Introduction
EphA2, first reported by Lindberg and Hunter in 1990
(1), was obtained from the cDNA
library screening of human keratin epithelial (HeLa cells) via a
degenerate primer. The human EphA2 gene is located at chromosome
1p36.1 and is composed of 17 exons (2). EphA2 protein is a tyrosine
kinase-containing transmembrane glycoprotein receptor with a
molecular weight of 130 kDa and it belongs to the Eph receptor
tyrosine kinases family. EphA2 is extensively expressed in the
epithelial and endothelial cells of normal vessels (3) with a low abundance in the
phosphorylated form (1). Compared
with normal tissues, the EphA2 receptor tyrosine kinase is
expressed relatively higher in the tissues of the prostate, breast,
endometrial, gliofibroma, ovarian, bladder and renal carcinomas in
the non-phosphorylated form (4–10). In
these cancers, high levels of EphA2 predict metastasis and a poor
survival rate (4–10). It was reported that the therapeutic
delivery of EphA2 siRNA into an orthotopic mouse model of ovarian
cancer diminished tumor growth in comparison to non-silencing siRNA
(8). The strong correlation between
the critical factors involved in angiogenesis and invasion,
including a greater microvessel density and higher matrix
metalloproteinase, with an enhanced expression of EphA2 was also
revealed in ovarian cancer samples (11). Additionally, the positive role of
EphA2 was demonstrated during mammary tumor onset and growth in the
MMTV-Neu transgenic mice model, but not in mice overexpressing the
polyomavirus middle T antigen. This indicates that, at least in
breast carcinoma, the role of EphA2 in tumor progression is
determined by the oncogene/tumor suppressor context (12). Previously, Taddei et al
(13) revealed that EphA2 in
prostate carcinoma cells activates the metastatic growth that
regulates amoeboid motility and clonogenic potential.
The aforementioned studies have supplied data that
increases the understanding of the role of EphA2 in malignant tumor
behavior. The aim of the present study was to introduce EphA2 into
a non-expressing prostate cancer cell line to investigate the
direct potential of EphA2 on promoting malignant behavior. It has
been previously demonstrated that the expression of EphA2 was at
undetectable levels in LNCaP human prostate cancer cells and was
highly overexpressed in PC-3 prostate cancer cells by western blot
analysis (7). To understand this
further, the exogenous EphA2 gene-transfected LNCaP cells were used
to generate an EphA2 overexpressed cell line, and the effect of
EphA2 overexpression on proliferation and invasion ability was
investigated in the LNCaP prostate cancer cells. An
immunohistochemical assay was also conducted to observe the effects
of EphA2 in prostate cancer tissues. Taken together, these data
indicate that EphA2 may be a novel and significant target for
clinical intervention against prostate cancer.
Materials and methods
Recombinant plasmid pcDNA3.1(+)-EphA2
identification and amplification
The Agarose Gel DNA Purification kit, version 2.0
(Takara Biotechnology Co., Ltd., Dalian, Liaoning, China) was used
to purify the HindIII/XhoI double-digested
pcDNA3.1(+) plasmid (Invitrogen Life Technologies, Carlsbad, CA,
USA). The purified linear pcDNA3.1(+) plasmid and EphA2 cDNA
(Invitrogen Life Technologies) were linked using the DNA Ligation
kit, version 2.0 (Takara Biotechnology Co., Ltd.). Following the
determination of the plasmid purity with UV spectrophotometry
(DU-800 spectrophotometer; Beckman Coulter, Fullerton, CA, USA),
the sample was sent to the Shanghai Gene Co., Ltd., (Shanghai,
China) for identification. According to the manufacturer’s
instructions for the endotoxic plasmid extraction kit (Tiangen
Biotech Co., Ltd., Beijing, China), the positive clone was
extracted. The plasmid purity and concentration of the DNA product
was determined by spectrophotometry.
Cell culture and generation of
EphA2-expressing stable LNCaP cells
The human prostate cancer cell lines, LNCaP and
PC-3, were obtained from the Shanghai Cell Culture Collection
(Shanghai, China). The zero-loaded plasmid, pcDNA3.1(+), and
recombinant plasmid, pcDNA3.1(+)-EphA2, were transfected into LNCaP
cells, respectively, using the Lipofectamine 2000™ transfection
reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. Furthermore, the transfected cells
were selected by adding G418 (500 μg/ml; Invitrogen Life
Technologies), while the EphA2 expression level was confirmed by
western blot analysis. Monoclones were subcultured and were labeled
as LNCaP (blank), LNCaP-pcDNA3.1(+) (negative) and LNCaP-EphA2
(test), respectively. The cells were maintained in RPMI-1640
(Invitrogen Life Technologies) supplemented with 10% fetal bovine
serum and 1% penicillin/streptomycin (both Invitrogen Life
Technologies).
Determination of the effect of EphA2 on
the proliferation and invasion activity of the LNCaP cells
Following 24 h of serum-free starvation of the three
cell lines in the exponential phase, the matrix was added.
