Introduction
Lung cancer is a leading cause of mortality
globally, with the most frequent type being adenocarcinoma.
Although platinum-based traditional chemotherapy is currently the
first-line therapy for advanced lung cancer, due to its clinical
benefits, its use is limited due to significant associated
toxicities. In an effort to overcome these limitations, targeted
therapies are currently an area of research focus due to our
progressive understanding of tumor molecular biology and the tumor
microenvironment (TME), including medications targeting the
epidermal growth factor receptor, such as gefitinib (1,2) and
erlotinib (1,3), and those targeting the vascular
endothelial growth factor (VEGF) signaling pathways, such as
bevacizumab (4). However, only a
small proportion of specific patients benefit from the current
targeting agents, with inevitable resistance. The identification of
alternative promising molecular targets would be a rational
consideration for individual patients with lung cancer.
Tumor growth and invasion is closely associated with
the TME. The main components of TME angiogenic endothelial cells
are regulated by various bio-mediators, including platelet
endothelial cell molecule 1 [PECAM-1; namely cluster of
differentiation 31 (CD31)] (5,6) and
VEGF (7–9). PECAM-1 is a biomarker of endothelial
cells (10–12). Experimental studies have indicated
that PECAM-1 regulates endothelial cell motility and angiogenesis
(13) and is a potential target on
TME endothelial cells (14,15). Although it has been shown that the
vascular inhibitor that targets the VEGF of the TME can be used an
efficacious therapy (4,9,16–19),
it remains uncertain whether PECAM-1 could be used as an angiogenic
inhibitor on the TME. In addition, the delivery system targeting
PECAM-1 in vivo requires further exploration.
RNA interference (RNAi) technology shows
considerable promise as a nucleic acid-based therapy (20). Small interfering RNA (siRNA)
consists of 19- to 23-nucleotide double-stranded RNA duplexes via
the formation of an RNA-induced silencing complex (RISC). RISCs
specifically identify homologous gene mRNA and induce
sequence-specific mRNA degradation leading to silencing of target
gene expression. The performance of siRNA-targeted therapy requires
a suitable and effective carrier delivery system. Cationic
liposomes have been used as effective siRNA carriers in
vitro and in vivo (21,22).
Achieving systemic RNAi in vivo requires that the siRNA
possesses the properties of stability, cellular delivery and tissue
bioavailability. Aside from siRNA alone (naked),
2′-O-methyl-modified siRNACD31 has the strongest
resistance towards degradation by exo- and endonucleases in the
serum and tissue homogenates (20,23),
leading to more effective therapeutic RNAi in vivo.
With respect to previous discussions regarding siRNA
delivery systems, the use of 2′-O-methyl-modified
siRNACD31, with cationic liposomes as carriers, would be
an attractive candidate technology for systemic delivery of PECAM-1
in vivo (20,22,23).
In the present study, the effects of the systemic delivery of
siRNACD31 on the growth of lung adenocarcinoma
xenografts were investigated with the application of
2′-O-methyl-modified siRNACD31-cationic liposome
complexes to silence PECAM-1.
Materials and methods
siRNA and RNAi-mate
The 2′-O-methyl-modified siRNACD31
molecules used in the present study are described in Table I. siRNACD31,
3′-fluorescein amidite (FAM) fluorescence-labeled
siRNACD31 (siRNACD31-FAM; described in
Table I), stable negative control
RNA (SNC; described in Table I) and
RNAi-mate were all synthesized by GenePharma Co., Ltd. (Shanghai,
China). The primers of PECAM-1 mRNA for reverse transcription
polymerase chain reaction (RT-PCR) were also synthesized by
GenePharma Co., Ltd. (Table
II).
 | Table IsiRNA sequence for EOMA cells. |
Table I
siRNA sequence for EOMA cells.
| siRNA | Sequence (5′ to
3′) |
|---|
| CD31 |
| Sense | CAGAUACUCUAGAACGGAA |
| Antisense | UUCCGUUCUAGAGUAUCUG |
| CD31-FAM |
| Sense | CAGAUACUCUAGAACGGAA |
| Antisense | UUCCGUUCUAGAGUAUCUG-FAM |
| SNC |
| Sense |
UUCUCCGAACGUGUCACGUTT |
| Antisense |
ACGUGACACGUUCGGAGAATT |
 | Table IIPrimers sequence for PECAM-1
RT-PCR. |
Table II
Primers sequence for PECAM-1
RT-PCR.
| Gene | Sequence (5′ to
3′) |
|---|
| PECAM-1
(murine) | |
| Sense |
TCCAGGCCAGCTGCTCCACTT |
| Antisense |
GCCTTCCGTTCTCTTGGTGAGGC |
| GAPDH (murine) | |
| Sense |
AACTTTGGCATTGTGGAAGG |
| Antisense |
GGATGCAGGGATGATGTTCT |
Cell lines and cell treatment
EOMA cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and grown in endothelial
growth MED-0002 media (PriCells Biomedical Technology Co., Ltd,
Wuhan, China) containing 10% fetal bovine serum (Gibco, Invitrogen
Life Technologies, Carlsbad, CA, USA), in 6-well plates at 37°C, in
a 100% humidity cell incubator containing 5% CO2, and
identified with human anti-factor VIII antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The cells cultured were
harvested for assays during the exponential growth phase. The
exponential growth EOMA cells (5×104/well) were seeded
in 24-well plates containing various agents for 24 h as follows:
Naked siRNACD31-RNAi-mate (siRNACD31 group),
FAM-labeled siRNACD31-RNAi-mate
(siRNACD31-FAM group), SNC-RNAi-mate (SNC group) and
Opti-minimum essential medium (MEM; reduced-serum cell culture
medium; Gibco) as a blank control (control group). In brief, the
siRNACD31-RNAi-mate transfection procedures were as
follows: Firstly, 50 μl Opti-MEM and 20 pmol siRNACD31
(or siRNACD31-FAM, SNC or Opti-MEM) were completely
mixed, then 50 μl Opti-MEM diluted with 2 μl RNAi-mate reagent was
added and the mixture was kept at room temperature for 5 min.
Secondly, the diluted siRNACD31 (or
siRNACD31-FAM or SNC) and RNAi-mate reagent were mixed
gently to form siRNA-lipoplexes at room temperature for 20 min.
Finally, 100 μl complexes involving siRNACD31-RNAi-mate
or siRNACD31-FAM-RNAi-mate were respectively added to
each well containing the cells and the medium used for
transfection, and 100 μl SNC-RNAi-mate and Opti-MEM medium were
added respectively to the SNC and control wells. The
FAM-fluorescence detection was performed with a confocal microscope
(excitation wavelength of 495 nm, emission wavelength of 525 nm;
Leica, Mannheim, Germany) after transfection efficiency had been
reached for 6 h at 37°C in a CO2 incubator. The cell
transfection rate was ~80%, and the transfection process continued
for 48 h. Assessment of the various specimens were carried out for
RT-PCR and western blot analysis, and the MTT assay of the cell
proliferation rate was performed as previously described (24). Each assay was performed in
triplicate and independently repeated three times. The rabbit
anti-PECAM-1 and mouse anti-glyceraldehyde-3-phosphate
dehydrogenase (GADPH) antibodies were obtained from Santa Cruz
Biotechnology, Inc., and the goat anti-rabbit immunoglobulin G
monoclonal antibody was purchased from Maixin Technology Co., Ltd.,
(Shenzhen, Guangdong, China) for the western blot assay. The primer
sequences of PECAM-1 used for amplification in the RT-PCR are
listed in Table II. The inhibition
rate of EOMA cell proliferation was calculated as follows:
Inhibition rate of proliferation (%) = [1 - optical density (OD)
experimental wells / OD control wells] × 100. The wells containing
Opti-MEM were used as the control.
