|
4
|
Ferrari S, Smeland S, Mercuri M, et al;
Italian and Scandinavian Sarcoma Groups. Neoadjuvant chemotherapy
with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and
doxorubicin for patients with localized osteosarcoma of the
extremity: a joint study by the Italian and Scandinavian Sarcoma
Groups. J Clin Oncol. 23:8845–8852. 2005.
|
|
5
|
Yang H, Zhang Y, Zhou Z, et al: Snail-1
regulates VDR signaling and inhibits 1,25(OH)-D3 action
in osteosarcoma. Eur J Pharmacol. 670:341–346. 2011.
|
|
6
|
Perez-Moreno M, Jamora C and Fuchs E:
Sticky business: orchestrating cellular signals at adherens
junctions. Cell. 112:535–548. 2003.
|
|
7
|
Takeichi M: Morphogenetic roles of classic
cadherins. Curr Opin Cell Biol. 7:619–627. 1995.
|
|
8
|
Huber O, Bierkamp C and Kemler R:
Cadherins and catenins in development. Curr Opin Cell Biol.
8:685–691. 1996.
|
|
9
|
De Wever O and Mareel M: Role of tissue
stroma in cancer cell invasion. J Pathol. 200:429–447. 2003.
|
|
10
|
Vleminckx K, Vakaet L Jr, Mareel M, et al:
Genetic manipulation of E-cadherin expression by epithelial tumor
cells reveals an invasion suppressor role. Cell. 66:107–119.
1991.
|
|
11
|
Perl AK, Wilgenbus P, Dahl U, Semb H and
Christofori G: A causal role for E-cadherin in the transition from
adenoma to carcinoma. Nature. 392:190–193. 1998.
|
|
12
|
Batlle E, Sancho E, Franci C, et al: The
transcription factor snail is a repressor of E-cadherin gene
expression in epithelial tumour cells. Nat Cell Biol. 2:84–89.
2000.
|
|
13
|
Kansara M and Thomas DM: Molecular
pathogenesis of osteosarcoma. DNA Cell Biol. 26:1–18. 2007.
|
|
14
|
Tang N, Song WX, Luo J, et al:
Osteosarcoma development and stem cell differentiation. Clin Orthop
Relat Res. 466:2114–2130. 2008.
|
|
15
|
Meyers PA, Schwartz CL, Krailo MD, et al;
Children’s Oncology Group. Osteosarcoma: the addition of muramyl
tripeptide to chemotherapy improves overall survival - a report
from the Children’s Oncology Group. J Clin Oncol. 26:633–638.
2008.
|
|
16
|
Kager L, Zoubek A, Potschger U, et al;
Cooperative German-Austrian-Swiss Osteosarcoma Study Group. Primary
metastatic osteosarcoma: presentation and outcome of patients
treated on neoadjuvant Cooperative Osteosarcoma Study Group
protocols. J Clin Oncol. 21:2011–2018. 2003.
|
|
17
|
Klymkowsky MW and Savagner P:
Epithelial-mesenchymal transition: a cancer researcher’s conceptual
friend and foe. Am J Pathol. 174:1588–1593. 2009.
|
|
18
|
Peinado H, Del Carmen Iglesias-de la Cruz
M, Olmeda D, et al: A molecular role for lysyl oxidase-like 2
enzyme in snail regulation and tumor progression. EMBO J.
24:3446–3458. 2005.
|
|
19
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004.
|
|
20
|
Tlsty TD and Coussens LM: Tumor stroma and
regulation of cancer development. Annu Rev Pathol. 1:119–150.
2006.
|
|
21
|
Yehiely F, Moyano JV, Evans JR, et al:
Deconstructing the molecular portrait of basal-like breast cancer.
Trends Mol Med. 12:537–544. 2006.
|
|
22
|
Lacroix M, Toillon R and Leclercq G:
Stable ‘portrait’ of breast tumors during progression: data from
biology, pathology and genetics. Endocr Relat Cancer. 11:497–522.
2004.
|
|
23
|
Zhuang Z, Lininger RA, Man YG, et al:
Identical clonality of both components of mammary carcinosarcoma
with differential loss of heterozygosity. Mod Pathol. 10:354–362.
1997.
|
|
24
|
Berx G, Cleton-Jansen AM, Strumane K, et
al: E-cadherin is inactivated in a majority of invasive human
lobular breast cancers by truncation mutations throughout its
extracellular domain. Oncogene. 13:1919–1925. 1996.
|
|
25
|
Castro Alves C, Rosivatz E, Schott C, et
al: Slug is overexpressed in gastric carcinomas and may act
synergistically with SIP1 and Snail in the down-regulation of
E-cadherin. J Pathol. 211:507–515. 2007.
|
|
26
|
Saegusa M, Hashimura M, Kuwata T and
Okayasu I: Requirement of the Akt/beta-catenin pathway for uterine
carcinosarcoma genesis, modulating E-cadherin expression through
the transactivation of slug. Am J Pathol. 174:2107–2115. 2009.
|
|
27
|
Moll R, Mitze M, Frixen UH and Birchmeier
W: Differential loss of E-cadherin expression in infiltrating
ductal and lobular breast carcinomas. Am J Pathol. 143:1731–1742.
1993.
|
|
28
|
Côme C, Magnino F, Bibeau F, et al: Snail
and slug play distinct roles during breast carcinoma progression.
Clin Cancer Res. 12:5395–5402. 2006.
|
|
29
|
Peinado H, Ballestar E, Esteller M and
Cano A: Snail mediates E-cadherin repression by the recruitment of
the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell
Biol. 24:306–319. 2004.
|
|
30
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007.
|
|
31
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006.
|
|
32
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: implications in
development and cancer. Development. 132:3151–3161. 2005.
|
|
33
|
Martin TA, Goyal A, Watkins G and Jiang
WG: Expression of the transcription factors snail, slug, and twist
and their clinical significance in human breast cancer. Ann Surg
Oncol. 12:488–496. 2005.
|
|
34
|
Parker BS, Argani P, Cook BP, et al:
Alterations in vascular gene expression in invasive breast
carcinoma. Cancer Res. 64:7857–7866. 2004.
|