Following incubation for 48 h, the cells were collected.
Subsequently, the samples were washed with PBS at an ambient
temperature, immobilized with 75% ethanol and stored at −20°C prior
to the removal of ethanol via centrifugation for 5 min at 1,000 ×
g. The samples were resuspended with PBS for 15 min at an ambient
temperature and then centrifuged. Subsequently, 1 ml propidium
iodide (MultiSciences (LIANKE) Biotech Co., Ltd., Hangzhou, China)
was added, incubated for 30 min at an ambient temperature and flow
cytometry was performed using BD FACSCanto II flow cytometer (BD
Biosciences, San Jose CA, USA).
According to the manufacturer’s instructions for the
Cell Counting Kit-8 (CCK8; Nanjing Kaiji Biology Development Co.,
Ltd., Nanjing, China), the three LNCaP cell lines were collected at
the exponential phase and inoculated onto a 96-well plate at a
density of 3,000 cells/well, with each cell line in six wells.
Following inoculation, each well was replaced with 100 μl RPMI-1640
supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin, and 10 μl CCK8 for 1–5 days. Subsequently,
the cells were incubated at 37°C for 3 h, followed by ELISA for
A450 determination.
Invasion activities of the three cell lines were
determined according to the BioCoat Matrigel Invasion Chamber guide
(BD Biosciences). The cells were inoculated at a density of
5×104 cells/well and incubated at 37°C for 24 h. The
cells were fixed with formaldehyde (Shanghai Bogoo Biotech. Co.,
Ltd., Shanghai, China), stained and counted under a microscope
(Olympus IX51; Olympus, Nagano, Japan) with five fields of vision.
The invasion activity of the carcinoma cells was characterized with
the average transmembrane cell numbers. Each experiment was
repeated three times.
Western blot analysis
Western blotting was performed as described
previously (14). Equal amounts of
protein (30 μg) were size-fractionated on 10% SDS-PAGE (Solarbio
Biotech Co., Ltd., Beijing, China) and transferred to
nitrocellulose membranes (Pierce Biotechnology, Inc., Rockford, IL,
USA) overnight. Samples were incubated with EphA2 rat anti-human
monoclonal antibody (1:500; Abcam, Cambridge, UK) and β-actin mouse
anti-human monoclonal antibody (1:1,000; MultiSciences (LIANKE)
Biotech Co., Ltd.) at 4°C overnight. Subsequently, horseradish
peroxidase-labeled goat anti-rat (1:20,000; Abcam) and goat
anti-mouse secondary polyclonal antibodies (1:3,000; MultiSciences
(LIANKE) Biotech Co., Ltd.) were added respectively, followed by
Immobilon enhanced chemiluminescence (Merck Millipore, Billerica,
MA, USA).
Tissue specimens
A total of 86 patients with prostate cancer and 40
benign prostate hyperplasia control subjects were recruited between
January 2003 and December 2012 at the Zhongshan Hospital, Xiamen
University (Xiamen, Fujian, China). None of the patients received
any anticancer therapy prior to the sample collection. The median
age of patients was 68 years (range, 54–79 years), and the median
level of serum total prostate-specific antigen (tPSA) was 31.52
ng/ml (range, 5.35–95.7 ng/ml). The clinical and pathological
characteristics are shown in Table
I. Clinical stage is classified according to the
Jwett-Whitmore-prout classification (15).
 | Table IAssociation between the expression of
EphA2 and patient characteristics of prostate cancer. |
Table I
Association between the expression of
EphA2 and patient characteristics of prostate cancer.
| Characteristics | n | Rate of positive
EphA2 immunoreactivity, % | P-value |
|---|
| Age, years |
| <60 | 36 | 83.33 | >0.05 |
| ≥60 | 50 | 84.00 | |
| tPSA, ng/ml |
| <40 | 60 | 82.00 | <0.05 |
| ≥40 | 26 | 96.24 | |
| Lymph node
metastasis |
| Negative | 64 | 76.17 | <0.05 |
| Positive | 22 | 100.00 | |
| Gleason grade |
| <7 | 50 | 84.00 | <0.05 |
| ≥7 | 36 | 95.67 | |
| Clinical stage |
| A + B | 60 | 75.86 | <0.05 |
| C + D | 26 | 100.00 | |
Immunohistochemical analysis
The EphA2 status was found using the established
immunohistochemistry method of the avidin-biotin-peroxidase complex
assay, as described by Zeng et al (16). Immunohistochemical analysis of EphA2
expression in prostate carcinoma and benign prostate hyperplasia
samples was performed with a monoclonal mouse anti-human-EphA2
antibody (1:100; Abnova Corporation, Taipei, Taiwan), followed by
biotinylated goat anti-mouse immunoglobulin G (1:1,000; Maixin
Biotechnology Development Co., Ltd., Fuzhou, China) and
peroxidase-labeled streptavidin. The controls were obtained from
the Department of Pathology of the Zhongshan Hospital (Xiamen
University, Xiamen, China). The positive controls consisted of
human breast cancer samples that have been shown to express EphA2
and LnCaP cells were used as negative controls. Semi-quantitative
assessment of immunohistochemical expression was performed as
previously described (17) by
assessing the percentage of stained tumor cells and staining
intensity. Briefly, the percentage of positively stained cells was
rated as follows: 1 point, 0–10%; 2 points, 11–50%; 3 points,
51–75%; and 4 points, >75%. The staining intensity was set from
0–3 points (0, no staining; 1, weak staining; 2, moderate staining;
and 3, strong staining). When the product of the scores for
intensity and the percentage of positive cells was >3 points,
EphA2 immunoreactivity was considered positive. For each sample, at
least five fields were observed at a high power (x400) under an
Olympus IX51 microscope (Olympus) to derive a score for EphA2
expression. The pathological and immunohistochemical outcomes were
checked and approved by two pathologists independently in the
Department of Pathology of the Zhongshan Hospital, Xiamen
University.
