Introduction
Although esophageal cancer is not as common as lung,
breast or colon malignancies, it is associated with a high
mortality rate, with an incidence rate that is close to its
cancer-specific mortality (1). Due
to presentation with severe comorbidity and an advanced stage of
disease, over half of patients with esophageal cancer in the
Western world are not able to undergo surgery (2). The 5-year survival rate for resectable
disease ranges from 10–25% (3),
while recurrent and metastatic esophageal cancer have even poorer
prognoses. Progression-free survival for esophageal cancer is
reportedly 5.6 months (95 % confidence interval [CI], 2.8–8.4
months), with a median survival time of 17.0 months (95 % CI,
12.3–21.7 months) (4).
The main aim when treating patients with inoperable
esophageal cancer is to sustain oral nutrition. Minimizing the
hospital stay, relieving pain and eliminating reflux and
regurgitation are also essential, and therefore treatment is
modified to the individual depending on the tumor stage and
location, and the overall health of the patient. Although
combination chemoradiotherapy has been associated with a high rate
of complete pathological remission (5), the majority of targeted drugs,
including avastin and cetuximab (6–7), have
limited roles in the treatment of esophageal squamous cancer. While
cetuximab may provide certain benefits in the treatment of this
type of cancer (7), it is an
expensive treatment. These observations underline the requirement
for exploration of other targeted drugs for the treatment of
esophageal squamous cancer.
Radiotherapy together with chemotherapy and surgery
represents the main treatment modality for esophageal cancer.
However, technological advances in radiation treatment, including
positron emission tomography-based, planning intensity-modulated
(IMRT) and image-guided radiotherapies, have developed the
practice, particularly for the treatment of esophageal cancer. The
main goal of these novel approaches is precise irradiation of the
tumor, while minimizing the risk of damage to healthy tissues
(8). Post-treatment complications
are often associated with esophageal cancer radiotherapy and
patients have to endure radiotherapy for long periods of time. In
addition, certain patients present with general radiotherapy
contraindications, including chronic obstructive pulmonary disease
(COPD) and cardiopathy. Although stereotactic body radiation
therapy (SBRT) is increasingly used to treat numerous solid tumors,
it is usually prohibited for use in the treatment of esophageal
cancer due to the potential for esophageal perforation and
stenosis. Thus, more efficient management should be explored to
improve survival and quality of life for patients with advanced
esophageal cancer.
In the present study, a combination of chemotherapy
and recombinant human endostatin (endostar) with sequential SBRT is
shown to be successful as a salvage treatment for recurrent
esophageal cancer accompanied by grade III dyspnea and difficulty
in swallowing a semi-liquid diet. The patient had a relatively long
progression-free survival time of >8 months. Patient provided
written informed consent. This study was approved by the ethics
committee of Daping Hospital (Chongqing, China).
Case report
A 71-year-old female with a 2-year history of
esophageal squamous cell carcinoma was referred to the Cancer
Center of Daping Hospital (Chongqing, China). The patient had
severe grade III dyspnea for 6 days and required a semi-liquid diet
for 2 years. The performance status (PS) upon admission was 4
points according to the Eastern Cooperative Oncology Group scale.