The human lung adenocarcinoma (HLAC) A549 cell line
was obtained from the Chinese Academy of Sciences Type Culture
Collection (CASTCC; Shanghai, China) and cultivated according to
the CASTCC recommendations. The cultured cells were harvested for
treatment in vivo during the exponential growth phase.
Tumor implantation
Male, 4–5-week-old BALB/c nude mice [experiment
animal number, SCXK (Hu) 2012-002)], weighing ~20 g, were obtained
from Shanghai SLAC Laboratory Animal, Co., Ltd., (Shanghai, China)
and housed in a specific pathogen-free environment. Abdominal skin
tumor xenografts of nude mice were established by subcutaneous
injection of 200 μl phosphate-buffered saline [PBS; 13 mM NaCl, 2.7
mM KCl, 1.5 mM KH2PO4 and 8 mM
K2HPO4 (pH 7.2)] containing a total of
2×105 exponential growth HLAC cells
(1×105/100 μl). All animal manipulations were performed
in accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals, and were approved by the
Wenzhou Medical University Animal Care and Use Committee (Wenzhou,
Zhejiang, China).
Delivery of
siRNACD31-RNAi-mate lipoplexes in tumor-bearing nude
mice
For in vivo delivery, the treatments were
initiated when the tumor xenografts reached ~85 mm3 (day
0). siRNACD31-RNAi-mate complexes were created by
administering siRNA-lipoplexes intravenously through single tail
vein injections of the total 100 μl solution involving 50 μl (20
μM) siRNACD31, 10 μl RNAi-mate (1 mg/ml) and 40 μl PBS,
while the control mice underwent a 100 μl saline injection. Each
nude mouse underwent vein injection every other day, a total of
five times. The tumor xenograft volumes were measured on days 0, 2,
4, 6 and 10 and were calculated according to the following formula:
V = (W2 × L)/2, where V is the tumor volume, W is the
width and L is the length. The mice were sacrificed by cervical
dislocation following the last measurement of the tumor xenograft
volumes on day 10. The two divided half-tissues of the tumor
xenograft and the lung, brain, liver, heart and kidney tissues were
kept in PBS at −80°C for PECAM-1 (Santa Cruz Biotechnology, Inc.)
and VEGF (Boster Biological Technology Co., Ltd., Wuhan, China)
ELISA examination, respectively, and fixed with formalin for
paraffin-embedded tissue sections for PECAM-1 immunohistochemical
examination.
ELISA estimations for PECAM-1 and
VEGF
The extract of the homogenate from the tumor
xenografts and the lung, brain, liver, heart and kidney tissues was
used for measuring the protein concentrations of PECAM-1 and VEGF
according to the ELISA kit instructions. Subsequent to stopping the
reaction, the plates were read on a KHB-ST-360 microplate reader
purchased from Jingong Industrial Co., Ltd., (Shaoxing, Zhejiang,
China). The PECAM-1 ELISA kit was obtained from Abgent (San Diego,
CA, USA), and the VEGF ELISA kit was purchased from EIAab (Wuhan,
Hubei, China). The bicinchoninic acid (BCA; Beyotime Institute of
Biotechnology, Shanghai, China) determination of total protein
homogenate was used for correcting the value of PECAM-1 and VEGF,
and the BCA correction values of PECAM-1 or VEGF in the homogenates
were calculated as follows: BCA correction value = measured value /
BCA value.
Statistical analysis
Each assay was performed in triplicate and was
independently repeated three times. Results are expressed as the
mean ± standard deviation. The difference between two group means
was tested by one-way analysis of variance or Student’s t-test
according to the character of the experimental data. P<0.05 was
considered to indicate a statistically significant difference. All
data were processed by SPSS version 16.0 for Windows (SPSS, Inc.,
Chicago, IL, USA).
Results
siRNACD31 is transfected
effectively into EOMA cells in vitro
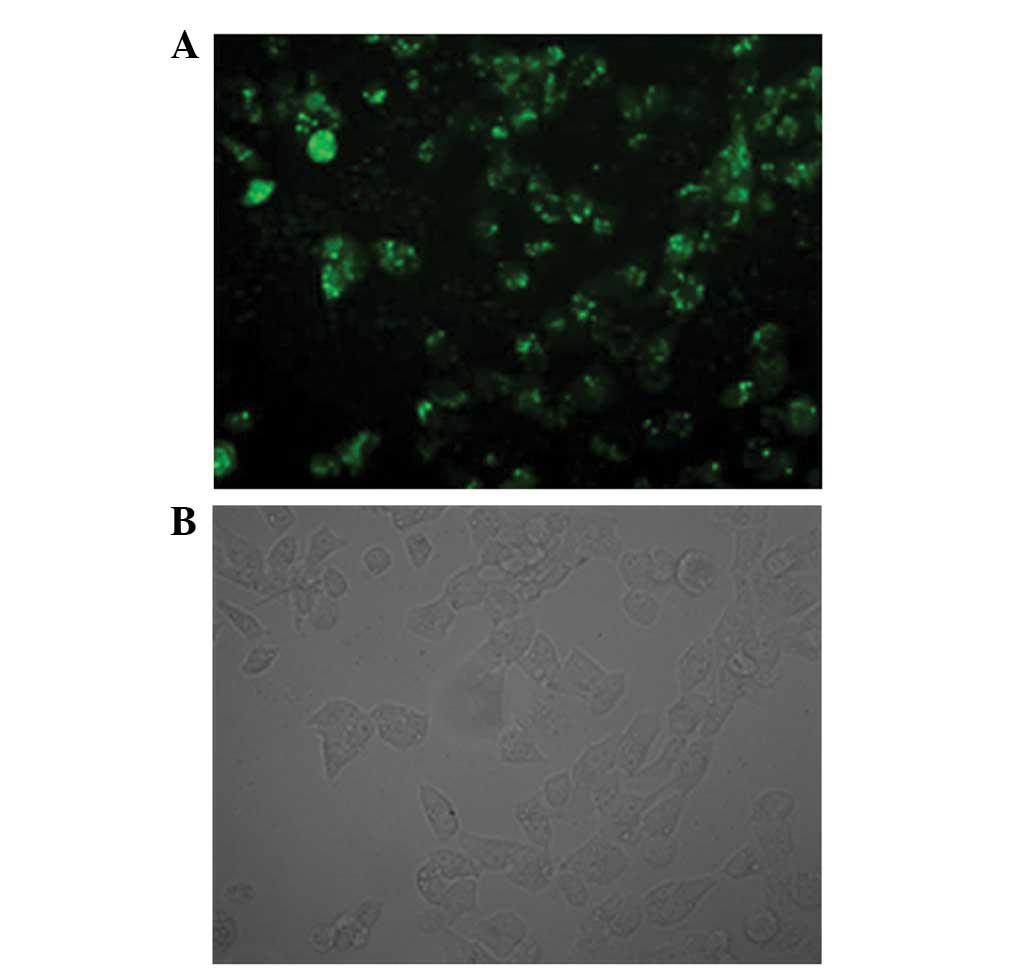
The results of FAM-fluorescence detection by
confocal fluorescence microscopy showed that siRNACD31,
with RNAi-mate as a carrier, was successfully transfected into the
EOMA cells. The bright fluorescence was emitted from the EOMA cells
transfected by the fluorescence FAM-labeled siRNACD31
(Fig. 1A). Fig. 1B shows the same cells observed by
optical microscopy (Olympus BX-51; Olympus Tokyo, Japan).