Statistical analysis
Data were processed with SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). Measurement variables were presented as
the mean ± standard deviation and compared using Student’s t-test.
Categorized variables were compared using the χ2 test,
or Fisher’s exact test when the χ2 test was unavailable.
P<0.05 was considered to indicate a statistically significant
difference.
Results
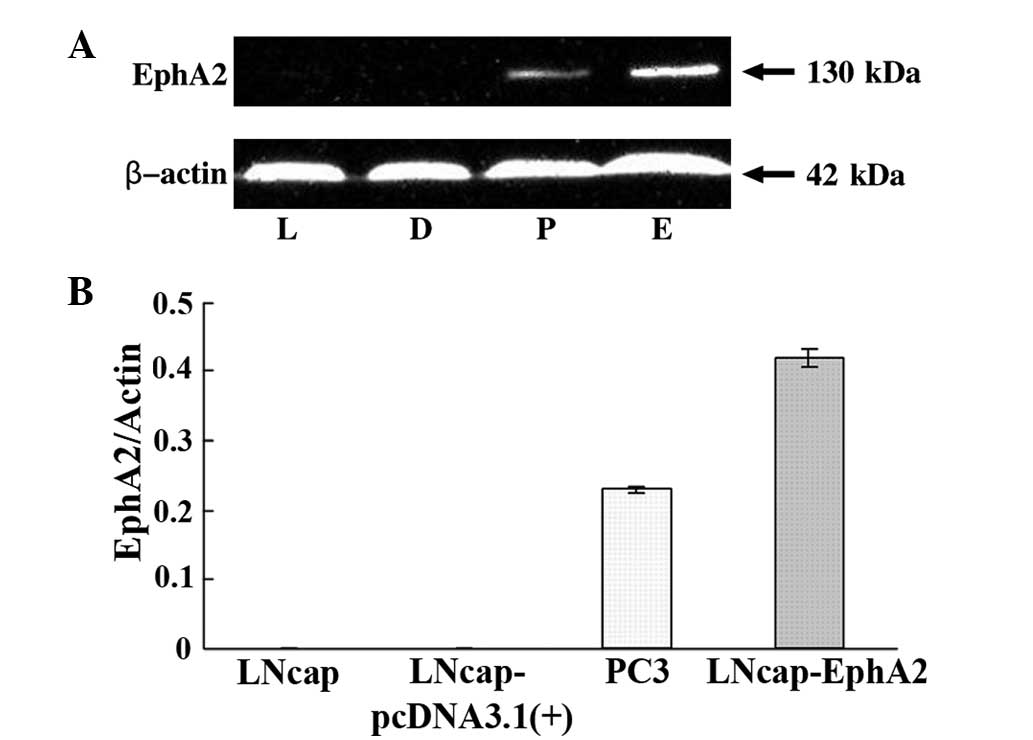
EphA2 overexpression in LNCaP-EphA2
cells
It has been previously demonstrated that EphA2 is
expressed at high levels in the PC-3 prostate cancer cells compared
with LNCaP cells (7). In the
present study, whether the introduction of EphA2 into the LNCaP
cells would promote proliferation and invasion behavior was
investigated. Therefore, the recombinant plasmid,
pcDNA3.1(+)-EphA2, or the zero-loaded plasmid, pcDNA3.1(+) control,
was transfected by means of Lipofectamine 2000 transfection reagent
into the LNCaP cells. Following G418 selection, EphA2 expression
was confirmed by western blot analysis (Fig. 1A and B).