The medical history of the patient included COPD, diagnosed in
1999, and diabetes, diagnosed in 2008, with no history of smoking
or drinking. The patient had undergone surgery 2 years prior,
followed by four cycles of cisplatin plus 5-fluorouracil as a
post-operative adjuvant therapy. At the time of the initial
surgery, the cancer was classified as T2N0M0 stage. A computed
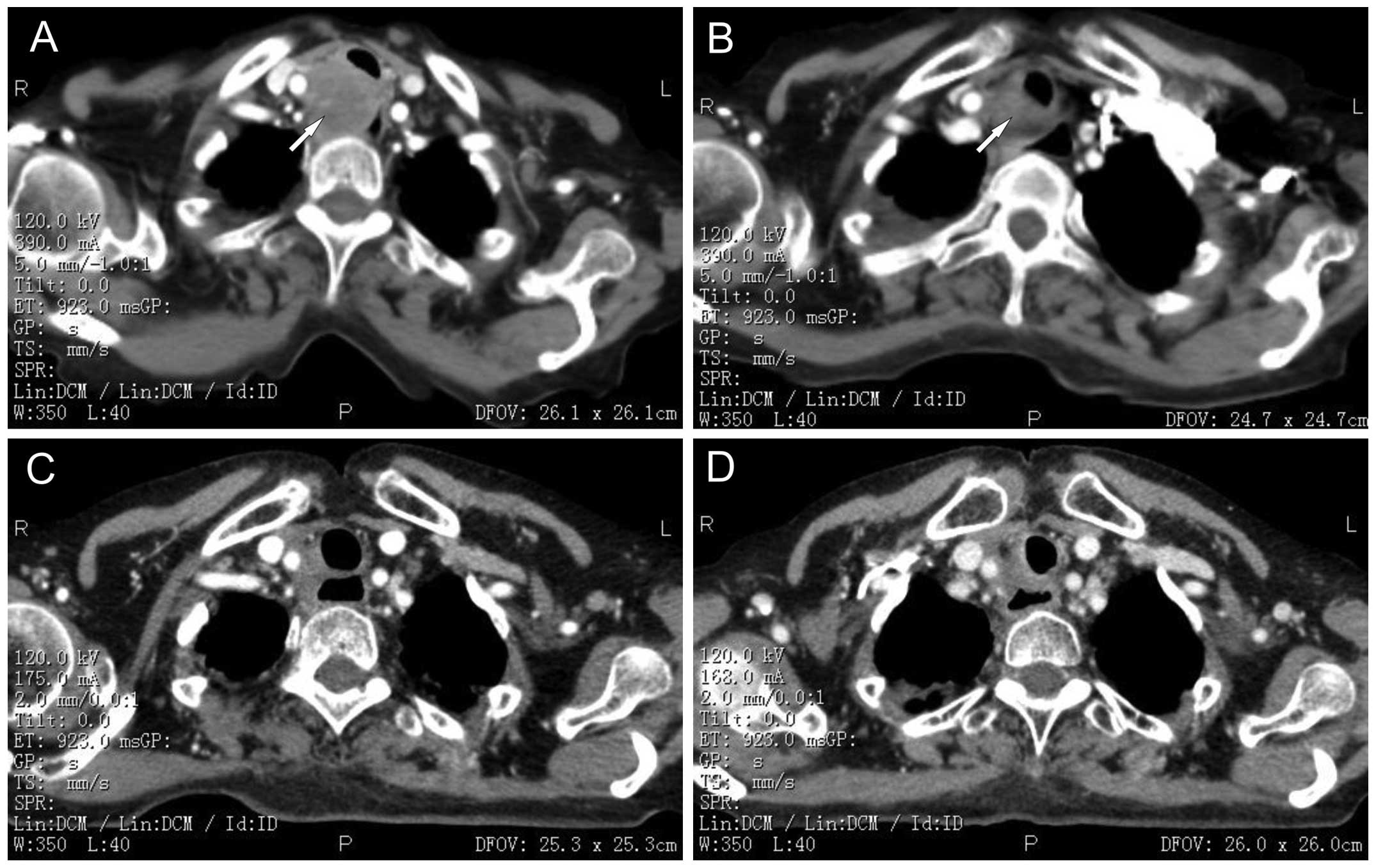
tomography (CT) of the chest (Fig.
1A) prior to admission revealed the presence of a paratracheal
lymph node metastasis, measuring 4.5×3.5×2.5 cm, that was squeezing
the trachea and esophagus. No other metastasis in the abdominal
organs or lymph nodes were detected by CT.