In vitro siRNACD31 inhibits
the proliferation of EOMA cells
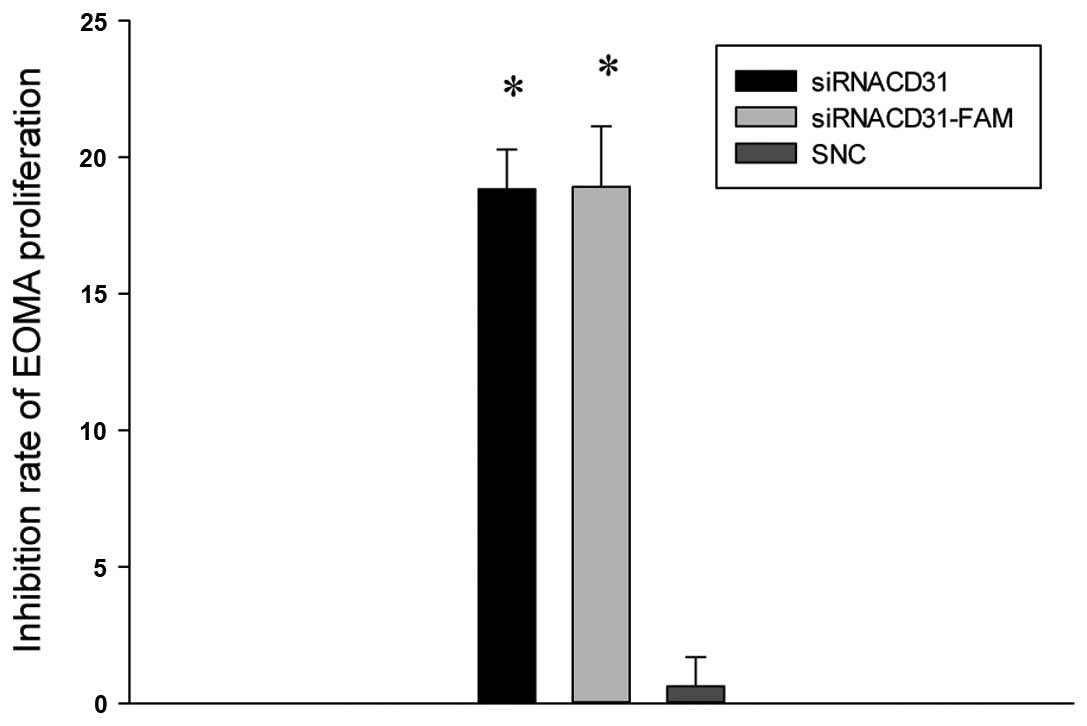
The results of the MTT assay for the inhibition
rates of EOMA proliferation showed that the inhibition rates of the
siRNACD31 and siRNACD31-FAM groups increased
compared with those of the SNC group (all P<0.01 vs. SNC;
Fig. 2), and the inhibition rates
of EOMA proliferation were not different between the
siRNACD31 and siRNACD31-FAM groups
(P>0.05; Fig. 2). These results
indicate that siRNACD31 inhibited the proliferation of
the EOMA cells, and that the fluorescence label, FAM, did not
impair the transfection rate of the siRNACD31.
In vitro siRNACD31
downregulates PECAM-1 mRNA and protein expression
The use of in vitro siRNACD31 and
siRNACD31-FAM, with RNAi-mate as a carrier, weakened the
expression of the PECAM-1 mRNA (Fig.
3A, B and E) and protein (Fig. 3C,
D and E) compared with the EOMA cells treated by SNC and
Opti-MEM (i.e., control groups) (all P<0.01 vs. SNC or control),
and the effects were not weakened for the fluorescence FAM-labeled
siRNACD31 (Fig. 3A–E)
(P>0.05, siRNA vs. siRNACD31-FAM). There was no
difference between the SNC and control groups (P>0.05). The
results indicated that siRNACD31 and
siRNACD31-FAM downregulated the expression of PECAM-1
mRNA and protein in vitro. The transfection efficiency of
siRNACD31 was not weakened by the fluorescence label,
FAM.
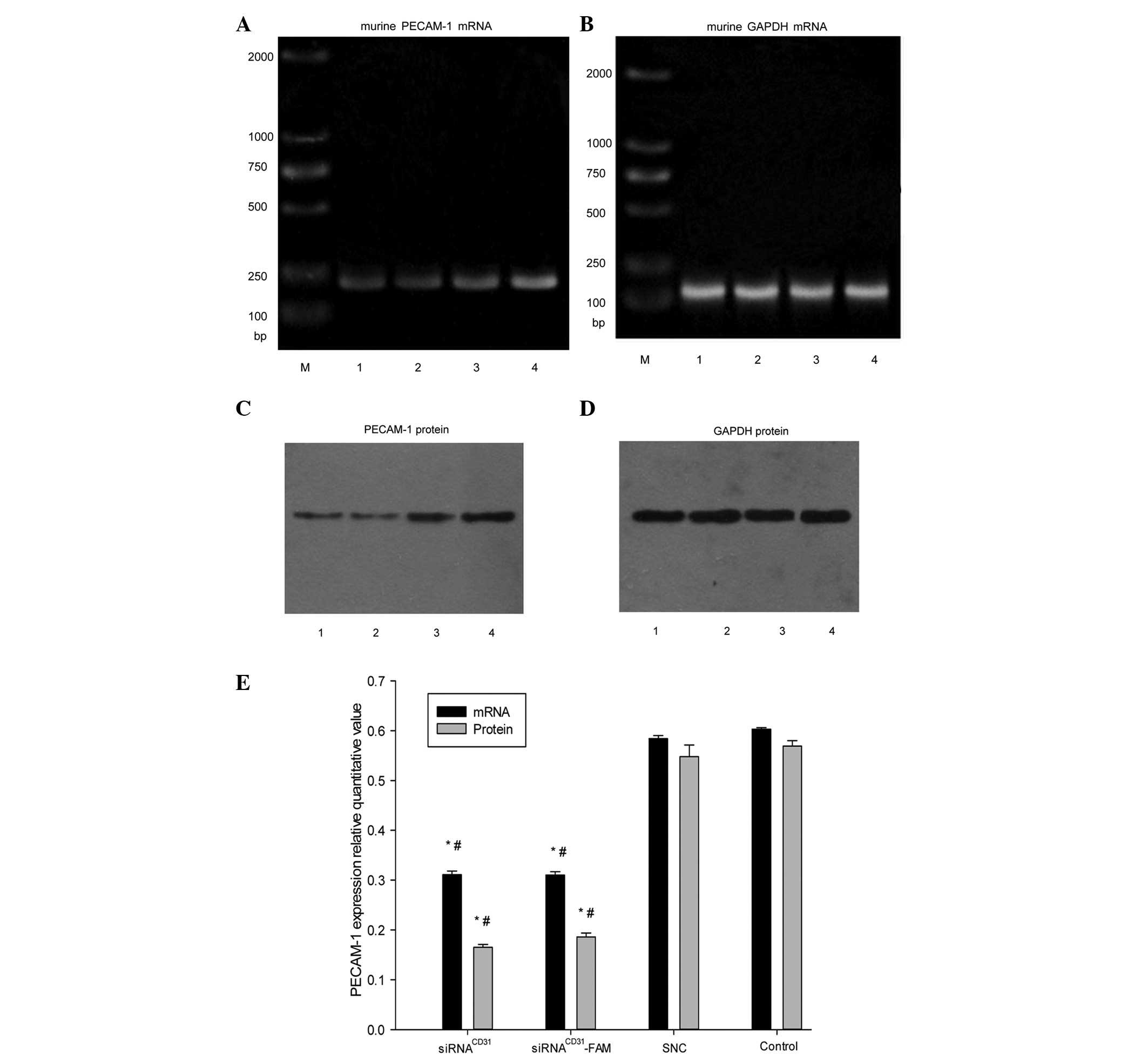 | Figure 3In vitro siRNACD31
downregulates the expression of PECAM-1 mRNA and protein in EOMA
cells. RT-PCR analysis of (A) PECAM-1 mRNA and (B) GADPH mRNA
expression was used as an internal control. Western blot analysis
of (C) PECAM-1 protein and (D) GADPH protein was used as an
internal control. (E) Bar diagram showing the relative quantitative
values of PECAM-1 mRNA and protein determined with RT-PCR and
western blot analysis, respectively. siRNACD31 and
siRNACD31-FAM, with RNAi-mate as a carrier,
downregulated the expression of PECAM-1 mRNA and protein compared
with EOMA cells treated with SNC and Opti-MEM (i.e., control group)
(all P<0.01 vs. SNC or control) and the effects were not
weakened for fluorescence FAM-labeled siRNACD31
(P>0.05, siRNACD31 vs. siRNACD31-FAM).