EphA2 overexpression increases cell
growth in vitro
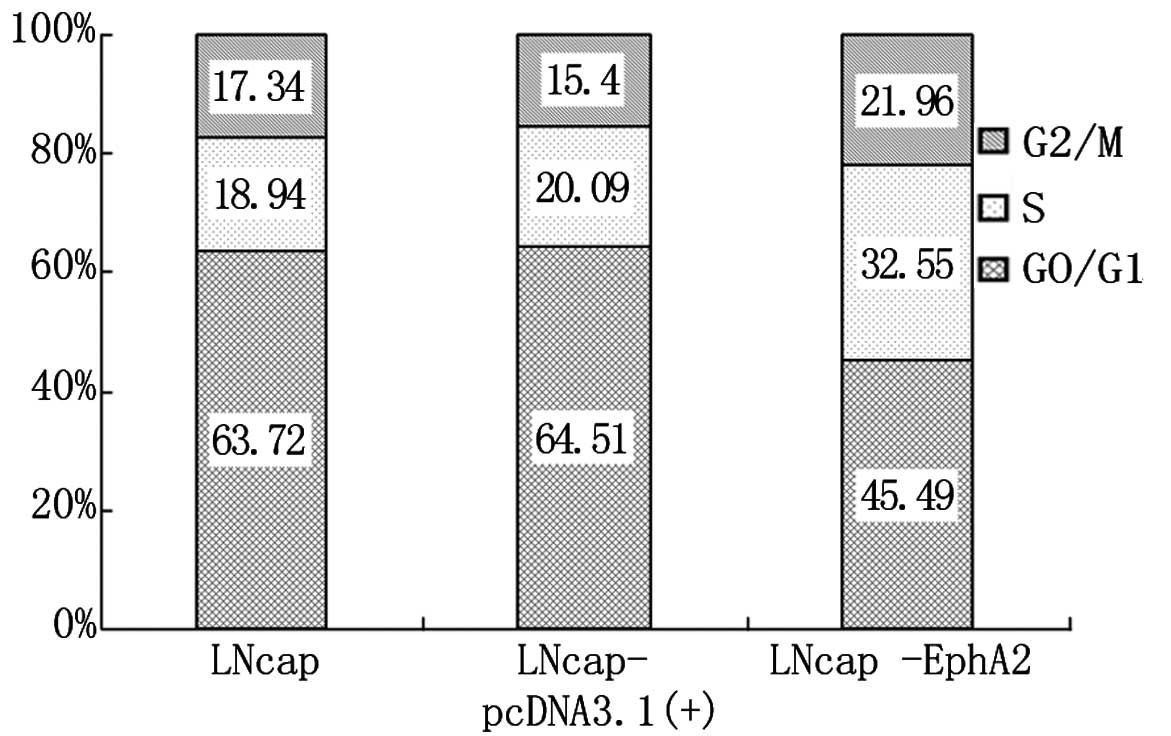
DNA cell cycle analysis was performed by cytometry
for the three cell lines, LNCaP, LNCaP-pcDNA3.1(+) and LNCaP-EphA2.
As shown in Fig. 2, the percentage
of cells at the G0/G1 phase was significantly lower in LNCaP-EphA2
compared with LNCaP and LNCaP-pcDNA3.1 cells. The cell percentage
at phases S and G2/M was clearly increased, and the difference was
statistically significant (P<0.01). The cell percentage for
LNCaP and LNCaP-pcDNA3.1(+) was similar at all phases, and its
difference was statistically insignificant (P>0.05). The results
show that EphA2 can enhance the proliferation of LNCaP cells.
EphA2 overexpression promotes cell
proliferation
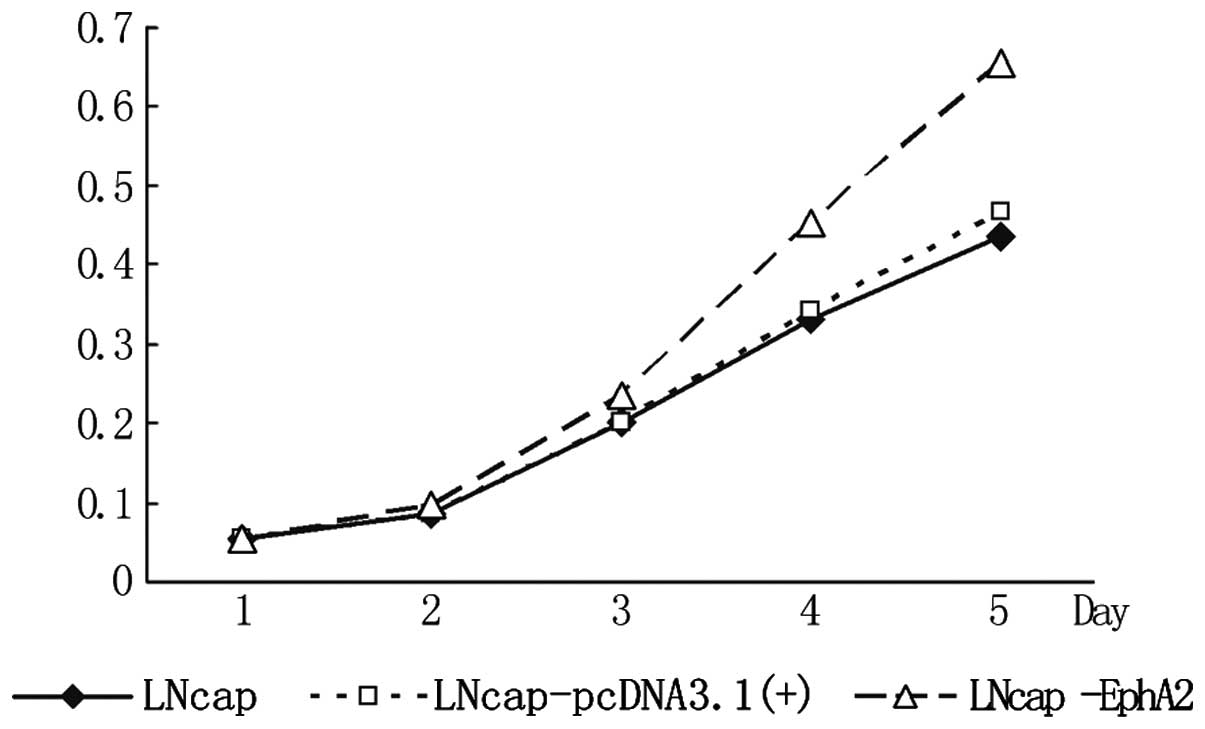
As shown in Fig. 3,
the optical density (OD) values of the three cell lines showed no
significant difference from day 1 to 2. From day 3, the OD value of
the LNCaP-EphA2 cell line was significantly higher compared with
that of the other two groups. This became clearer on days 4 and 5