Upon referral, the airway of the patient was so
narrow that endotracheal intubation was unsuccessful and,
consequently, the patient was unsuitable for tracheotomy due to the
location of the stenosis. For similar reasons, the patient was also
unsuitable for surgical resection. In addition, dyspnea is a
contraindication for radiotherapy, as radiation edema may aggravate
the condition. As the life of the patient was in danger,
methylprednisolone acetate was administered at 80 mg per day for
temporary relief of the dyspnea, and this was followed by initial
treatment with chemotherapy combined with endostar. As the PS of
the patient was 4, the chemotherapy regimen, which included
intravenous (IV) administration of docetaxel (40 mg/m2;
days 1 and 8), 5-fluorouracil (400 mg/m2; days 1–5 and
8–12), nedaplatin (40 mg/m2; days 1 and 8) and endostar
(3 mg; days 1–14), was divided into 2 weeks. The dyspnea of the
patient was downgraded to grade II after 1 week, and the
methylprednisolone acetate dose was reduced to 40 mg per day. One
month following chemotherapy, the dyspnea of the patient was
alleviated. The dyspnea was downgraded to grade I and a CT scan
showed that the lesions were 3.0×2.5×1.5 cm (Fig. 1B). Objective efficacy evaluation
indicated partial remission.
The patient was then administered SBRT treatment
combined with methylprednisolone acetate at 40 mg per day until the
end of radiotherapy. The stereotactic γ-ray whole-body therapeutic
system (body γ-knife radiosurgery) with 30 rotary conical-surface
Co (60) sources was focused on the target volume. Low-speed CT
simulation was conducted, followed by three-dimensional conformal
radiotherapy (3D-CRT) planning. A total dose of 33 Gy was delivered
at 3.3 Gy/fraction to the 60% isodose line covering the planning
target volume. The biological equivalent dose (BED) was 43.89 Gy.
The radiotherapy course was delivered in 2 weeks. During the
radiotherapy, the patient experienced grade II esophagitis. One
month following radiotherapy, the patient showed rapid improvement
in dyspnea and dysphagia, and CT revealed that the paratracheal
lymph node lesions had disappeared (Fig. 1C). Objective efficacy evaluation
indicated almost complete remission.
The patient then received another three cycles
(separated by a 3-week interval) of IV systemic chemotherapy
combined with endostar (docetaxel: 75 mg/m2, day 1;
5-fluorouracil: 800 mg/m2, days 1–5; nedaplatin: 75
mg/m2, day 1; and endostar: 3 mg; days 1–14). Four
months following radiotherapy, CT imaging revealed that the cancer
was in complete remission (Fig.
1D). The patient was virtually asymptomatic following this
treatment, and was able to breathe, eat normally and gain weight.
Significantly, radiation-induced esophageal perforation and
stenosis was not observed. The patient continued to exhibit no
symptoms of dyspnea and dysphagia and has had no evidence of
metastatic disease, with a progression-free survival of >8
months at the time of writing.
Discussion
In the present study, the value of SBRT in patients
with esophageal cancer, who are not suitable for surgery due to the
advanced disease stage or comorbidity was assessed. The case of a
patient with esophageal cancer who responded to SBRT treatment is
presented, including the use of combined chemotherapy plus endostar
with sequential SBRT for the treatment of esophageal squamous
cancer.
Esophageal carcinoma is one of the most common
malignant tumors, particularly in China, which is a high-incidence
area. Due to the mild symptoms associated with early-stage
esophageal cancer, the majority of patients cannot be diagnosed
until they progress to advanced cancer. The treatment outcomes for
surgery or chemoradiotherapy for advanced-stage patients remain
unsatisfactory (9). Although
esophagectomy has historically been considered the standard of
care, ~60% of patients are unsuitable for surgical resection due to
advanced-stage disease or the presence of comorbidity (10). This involves the infiltration of
surrounding tissues by the esophageal cancer, causing esophageal
stenosis and possibly esophagotracheal fistula in certain cases.