There was no significant difference between the SNC and control
groups (P>0.05). The relative quantification value (RQ) of
PECAM-1 mRNA (protein) was calculated according to the following
equation: RQ PECAM-1 RNA (protein) = IOD PECAM-1 mRNA (protein) /
IOD GADPH mRNA (protein). The RT-PCR and western blot analysis data
shown were obtained from assays performed in triplicate and
independently repeated three times. siRNA, small interfering RNA;
PECAM-1, platelet endothelial adhesion molecule 1; CD31, cluster of
differentiation 31; EOMA, murine hemangioendothelioma; RT-PCR,
reverse transcription polymerase chain reaction; RNAi, RNA
interference; SNC, stable negative control; MEM, mimimum essential
medium; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IOD,
integrated optical density. M, marker; lane 1,
siRNACD31; lane 2, siRNACD31-FAM; lane 3,
SNC; lane 4, Opti-MEM used as control. Error bars shows the
standard error of the mean. *P<0.01 vs. control;
#P<0.01 vs. SNC. |
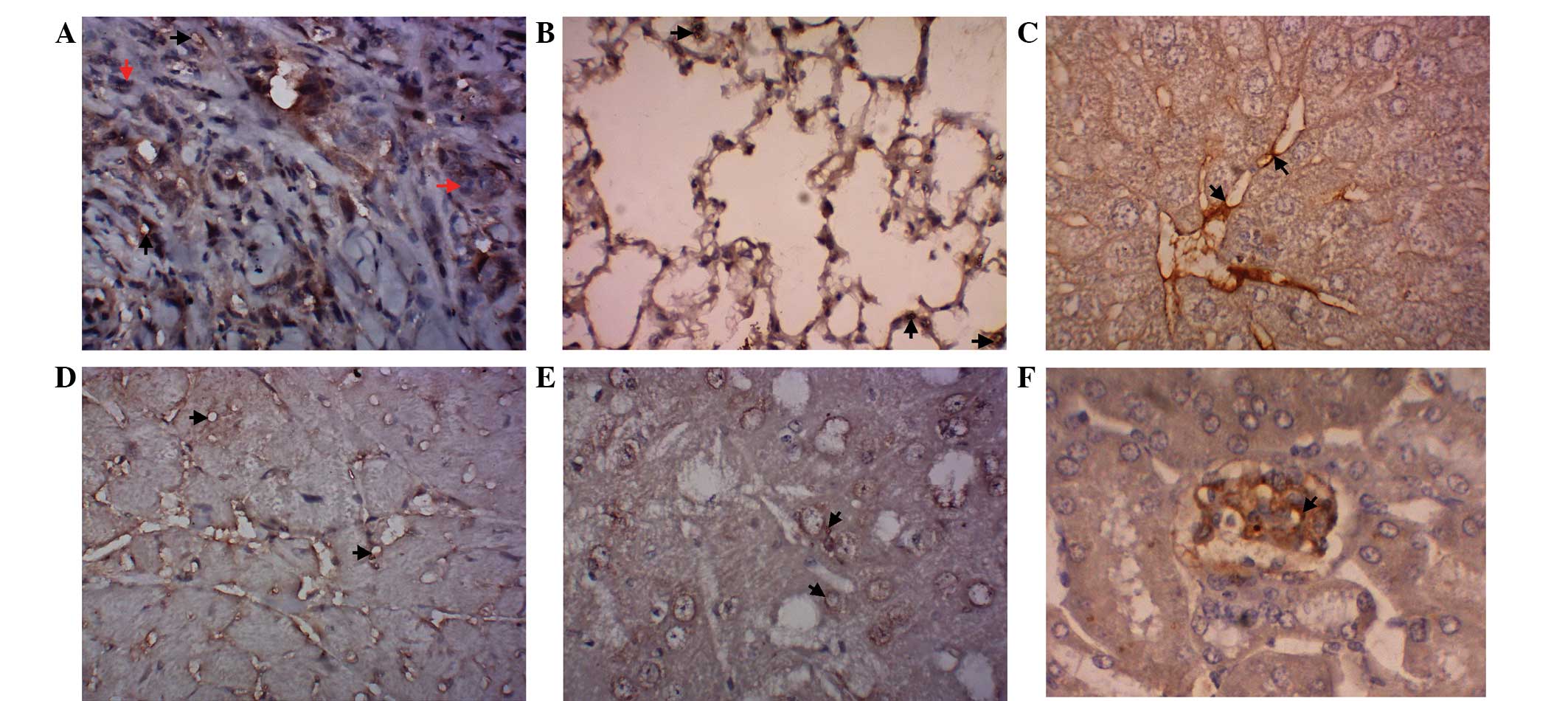
The expression of PECAM-1 was observed in the
vasculature of various tissues. The results of the
immunohistochemical examination indicated that PECAM-1 expression
was observed in the vasculature of the lung adenocarcinoma
xenograft and the lung, liver, heart, brain and kidney tissues
(Fig. 4A–F).