and the difference was statistically significant (P<0.01). This
result shows that EphA2 can significantly enhance the proliferation
of the LNCaP cells.
EphA2 overexpression enhances the
invasion ability of the prostate cancer LNCaP cells
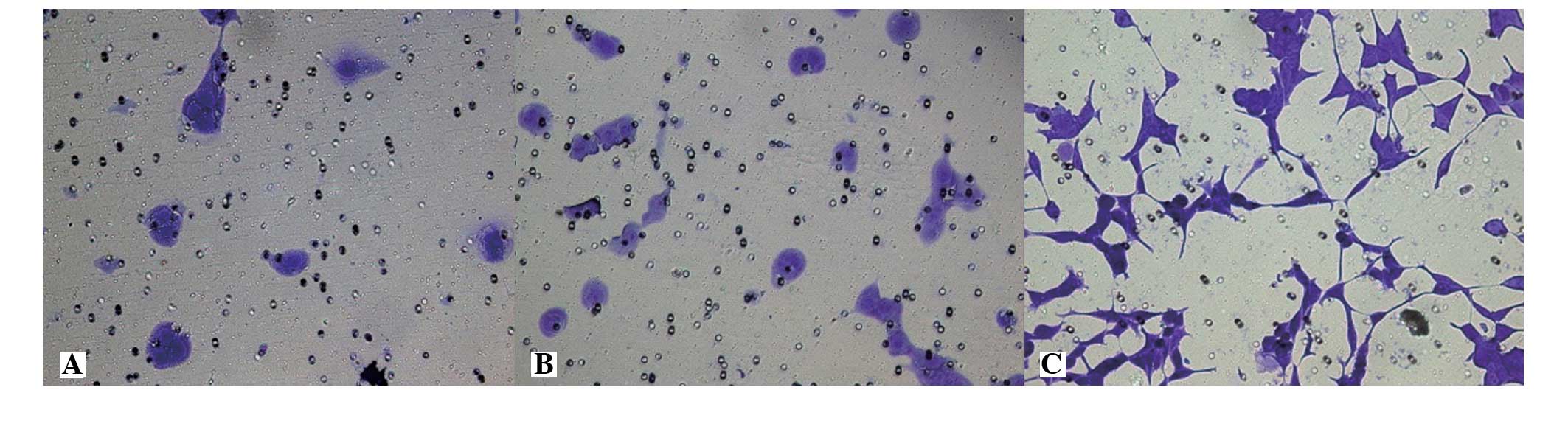
The membrane-penetrating cell numbers for the LNCaP,
LNCaP-pcDNA3.1(+) and LNCaP-EphA2 cell lines were 20.42±4.35,
22.07±5.74 and 89.64±6.81 cells, respectively. The invasion
activity difference of the first two groups was not statistically
significant (P>0.05), while the invasion cell numbers of
LNCaP-EphA2 was significantly higher compared with the first two
groups and was statistically significant (P<0.01). The result
shows that the cell invasion activity of EphA2-transfected LNCaP
cells was significantly increased (Fig.
4).
EphA2 overexpression is associated with
aggressive features in patients with prostate cancer
Based on the findings that EphA2 enhances
proliferation and invasion ability of the LNCaP prostate cancer
cells, whether EphA2 overexpression is associated with aggressive
features in patients with prostate cancer was assessed.