The median survival time for patients with advanced esophageal
cancer in a case-control study was only 3–5 months (11). These patients can only be treated
with palliative procedures, including radiotherapy, chemotherapy
and esophageal stent placement, to improve their quality of life.
The standard scheme for concurrent chemoradiotherapy is
radiotherapy at a dose of 50–50.4 Gy over 5–5.5 weeks and
5-fluorouracil and cisplatin-based concurrent chemotherapy
(12–13).
Radiotherapy methods for treatment of esophageal
cancer are 3D-CRT, IMRT and afterloading intracavitary
radiotherapy. Although the current worldwide standard of esophageal
cancer radiation treatment is 3D-CRT, overall survival,
locoregional control and non-cancer-related mortality were
significantly improved following IMRT compared with those following
3D-CRT (14). Nutting et al
(15) concluded that IMRT using
conventional beam angles can provide acceptable dose homogeneity
within the planned target volume and reduce lung irradiation
compared to 3D-CRT. Additionally, numerous studies have revealed
the benefits of IMRT in various tumor sites in terms of the
feasibility of normal tissue sparing (16–18).
Postponement complications for esophageal cancer radiotherapy
include esophagitis and toxicity of the lung, heart and spinal
cord, while patients with severe comorbidity, advanced age and
advanced-stage disease cannot endure the long treatment time and
are not suitable for IMRT. Afterloading intracavitary radiotherapy
offers effective palliation as a method of brachytherapy, but is
also associated with multiple endoscopies, transient acute mucosal
edema causing symptom exacerbation and an increased risk of
esophageal fistula formation (19–20).
Localized toxicity may be the consequence of difficult
catheter-luminal centering and the resulting poor dosimetry of
high-dose rate brachytherapy. Additionally, brachytherapy is often
complicated by the difficulty in confirmation of the final catheter
position (21).
SBRT has been accepted as an effective cancer
therapy by patients and physicians. The goal of SBRT is precise,
and complete destruction of chosen target structures without
significant concomitant or late-radiation damage to adjacent
tissues. This effect is obtained by the precise focusing of
multiple low-energy radiation beams crossing at the target,
operating by the radiobiological effect of stereotactically
directed, highly focused ionizing γ-beams of the 201 cobalt-60
sources. The mechanical accuracy is ~0.3 mm, which was originally
developed primarily to treat brain tumors, but was soon used for
the treatment of other tumors.
Although tumor location in the cavity is usually
prohibitive for SBRT treatment, radiation esophagitis was slight in
the present study, and radiation-induced esophageal perforation and
stenosis were not observed. The dose of only 33 Gy (BED, 43.89 Gy)
used in the present study was able to achieve local control and
provide symptom relief. These findings indicate that tumor invasion
of the whole layer of the esophagus, and not SBRT, is the primary
factor responsible for radiation-induced esophageal perforation,
and everyday esophageal movement itself can prevent esophageal
stenosis. Thus, if the single-dose radiation is not too high,
esophagus cancer is not an absolute contraindication to SBRT
treatment. Additionally, although SBRT is not a standard treatment
option for esophageal cancer, it appears to be a reasonable option
in patients who have become refractory to traditional therapy, and
it can rapidly relieve patient symptoms when administered at the
appropriate dose. More specifically, SBRT is a good treatment
option for certain cases with severe comorbidity, advanced age and
advanced disease stage. However, further clinical trials are
required to explore the safety and efficacy of SBRT for the
treatment of esophageal cancer, particularly with regard to late
complications.