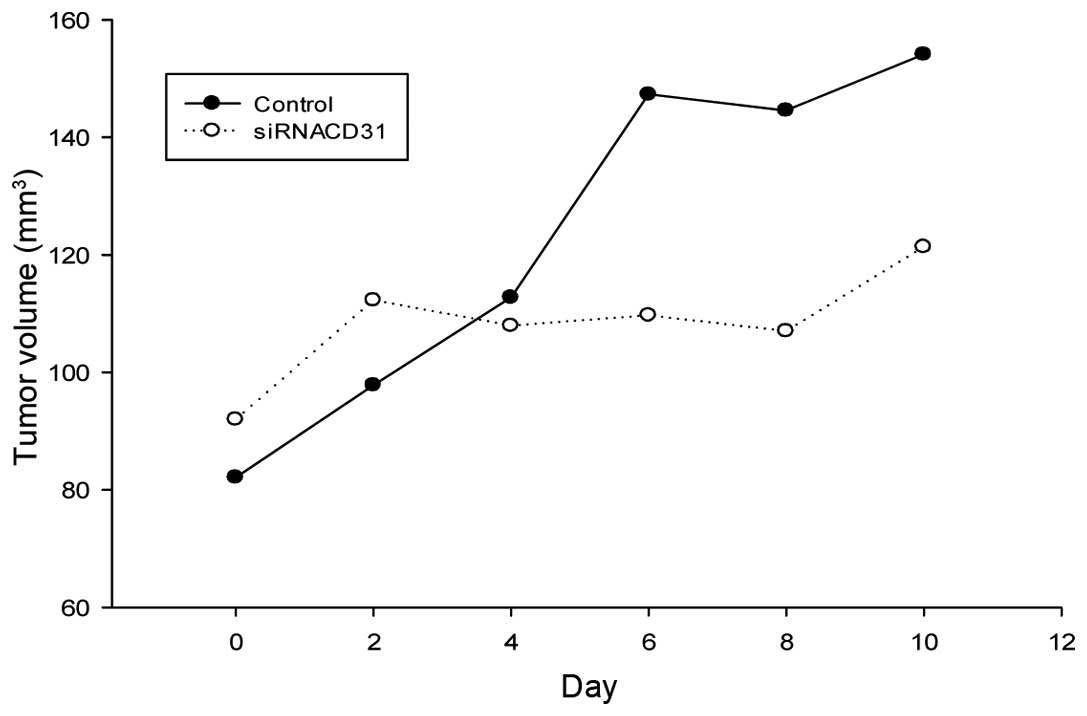
In vivo siRNACD31 inhibits
tumor growth
The volumes of the tumor xenografts in the nude mice
treated by siRNACD31, with RNAi-mate (the
siRNACD31 group) as the carrier, were smaller than those
in the control nude mice (Table
III) (Pday 10<0.05 vs. control; Pdeviation
of tumor xenograft volume (DV)<0.01 vs. control). The
growth of the tumor xenograft in the nude mice of the
siRNACD31 group was slower than in the control group
from day 4, and the DV increased in the latter days (Fig. 5). These results indicated that
siRNACD31, with RNAi-mate as a carrier, may effectively
inhibit the growth of lung adenocarcinoma in vivo.
 | Table IIIChange of tumor xenograft
volumes. |
Table III
Change of tumor xenograft
volumes.
| Group | V0,
mm3 | V10,
mm3 | DV,
mm3 |
|---|
|
siRNACD31 | 91.990±6.562 |
121.346±5.935a |
29.356±2.917b |
| Control | 82.127±17.033 | 154.082±28.563 | 71.954±19.938 |
In vivo siRNACD31
downregulates PECAM-1 and VEGF expression in tumor xenografts
The results of the PECAM-1 protein expression
analysis with ELISA indicated that the measured values (MVs) and
BCA correction values of PECAM-1 in the tumor xenografts of the
nude mice treated with siRNACD31-RNAi-mate complexes
(the siRNACD31 group) were decreased compared with the
values of the tumor xenografts of the nude mice treated with saline
(control group) (all P<0.01; Fig.
6). However, the MVs and BCA correction values of PECAM-1 of
the other tissues (lung, liver, brain, heart and kidney) in the
nude mice treated with siRNACD31-RNAi-mate complexes
were not significantly different compared with the values from the
control nude mice treated with saline (all P>0.05; Fig. 6). The VEGF ELISA assay achieved
similar results to those of PECAM-1 (Fig. 7).
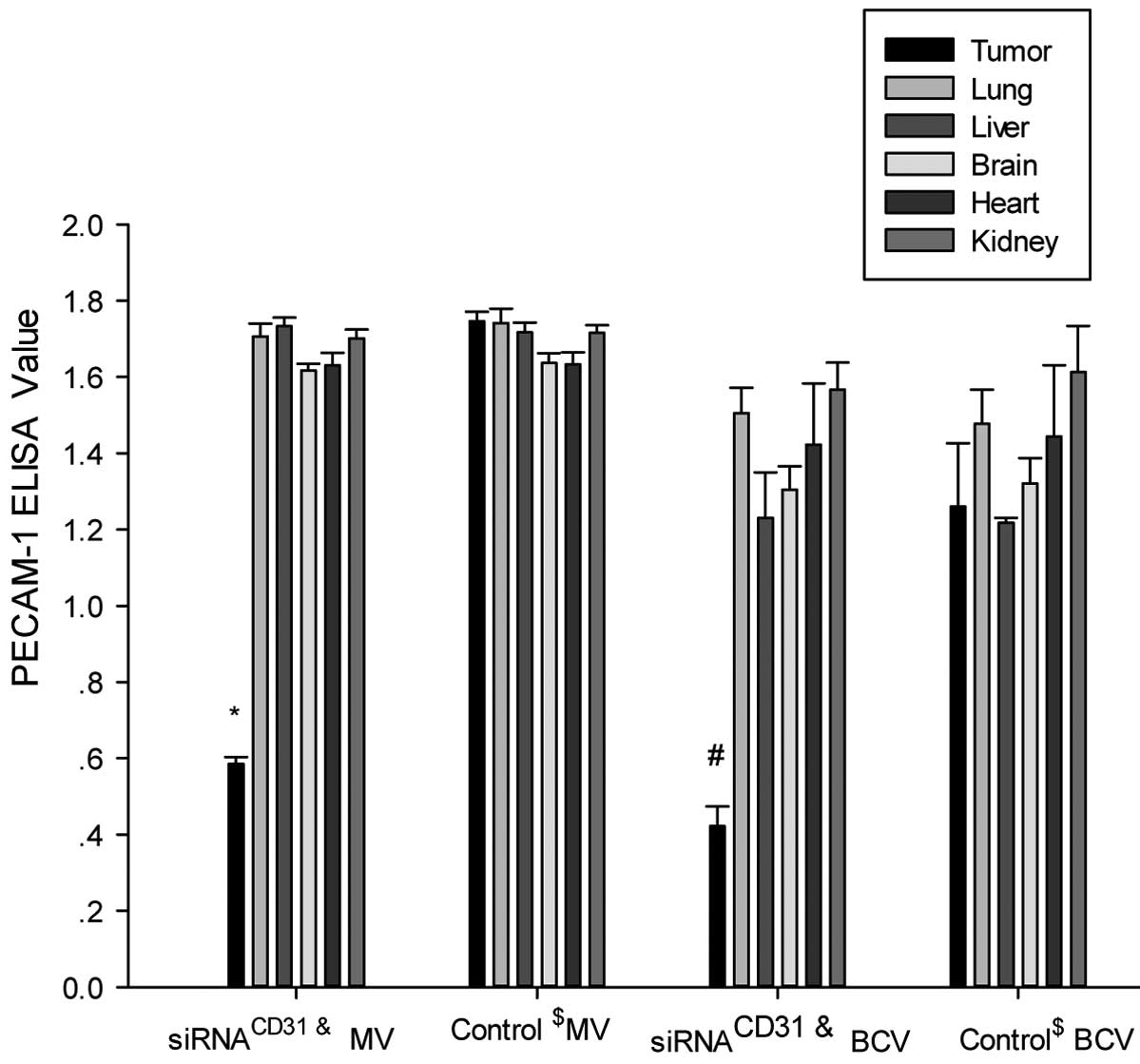 | Figure 6siRNACD31 downregulates
PECAM-1 protein expression of tumor xenografts using RNAi-mate as a
carrier. The measured values (MV, μg/ml) and BCA correction values
(BCV, μg/mg) of PECAM-1 in the tumor xenografts of the nude mice
treated with siRNACD31-RNAi-mate complexes
(siRNACD31 group) decreased compared with those of the
tumor xenograft of the control nude mice treated with saline
(control group) (all P<0.01). The MV and BCV of PECAM-1 in other
tissues (lung, liver, brain, heart and kidney) of the
siRNACD31 group were not different compared with the
values of the control group (all P>0.05). Each assay was
performed in triplicate and was independently repeated three times.