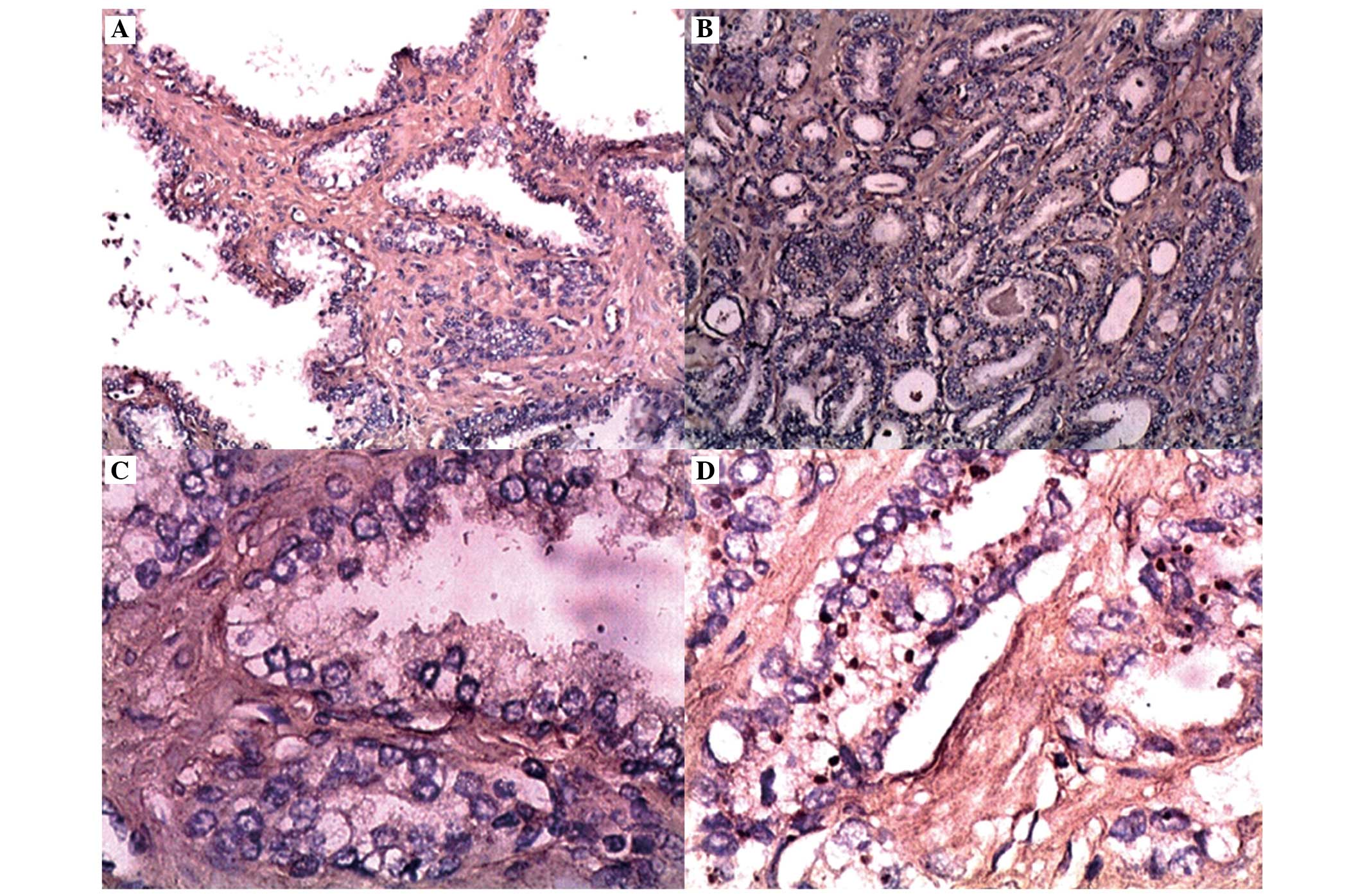
Representative photomicrographs illustrating EphA2 expression in
prostate cancer and benign prostate hyperplasia are presented in
Fig. 5. The EphA2 immunoreactivity
was distributed diffusely throughout the cytoplasm in the cells,
with 12 prostate cancer samples observing both cytoplasmic and
membrane staining. In the benign tissues, 10% of the samples
demonstrated weakly positively stained cells (score of 1). The
staining intensity of EphA2 was significantly higher (P<0.05) in
carcinoma cells compared with that in benign tissues (Table II). In the prostate cancer cells,
72 (83.72%) samples had positive EphA2 immunoreactivity, with
65.74±4.51% as the mean of positively stained cells and 2.26±0.10
as the mean staining intensity.
 | Table IIIntensity of EphA2 antibody staining
in the tissues of benign and malignant prostate cancer. |
Table II
Intensity of EphA2 antibody staining
in the tissues of benign and malignant prostate cancer.
| EphA2 staining
intensity |
|---|
|
|
|---|
| Variable | 0 | 1 | 2 | 3 |
|---|
| Benign prostate
hyperplasia, n=40 | 36 | 4 | 0 | 0 |
| Prostate cancer,
n=86 | 0 | 10 | 44 | 32 |
Subsequent to finding high levels of EphA2 in
prostate cancer, the level of EphA2 associated with known
prognostic variables was assessed. The correlations between EphA2
overexpression and various clinical and pathological variables are
listed in Table I. There was an
increase in immunoreactivity with an increase in the stage and
grade of the prostate carcinoma. The EphA2-positive
immunoreactivity was significantly greater in C + D stages compared
with A + B stages of the prostate cancer (P<0.05). Notably, high
levels of EphA2 expression were also associated with higher-grade
tumors (Gleason score ≥795.67%, P<0.05) and increasing level of
tPSA (P<0.05). There was no significant difference in EphA2
expression among patients of various ages.