SBRT was combined with endostar, a proteolytic
C-terminal fragment of the vascular and epithelial basement
membrane collagen type XVIII that has been proven to be effective
for anti-angiogenesis and tumor growth inhibition (22), to treat the patient with esophageal
cancer in the present study. Endostar combined with radiation or
chemotherapy has proven effective in treating other cancers,
including nasopharyngeal carcinoma, colorectal, non-small cell lung
and cervical cancers (23–25). In addition, Chang et al
(26) investigated the efficacy of
endostar combined with chemotherapy on human esophageal squamous
cell carcinoma Eca-109 cells in mice, and demonstrated that the
tumor volumes and weight were significantly lower in the treatment
group compared with the control. The cellular proliferation of the
tumor xenograft in the combined treatment group was significantly
lower. These findings indicate that endostar combined with
chemotherapy can evidently enhance the inhibitory effect on
esophageal squamous cell carcinoma Eca-109 cells in mice. A review
of the outcomes of endostar combined with chemoradiotherapy as a
first-line treatment for patients with unresectable esophageal
cancer showed that chemoradiotherapy combined with endostatin
increased the complete response rate (44.4 vs. 30% in the
chemoradiotherapy-alone group) and the 1-year and 3-year overall
survival rates (72 vs. 50.0% and 32 vs. 22.0%, respectively)
(27). Although cetuximab combined
with chemotherapy is also effective in the treatment of esophageal
squamous cell cancer according to the National Comprehensive Cancer
Network guidelines (9), endostar
combined with chemotherapy was chosen for the present study due to
economic reasons. The results show that this regimen was effective,
safe and reversed the development of the disease with the initial
treatment. In addition, endostar prolonged the progression-free
survival of the patient. However, further clinical trials are
required to confirm its role in the treatment of esophageal
cancer.
References
|
1
|
Karaosmanoğlu AD and Blake MA:
Applications of PET-CT in patients with esophageal cancer. Diagn
Interv Radiol. 18:171–182. 2012.
|
|
2
|
Koshy M, Esiashvilli N, Landry JC, et al:
Multiple management modalities in esophageal cancer: epidemiology,
presentation and progression, work-up, and surgical approaches.
Oncologist. 9:137–146. 2004.
|
|
3
|
Tytgat GN, Bartelink H, Bernards R, et al:
Cancer of the esophagus and gastric cardia: recent advances. Dis
Esophagus. 17:10–26. 2004.
|
|
4
|
Huang J, Zhou Y, Zhang H, et al: A phase
II study of biweekly paclitaxel and cisplatin chemotherapy for
recurrent or metastatic esophageal squamous cell carcinoma: ERCC1
expression predicts response to chemotherapy. Med Oncol.
30:3432013.
|
|
5
|
Emi M, Hihara J, Hamai Y, et al:
Neoadjuvant chemoradiotherapy with docetaxel, cisplatin, and
5-fluorouracil for esophageal cancer. Cancer Chemother Pharmacol.
69:1499–1505. 2012.
|
|
6
|
Idelevich E, Kashtan H, Klein Y, et al:
Prospective phase II study of neoadjuvant therapy with cisplatin,
5-fluorouracil, and bevacizumab for locally advanced resectable
esophageal cancer. Onkologie. 35:427–431. 2012.
|
|
7
|
Lorenzen S, Schuster T, Porschen R, et al:
Cetuximab plus cisplatin-5-fluorouracil versus
cisplatin-5-fluorouracil alone in first-line metastatic squamous
cell carcinoma of the esophagus: a randomized phase II study of the
Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol.
20:1667–1673. 2009.
|
|
8
|
Vosmik M, Petera J, Sirak I, et al:
Technological advances in radiotherapy for esophageal cancer. World
J Gastroenterol. 16:5555–5564. 2010.
|
|
9
|
Gao XS: Considerations of treatment
standardization from the procession of NCCN guideline of esophageal
cancer. Chin J Cancer. 29:860–864. 2010.
|
|
10
|
Katsoulis IE, Karoon A, Mylvaganam S and
Livingstone JI: Endoscopic palliation of malignant dysphagia: a
challenging task in inoperable oesophageal cancer. World J Surg
Oncol. 4:382006.
|
|
11
|
Wong SK, Chiu PW, Leung SF, et al:
Concurrent chemoradiotherapy or endoscopic stenting for advanced
squamous cell carcinoma of esophagus: a case-control study. Ann
Surg Oncol. 15:576–582. 2008.