Error bars show the standard error, n=6;
*PMV<0.01 vs. control MV;
#PBCV<0.01 vs. control BCV;
&siRNACD31 group, the nude mice treated
with an injection of siRNA-RNAi-mate via the tail-vein;
$control group, the nude mice treated with an injection
of saline via the tail-vein; siRNA, small interfering RNA; PECAM-1,
platelet endothelial adhesion molecule 1; CD31, cluster of
differentiation 31; RNAi, RNA interference; BCA, bicinchoninic
acid, for the determination of total protein in homogenate (BCA
correction value was calculated by: BCA correction value = measured
value / BCA value). |
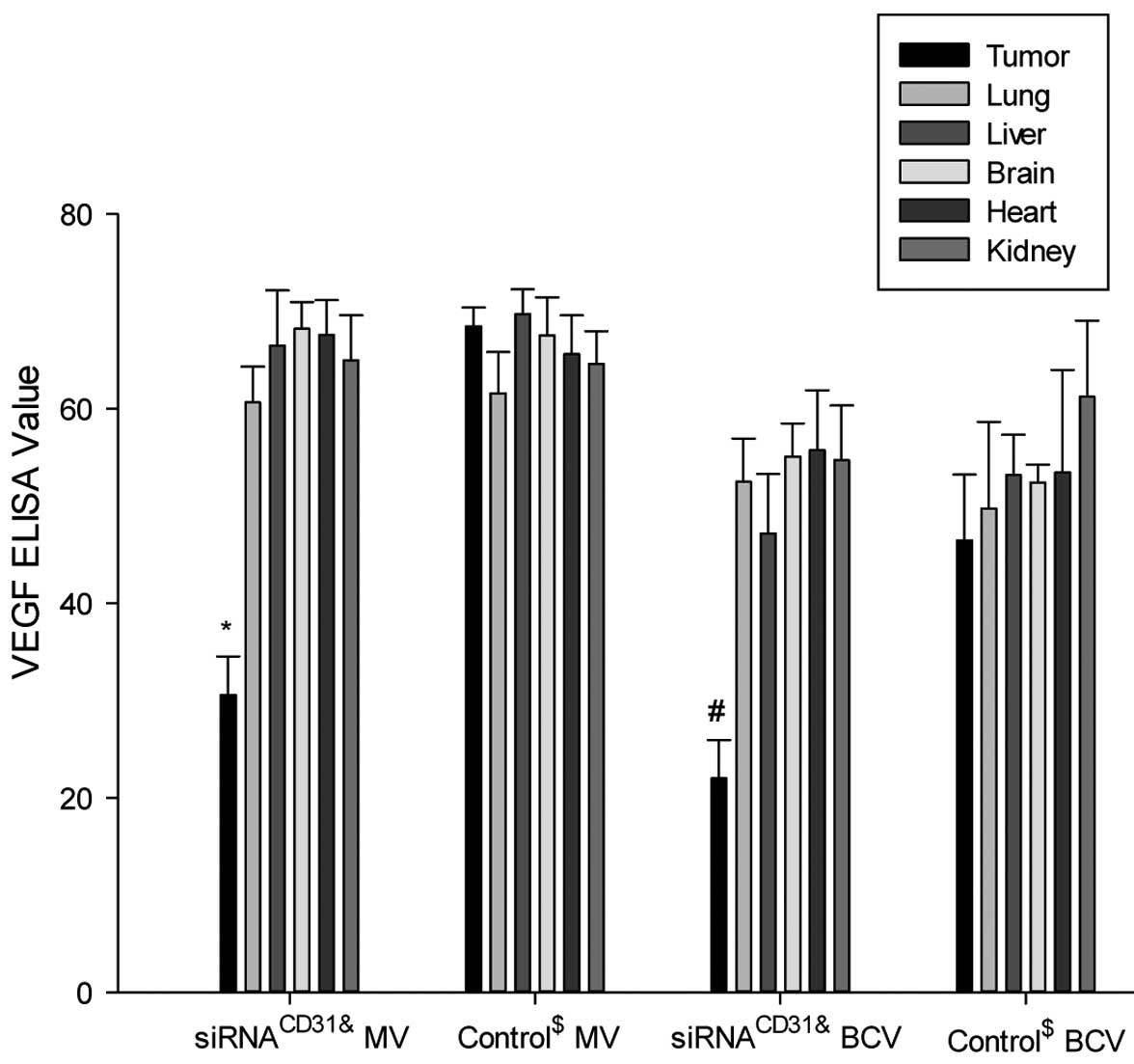 | Figure 7siRNACD31 weakens the VEGF
protein expression of tumor xenografts using RNAi-mate as carrier.
Measured values (MV, ng/ml) and BCA correction values (BCV, ng/mg)
of VEGF in the tumor xenografts of the nude mice treated with
siRNACD31-RNAi-mate complexes (siRNACD31
group) decreased compared with those of the tumor xenografts of the
control nude mice treated with saline (group control) (all
P<0.01). The MV and BCV of VEGF in the other tissues (lung,
liver, brain, heart and kidney) of the siRNACD31 group
were not different compared with the values of the control group
(all P>0.05). Each assay was performed in triplicate and was
independently repeated three times. Error bars show the standard
error, n=6; *PMV<0.01 vs. control MV;
#PBCV<0.01 vs. control BCV;
&siRNACD31 group, the nude mice treated
with an injection of siRNA-RNAi-mate via the tail vein;
$control group, the nude mice treated with an injection
of saline via the tail vein; siRNA, small interfering RNA; CD31,
cluster of differentiation 31; VEGF, vascular endothelial growth
factor; RNAi, RNA interference; BCA, bicinchoninic acid, for the
determination of total protein in homogenate (BCA correction value
was calculated by: BCA correction value = measure value / BCA
value). |
Discussion
RNAi is a promising therapeutic approach to
silencing disease-causing genes, and is usually mediated by siRNA
consisting of 19- to 23-nucleotide double-stranded RNA duplexes.
The delivery system is the main complication in achieving gene
silencing by siRNA technologies in vivo. Previous studies
have reported that 2′-O-methyl-modified siRNA possess a strong
resistance to the degradation by nuclease in the serum and tissues
(20,23). The administration of cationic lipids
has been applied for siRNA delivery in vivo (22). The endothelial specific marker,
PECAM-1 (i.e., CD31) (10,12,26),
is closely associated with angiogenesis (27), and the vasculature of the TME plays
a significant role in the proliferation and invasion of tumor
cells. PECAM-1 could be used as a potential therapeutic target on
the TME with respect to its activity in the pathogenesis of tumors,
including lung cancer (28,29).