Discussion
A major finding of the present study was that the
plasmid delivery of the exogenous EphA2 gene (pcDNA3.1(+)-EphA2) in
prostate cancer cells was sufficient to enhance LNCaP prostate
cancer cell growth and invasion. In accordance with this data,
Taddei et al (13) reported
that EphA2 influences the metastatic growth that regulates amoeboid
motility and the clonogenic potential in the prostate carcinoma
cells. In fact, LNCaP-EphA2 enhanced the growth of the prostate
cancer cells in vitro by >50% (Figs. 2 and 3). In a breast carcinoma study, Margaryan
et al (18) reported that
the expression of silence EphA2 can suppress carcinoma cell growth,
invasion and vasculogenesis. This further indicates that EphA2 can
be upregulated and that EphA2 may be a significant target in
prostate cancer treatment. Parri et al (19) showed that EphA2 reexpression in
melanoma cells converted their migration style from mesenchymal to
amoeboid-like, conferring a plasticity in tumor cell invasiveness.
Following reexpression and activation of EphA2, melanoma cells
initiate a non-proteolytic invasive program that activates
cytoskeleton motility, retracts cell protrusions, rounds the cell
body and forces through the three-dimensional matrix to produce a
successful lung and peritoneal lymph node metastases. The
overexpression of EphA2 can enhance carcinoma cell invasion
activity. This is realized through the weakening of intercellular
connection and enhanced adhesion of carcinoma cells to the
extracellular matrix and matrix invasion (20). This phenomenon may be relevant to
the function of EphA2 in carcinoma cells. Although the EphA2
receptor is overexpressed, its distribution and phosphorylation are
abnormal and it has a decreased affinity to its ligand. Therefore,
it can not function properly. However, overexpressed EphA2 has a
decreased sensitivity to its ligand, and so it can not negatively
regulate the carcinoma growth and transport but it can enhance
carcinoma cell migration and metastasis (21,22).
To the best of our knowledge, this is the first in vitro
study to show that the overexpression of the receptor by using a
vector that contained EphA2 significantly upregulated the
proliferation and invasion behavior in the LNCaP prostate cancer
cell line.
Another novel outcome of the present study is that
the overexpression of EphA2 in human prostate carcinoma cells is
associated with aggressive features, including Gleason grade, tPSA
and advanced clinical stage. Immunohistochemical analyses for EphA2
showed a much higher staining intensity in prostate carcinoma
compared with normal tissues and a significantly increased
expression with increasing stage of the disease and lymph node
metastasis. The carcinoma pathological grade and invasion is
correlated positively (23). Due to
the overexpression of EphA2 in the majority of tumors and its
suspected role in tumor growth and progression, EphA2 is being
investigated as a novel therapeutic target. For example, EphA2
antibody treatment of athymic mice bearing MDA231 xenografts was
sufficient to cause tumor regression with no adverse toxicity
(24). Similar data in association
with the growth inhibitory effects of EphA2 suppression have been
illustrated in breast cancer, glioma and malignant mesothelioma
(12,25,26),
supporting the idea that EphA2 should be investigated as a target
in other types of cancer, including prostate cancer.
In conclusion, the present study contributes more
information on the function of EphA2 tumor growth and progression
to further the understanding of the mechanism. The results of the
present study show that the overexpression of EphA2 enhances the
proliferation and invasion ability of the LNCaP prostate cancer
cells. In addition, these results show that EphA2 overexpression is
associated with aggressive features in patients with prostate
cancer. The findings of the present study provide further support
for developing novel therapeutic approaches targeted against EphA2
for patients with prostate cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 21035006; http://www.nsfc.gov.cn).
References
|
1
|
Lindberg RA and Hunter T: cDNA cloning and
characterization of eck, an epithelial cell receptor
protein-tyrosine kinase in the eph/elk family of protein kinases.
Mol Cell Biol. 10:6316–6324. 1990.
|
|
2
|
Sulman EP, Tang XX, Allen C, et al: ECK, a
human EPH-related gene, maps to 1p36.1, a common region of
alteration in human cancers. Genomics. 40:371–374. 1997.
|
|
3
|
Cheng N, Brantley DM and Chen J: The
ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor
Rev. 13:75–85. 2002.
|
|
4
|
Kamat AA, Coffey D, Merritt WM, et al:
EphA2 overexpression is associated with lack of hormone receptor
expression and poor outcome in endometrial cancer. Cancer.