|
|
12
|
Cooper JS, Guo MD, Herskovic A, et al:
Chemoradiotherapy of locally advanced esophageal cancer: long-term
follow-up of a prospective randomized trial (RTOG 85-01). Radiation
Therapy Oncology Group. JAMA. 281:1623–1627. 1999.
|
|
13
|
Minsky BD, Pajak TF, Ginsberg RJ, et al:
INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial
of combined-modality therapy for esophageal cancer: high-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002.
|
|
14
|
Lin SH, Wang L, Myles B, et al: Propensity
score-based comparison of long-term outcomes with 3-dimensional
conformal radiotherapy vs intensity-modulated radiotherapy for
esophageal cancer. Int J Radiat Oncol Biol Phys. 84:1078–1085.
2012.
|
|
15
|
Nutting CM, Bedford JL, Cosgrove VP, et
al: A comparison of conformal and intensity-modulated techniques
for oesophageal radiotherapy. Radiother Oncol. 61:157–163.
2001.
|
|
16
|
Mundt AJ, Mell LK and Roeske JC:
Preliminary analysis of chronic gastrointestinal toxicity in
gynecology patients treated with intensity-modulated whole pelvic
radiation therapy. Int J Radiat Oncol Biol Phys. 56:1354–1360.
2003.
|
|
17
|
Uy NW, Woo SY, Teh BS, et al:
Intensity-modulated radiation therapy (IMRT) for meningioma. Int J
Radiat Oncol Biol Phys. 53:1265–1270. 2003.
|
|
18
|
Vicini FA, Sharpe M, Kestin L, et al:
Optimizing breast cancer treatment efficacy with
intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys.
54:1336–1344. 2002.
|
|
19
|
Homs MY, Eijkenboom WM, Coen VL, et al:
High dose rate brachytherapy for the palliation of malignant
dysphagia. Radiother Oncol. 66:327–332. 2003.
|
|
20
|
Homs MY, Steyerberg EW, Eijkenboom WM, et
al: Single-dose brachytherapy versus metal stent placement for the
palliation of dysphagia from oesophageal cancer: multicentre
randomised trial. Lancet. 364:1497–1504. 2004.
|
|
21
|
Russo JK and Rosen L: TomoTherapy
stereotactic body radiation therapy (SBRT) for the salvage
treatment of locally recurrent esophageal adenocarcinoma following
trimodality therapy: a case report. Tumori. 97:406–410. 2011.
|
|
22
|
O’Reilly MS, Boehm T, Shing Y, et al:
Endostatin: an endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997.
|
|
23
|
Rong B, Yang S, Li W, et al: Systematic
review and meta-analysis of Endostar (rh-endostatin) combined with
chemotherapy versus chemotherapy alone for treating advanced
non-small cell lung cancer. World J Surg Oncol. 10:1702012.
|
|
24
|
Zhou J, Wang L, Xu X, et al: Antitumor
activity of Endostar combined with radiation against human
nasopharyngeal carcinoma in mouse xenograft models. Oncol Lett.
4:976–980. 2012.
|
|
25
|
Jin F, Ji H, Jia C, et al: Synergistic
antitumor effects of endostar in combination with oxaliplatin via
inhibition of HIF and CXCR4 in the colorectal cell line SW1116.
PLoS One. 7:e471612012.
|
|
26
|
Chang L, Guo F, Lv Y, et al: The
inhibitory effects of Endostar combined with chemotherapy on human
esophageal squamous cell carcinoma xenograft in mice. Mol Biol Rep.
40:669–673. 2013.
|
|
27
|
Zhong Z, Gu X, Zhang Z, et al: Recombinant
human endostatin combined with definitive chemoradiotherapy as
primary treatment for patients with unresectable but without
systemic metastatic squamous cell carcinoma of the oesophagus. Br J
Radiol. 85:e1104–e1109. 2012.
|