In the present study, the targeted delivery of
2′-O-methyl-modified siRNACD31 and cationic liposome
RNAi-mate complexes on endothelial PECAM-1 in vitro and
in vivo were investigated. Three important findings were
noted in the present study. The first was that the
2′-O-methyl-modified siRNACD31-lipoplexes effectively
silenced the target gene, PECAM-1, in vitro and in
vivo. 2′-O-methyl-modified siRNACD31 successfully
downregulated the PECAM-1 mRNA and protein expression of the EOMA
cells using RNAi-mate as a carrier in vitro (Fig. 3), and the expression of PECAM-1 was
detected by immunohistochemical examinations in the vasculature of
the lung adenocarcinoma xenografts (Fig. 4A) and in the vascular tissues of the
lung, liver, heart, brain and kidney in vivo (Fig. 4B–F). These results provided a
molecular and cellular basis for targeted treatment with
2′-O-methyl-modified siRNACD31 and RNAi-mate complexes
in vivo. In the in vivo study, the growth of the lung
adenocarcinoma xenografts was effectively inhibited by injecting
the complexes of 2′-O-methyl-modified siRNA and RNAi-mate via the
tail veins of the nude mice (Table
III and Fig. 5). Although the
expression of the PECAM-1 protein in the lung, liver, heart, brain
and kidney tissues was not decreased (Fig. 6), a decrease in PECAM-1 expression
was obtained in the lung adenocarcinoma xenografts (Fig. 6). These findings indicated that the
2′-O-methyl-modified siRNA-lipoplexes achieved the targeted
silencing of the PECAM-1 gene in the vasculature of the lung
adenocarcinoma xenografts in vivo. The achievement of
specific targeted silencing of the PECAM-1 gene in tumor xenografts
is possibly due to the strong bioavailability of the
siRNACD31-lipoplexes of the neovascular cells in the TME
(22,30). Cationic liposome RNAi-mate may act
as a candidate carrier for the systemic administration of
siRNACD31 to other liposomes (21,30).
Lung adenocarcinoma with abundant vasculature is the most common
pathological type of lung cancer. With respect to the limit and
toxicity of traditional chemotherapy on lung cancer, target
medications have created a promising research area for the
bio-therapy of lung cancer (1–4).
Besides VEGF (4), PECAM-1 would
also be a potential target on the vasculature of the TME (14,15,28),
as it plays a significant role in angiogenesis (13,27).
Although PECAM-1 activity on the modulation of endothelial cells
and its effects in tumor angiogenesis are known (14,15),
the effects of targeted delivery of PECAM-1 on the growth of lung
cancer requires further investigation. It has been demonstrated
that 2′-O-methyl-modified siRNA and cationic lipids could be
applied as an effective targeted delivery for silencing a target
gene (22). On the basis of the
previous discussions regarding siRNA delivery systems, the present
study possibly provides an important target strategy involving
2′-O-methyl-modified siRNA targeting PECAM-1 using cationic lipids
RNAi-mate against the proliferation of lung carcinoma cells
(20,22,23).
The second important finding in the present study
was that the proliferation of the EOMA cells was inhibited by
2′-O-methyl-modified siRNACD31 using RNAi-mate as a
carrier (Fig. 2). PECAM-1 is a
membrane protein with signal transduction (10,31,32)
occurring via the initiation of downstream signaling pathways,
including mitogen-activated protein kinase (33), Erk (34,35)
and PI-3/Akt (36). This has
significant implications in the regulation of endothelial apoptosis
(37,38). Therefore, it is speculated that this
finding possibly contributes to the initiation of signal
transduction by PECAM-1 and to the induction of EOMA cell
apoptosis, leading to the inhibition of the proliferation of EOMA
cells by 2′-O-methyl-modified siRNACD31.
The third significant finding was that a
simultaneous decrease in PECAM-1 (Fig.
6) and VEGF (Fig. 7) was
observed when 2′-O-methyl-modified siRNACD31
downregulated PECAM-1 expression. It is well known that PECAM-1
initiates signal transduction to activate the downstream signaling
pathway (31,33–36,39)
and regulate the generation and release of bio-mediators (27,32,40,41).
On the basis of the aforementioned studies and the results of the
present study, we believe that the downregulation of PECAM-1
expression using siRNACD31 silencing of the PECAM-1 gene
possibly executed an effect on the signal transduction of PECAM-1,
leading to the decrease in VEGF protein expression.
In summary, the present study demonstrated that
2′-O-methyl-modified siRNACD31 and RNAi-mate complexes
may effectively silence the PECAM-1 gene in vitro and in
vivo, and downregulate the expression of PECAM-1 and VEGF
proteins. siRNACD31 targeting of PECAM-1 in the TME may
be a potential gene therapy for tumors. PECAM-1 regulated the
generation of VEGF possibly through the signaling pathway involving
PECAM-1. However, the improvement of the delivery system of
siRNACD31 for achieving complete dissolution of the
tumor xenografts with siRNACD31 mediated by lipoplexes
and the regulation mechanism of PECAM-1 on VEGF requires further
exploration. The combination of various cytokines, including
PECAM-1, VEGF, transforming GF and fibroblast GF, contributing to
tumor angiogenesis would be a possible candidate for future
study.
Acknowledgements
The present study was supported by a grant (no.
Y20100182) from the Wenzhou Science and Technology Bureau
Foundation.
References
|
1
|
Kim ST, Uhm JE, Lee J, et al: Randomized
phase II study of gefitinib versus erlotinib in patients with
advanced non-small cell lung cancer who failed previous
chemotherapy. Lung Cancer. 75:82–88. 2012.
|
|
2
|
Cohen MH, Williams GA, Sridhara R, Chen G
and Pazdur R: FDA drug approval summary: gefitinib (ZD1839)
(Iressa) tablets. Oncologist. 8:303–306. 2003.
|
|
3
|
Perez-Soler R: The role of erlotinib
(Tarceva, OSI 774) in the treatment of non-small cell lung cancer.
Clin Cancer Res. 10:4238s–4240s. 2004.
|
|
4
|
de Gramont A and Van Cutsem E:
Investigating the potential of bevacizumab in other indications:
metastatic renal cell, non-small cell lung, pancreatic and breast
cancer. Oncology. 69(Suppl 3): 46–56. 2005.
|
|
5
|
Park S, DiMaio TA, Scheef EA, Sorenson CM
and Sheibani N: PECAM-1 regulates proangiogenic properties of
endothelial cells through modulation of cell-cell and cell-matrix
interactions. Am J Physiol Cell Physiol. 299:C1468–C1484. 2010.
|
|
6
|
Woodfin A, Voisin MB and Nourshargh S:
PECAM-1: a multi-functional molecule in inflammation and vascular
biology. Arterioscler Thromb Vasc Biol. 27:2514–2523. 2007.
|
|
7
|
Bautch VL: VEGF-directed blood vessel
patterning: from cells to organism. Cold Spring Harb Perspect Med.
2:a0064522012.
|
|
8
|
Yang L, Guan H, He J, Zeng L, Yuan Z, Xu
M, Zhang W, Wu X and Guan J: VEGF increases the proliferative
capacity and eNOS/NO levels of endothelial progenitor cells through
the calcineurin/NFAT signalling pathway. Cell Biol Int. 36:21–27.
2012.
|
|
9
|
Holash J, Davis S, Papadopoulos N, et al:
VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl
Acad Sci USA. 99:11393–11398. 2002.
|
|
10
|
Ilan N and Madri JA: PECAM-1: old friend,
new partners. Curr Opin Cell Biol. 15:515–524. 2003.
|
|
11
|
Watt SM, Gschmeissner SE and Bates PA:
PECAM-1: its expression and function as a cell adhesion molecule on
hemopoietic and endothelial cells. Leuk Lymphoma. 17:229–244.
1995.
|
|
12
|
Müller AM, Hermanns MI, Skrzynski C,
Nesslinger M, Müller KM and Kirkpatrick CJ: Expression of the
endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro.