115:2684–2692. 2009.
|
|
5
|
Liu F, Park PJ, Lai W, et al: A
genome-wide screen reveals functional gene clusters in the cancer
genome and identifies EphA2 as a mitogen in glioblastoma. Cancer
Res. 66:10815–10823. 2006.
|
|
6
|
Lu M, Miller KD, Gokmen-Polar Y, et al:
EphA2 overexpression decreases estrogen dependence and tamoxifen
sensitivity. Cancer Res. 63:3425–3429. 2003.
|
|
7
|
Walker-Daniels J, Coffman K, Azimi M, et
al: Overexpression of the EphA2 tyrosinase kinase in prostate
cancer. Prostate. 41:275–280. 1999.
|
|
8
|
Landen CN Jr, Chavez-Reyes A, Bucana C, et
al: Therapeutic EphA2 gene targeting in vivo using neutral
liposomal small interfering RNA delivery. Cancer Res. 65:6910–6918.
2005.
|
|
9
|
Abraham S, Knapp DW, Cheng L, et al:
Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary
bladder. Clin Cancer Res. 12:353–360. 2006.
|
|
10
|
Herrem CJ, Tatsumi T, Olson KS, et al:
Expression of EphA2 is prognostic of disease-free interval and
overall survival in surgically treated patients with renal cell
carcinoma. Clin Cancer Res. 11:226–231. 2005.
|
|
11
|
Lin YG, Han LY, Kamat AA, et al: EphA2
overexpression is associated with angiogenesis in ovarian cancer.
Cancer. 109:332–340. 2007.
|
|
12
|
Brantley-Sieders DM, Zhuang G, Hicks D, et
al: The receptor tyrosine kinase EphA2 promotes mammary
adenocarcinoma tumorigenesis and metastatic progression in mice by
amplifying ErbB2 signaling. J Clin Invest. 118:64–78. 2008.
|
|
13
|
Taddei ML, Parri M, Angelucci A, et al:
EphA2 induces metastatic growth regulating amoeboid motility and
clonogenic potential in prostate carcinoma cells. Mol Cancer Res.
9:149–160. 2011.
|
|
14
|
Kinch MS, Clark GJ, Der CJ and Burridge K:
Tyrosine phosphorylation regulates the adhesions of ras-transformed
breast epithelia. J Cell Biol. 130:461–471. 1995.
|
|
15
|
Prout GR Jr: Proceedings: Diagnosis and
staging of prostatic carcinoma. Cancer. 32:1096–1103. 1973.
|
|
16
|
Zeng G, Hu Z, Kinch MS, et al: High-level
expression of EphA2 receptor tyrosine kinase in prostatic
intraepithelial neoplasia. Am J Pathol. 163:2271–2276. 2003.
|
|
17
|
Kamat AA, Fletcher M, Gruman LM, et al:
The clinical relevance of stromal matrix metalloproteinase
expression in ovarian cancer. Clin Cancer Res. 2:1707–1714.
2006.
|
|
18
|
Margaryan NV, Strizzi L, Abbott DE, et al:
EphA2 as a promoter of melanoma tumorigenity. Cancer Biol Ther.
8:279–288. 2009.
|
|
19
|
Parri M, Taddei ML, Bianchini F, et al:
EphA2 reexpression prompts invasion of melanoma cells shifting from
mesenehymal to amoeboid-like motility style. Cancer Res.
69:2072–2081. 2009.
|
|
20
|
Lu C, Shahzad MM, Wang H, et al: EphA2
overexpression promotes ovarian cancer growth. Cancer Biol Ther.
7:1098–2103. 2008.
|
|
21
|
Taddei ML, Parri M, Angelucci A, et al:
Kinase-dependent and-independent roles of EphA2 in the regulation
of prostate cancer invasion and metastasis. Am J Pathol.
174:1492–1503. 2009.
|
|
22
|
Petty A, Myshkin E, Qin H, et al: A small
molecule agonist of EphA2 receptor tyrosine kinase inhibits tumor
cell migration in vitro and prostate cancer metastasis in vivo.
PLoS One. 7:e421202012.
|
|
23
|
Van den Broeck A, Vankelecom H, Van
Eijsden R, et al: Molecular markers associated with outcome and
metastasis in human pancreatic cancer. J Exp Clin Cancer Res.
31:682012.
|
|
24
|
Coffman KT, Hu M, Carles-Kinch K, et al:
Differential EphA2 epitope display on normal versus malignant
cells. Cancer Res. 63:7907–7912. 2003.
|
|
25
|
Liu DP, Wang Y, Koeffler HP and Xie D:
Ephrin-A1 is a negative regulator in glioma through downregulation
of EphA2 and FAK. Int J Oncol. 30:865–871. 2007.
|
|
26
|
Mohammed KA, Wang X, Goldberg EP, et al:
Silencing receptor EphA2 induces apoptosis and attenuates tumor
growth in malignant mesothelioma. Am J Cancer Res. 1:419–431.
2011.
|