Exp Mol Pathol. 72:221–229. 2002.
|
|
13
|
DeLisser HM, Christofidou-Solomidou M,
Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda
C, Merwin JR, Madri JA and Albelda SM: Involvement of endothelial
PECAM-1/CD31 in angiogenesis. Am J Pathol. 151:671–677. 1997.
|
|
14
|
Zhou Z, Christofidou-Solomidou M, Garlanda
C and DeLisser HM: Antibody against murine PECAM-1 inhibits tumor
angiogenesis in mice. Angiogenesis. 3:181–188. 1999.
|
|
15
|
Tachezy M, Reichelt U, Melenberg T,
Gebauer F, Izbicki JR and Kaifi JT: Angiogenesis index CD105
(endoglin)/CD31 (PECAM-1) as a predictive factor for invasion and
proliferation in intraductal papillary mucinous neoplasm (IPMN) of
the pancreas. Histol Histopathol. 25:1239–1246. 2010.
|
|
16
|
Herbst RS, Ansari R, Bustin F, et al:
Efficacy of bevacizumab plus erlotinib versus erlotinib alone in
advanced non-small-cell lung cancer after failure of standard
first-line chemotherapy (BeTa): a double-blind, placebo-controlled,
phase 3 trial. Lancet. 377:1846–1854. 2011.
|
|
17
|
Prager GW, Lackner EM, Krauth MT, et al:
Targeting of VEGF-dependent transendothelial migration of cancer
cells by bevacizumab. Mol Oncol. 4:150–160. 2010.
|
|
18
|
Inai T, Mancuso M, Hashizume H, et al:
Inhibition of vascular endothelial growth factor (VEGF) signaling
in cancer causes loss of endothelial fenestrations, regression of
tumor vessels, and appearance of basement membrane ghosts. Am J
Pathol. 165:35–52. 2004.
|
|
19
|
Lagnien-Gaume V, Jehl J, Manzoni P, et al:
Bevacizumab and lung cancer: eligible patients in daily practice.
Rev Mal Respir. 28:25–31. 2011.(In French).
|
|
20
|
Soutschek J, Akinc A, Bramlage B, et al:
Therapeutic silencing of an endogenous gene by systemic
administration of modified siRNAs. Nature. 432:173–178. 2004.
|
|
21
|
Chien PY, Wang J, Carbonaro D, et al:
Novel cationic cardiolipin analogue-based liposome for efficient
DNA and small interfering RNA delivery in vitro and in vivo. Cancer
Gene Ther. 12:321–328. 2005.
|
|
22
|
Aleku M, Schulz P, Keil O, et al: Atu027,
a liposomal small interfering RNA formulation targeting protein
kinase N3, inhibits cancer progression. Cancer Res. 68:9788–9798.
2008.
|
|
23
|
Czauderna F, Fechtner M, Dames S, et al:
Structural variations and stabilising modifications of synthetic
siRNAs in mammalian cells. Nucleic Acids Res. 31:2705–2716.
2003.
|
|
24
|
Ouyang JS, Li YP, Li CY, et al:
Mitochondrial ROS-K+ channel signaling pathway regulated secretion
of human pulmonary artery endothelial cells. Free Radic Res.
46:1437–1445. 2012.
|
|
25
|
Bidwell GL III, Perkins E and Raucher D: A
thermally targeted c-Myc inhibitory polypeptide inhibits breast
tumor growth. Cancer Lett. 319:136–143. 2012.
|
|
26
|
Feng D, Nagy JA, Pyne K, Dvorak HF and
Dvorak AM: Ultrastructural localization of platelet endothelial
cell adhesion molecule (PECAM-1, CD31) in vascular endothelium. J
Histochem Cytochem. 52:87–101. 2004.
|
|
27
|
Dimaio TA, Wang S, Huang Q, Scheef EA,
Sorenson CM and Sheibani N: Attenuation of retinal vascular
development and neovascularization in PECAM-1-deficient mice. Dev
Biol. 315:72–88. 2008.
|
|
28
|
DeLisser H, Liu Y, Desprez PY, et al:
Vascular endothelial platelet endothelial cell adhesion molecule 1
(PECAM-1) regulates advanced metastatic progression. Proc Natl Acad
Sci USA. 107:18616–18621. 2010.
|
|
29
|
Delisser HM: Targeting PECAM-1 for
anti-cancer therapy. Cancer Biol Ther. 6:121–122. 2007.
|
|
30
|
Aleku M, Fisch G, Möpert K, Keil O, Arnold
W, Kaufmann J and Santel A: Intracellular localization of
lipoplexed siRNA in vascular endothelial cells of different mouse
tissues. Microvasc Res. 76:31–41. 2008.
|
|
31
|
Newman PJ and Newman DK: Signal
transduction pathways mediated by PECAM-1: new roles for an old
molecule in platelet and vascular cell biology. Arterioscler Thromb
Vasc Biol. 23:953–964. 2003.
|
|
32
|
Ilan N, Mahooti S, Rimm DL and Madri JA:
PECAM-1 (CD31) functions as a reservoir for and a modulator of
tyrosine-phosphorylated beta-catenin. J Cell Sci. 112:3005–3014.
1999.
|
|
33
|
Wang Y and Sheibani N: PECAM-1
isoform-specific activation of MAPK/ERKs and small GTPases:
implications in inflammation and angiogenesis. J Cell Biochem.
98:451–468. 2006.
|
|
34
|
Masuda M, Kogata N and Mochizuki N:
Crucial roles of PECAM-1 in shear stress sensing of vascular
endothelial cells. Nihon Yakurigaku Zasshi. 124:311–318. 2004.(In
Japanese).
|
|
35
|
Fujiwara K, Masuda M, Osawa M, Kano Y and
Katoh K: Is PECAM-1 a mechanoresponsive molecule? Cell Struct
Funct. 26:11–17. 2001.
|
|
36
|
Limaye V, Li X, Hahn C, Xia P, Berndt MC,
Vadas MA and Gamble JR: Sphingosine kinase-1 enhances endothelial
cell survival through a PECAM-1-dependent activation of PI-3K/Akt
and regulation of Bcl-2 family members. Blood. 105:3169–3177.
2005.
|
|
37
|
Bergom C, Goel R, Paddock C, Gao C, Newman
DK, Matsuyama S and Newman PJ: The cell-adhesion and signaling
molecule PECAM-1 is a molecular mediator of resistance to genotoxic
chemotherapy. Cancer Biol Ther. 5:1699–1707. 2006.
|
|
38
|
Wu N, Kurosu T, Oshikawa G, Nagao T and
Miura O: PECAM-1 is involved in BCR/ABL signaling and may
downregulate imatinib-induced apoptosis of Philadelphia
chromosome-positive leukemia cells. Int J Oncol. 42:419–428.
2013.
|
|
39
|
Masuda M, Osawa M, Shigematsu H, Harada N
and Fujiwara K: Platelet endothelial cell adhesion molecule-1 is a
major SH-PTP2 binding protein in vascular endothelial cells. FEBS
Lett. 408:331–336. 1997.
|
|
40
|
Enciso JM, Gratzinger D, Camenisch TD,
Canosa S, Pinter E and Madri JA: Elevated glucose inhibits
VEGF-A-mediated endocardial cushion formation: modulation by
PECAM-1 and MMP-2. J Cell Biol. 160:605–615. 2003.
|
|
41
|
Privratsky JR, Tilkens SB, Newman DK and
Newman PJ: PECAM-1 dampens cytokine levels during LPS-induced
endotoxemia by regulating leukocyte trafficking. Life Sci.
90:177–184. 2012.
|