Introduction
At present, breast cancer is the most common
malignant tumor in females, and the bone metastasis of breast
cancer has a direct effect on patient prognosis, which results in a
marked increase in the mortality rate (1). The molecular mechanisms of metastasis
remain unclear, as the factors involved in the metastatic process
are complicated. However, a recent study has revealed that
chemokine (C-X-C motif) receptor 4 (CXCR4) closely correlates with
the incidence, development, treatment and prognosis of breast
cancer (2–6). In the present study, the targeted
downregulation of CXCR4 expression in the MDA-MB-231BA-rfp breast
cancer cell line (with a high propensity to metastasize to bone)
via RNA interference (RNAi) techniques was performed to analyze the
effect of CXCR4 on the ability of cancerous cells to metastasize to
the bone, as well as to investigate the underlying mechanisms. The
results offer an enhanced understanding of the molecular mechanisms
that lead to breast cancer bone metastasis.
Materials and methods
Experimental materials
The human breast cancer cell line, MDA-MB-231BA-rfp,
was stored frozen in liquid nitrogen. Dulbecco’s modified Eagle’s
medium with 10% fetal calf serum was purchased from Gibco-BRL
(Carlsbad, CA, USA). The rabbit polyclonal antibodies against human
CXCR4, phosphatidylinositide 3-kinase (PI3K), protein kinase B
(AKT), and matrix metalloproteinase (MMP)-9 were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The western
blot analysis kits were purchased from Wuhan Boster Biological
Technology, Ltd. (Wuhan, China). This study was approved by the
Institutional Review Board of Tonji Hospital, Huazhong University
of Science and Technology (Wuhan, China).
CXCR4 small interfering RNA (siRNA)
construction and transfection
The two CXCR4 siRNA oligonucleotide sequences
purchased from Dharmacon, Inc., (Lafayelle, CA, USA) were
identified and matched with the following CXCR4 cDNA sequences
obtained from GeneBank through a BLAST search: Sense,
5′-UAAAAUCUUCCUGCCCACCdTdT-3′ for siRNA1; and sense,
5′-GGAAGCUGUUGGCUGAAAAdT dT-3′ for siRNA2. In addition, a negative
control siRNA sequence was formulated and synthesized, as follows:
5′-UUCUCCGAACGUGUCACGUUUGUGC-3′. The MDA-MB-231BA-rfp cells
(1×105 cells/ml) were then transfected with 100 nM CXCR4
siRNA mediated by oligofectamine (Invitrogen Life Technologies,
Carlsbad, CA, USA). The following groupings were then determined:
Con-B group, blank control group; Con-A group, empty vector group;
S1 group, siRNA1 transfection group; S2 group, siRNA2 transfection
group; and Sn group, negative control siRNA sequence transfection
group. With the exception of isocyanic phosphate-buffered saline
therapy for Con-B group and isocyatic oligofectamine therapy for
Con-A group, the follow-up procedures for the five groups were the
same. The siRNA sequence that exhibited the highest interfering
efficiency was then selected to continue the study.
Western blot analysis
The MDA-MB-231BA-rfp cells in the exponential growth
phase were centrifuged at 30,000 × g at 4°C for 5 min to separate
the supernatant from the cellular debris (centrifugation radius, 4
cm) following the application of radioimmunoprecipitation assay
protein lysis buffer (Wuhan Boster Biological Technology, Ltd.).
Next, the level of protein expression was determined using the
bicinchoninic acid assay method. Subsequently, 50 μg of protein was
harvested and 2× loading buffer was added to the protein samples,
which were then were then heated to 100°C for 5 min. Next,
following SDS-PAGE separation, the samples were loaded onto a
nitrocellulose filter and then combined with the specific
antibodies and corresponding diantibodies. Finally, the samples
were stained using enhanced chemiluminescence kit (Wuhan Boster
Biological Technology, Ltd.) prior to the X-ray films being
exposed, developed and fixed. Gray scale images were also captured
and analyzed using BandScan software (Glyko, Novato, CA, USA).
Cell invasion assay in vitro
Transwell chamber models (Chemicon, Temecula, CA,
USA) were performed to prepare a cell suspension containing
1×105 cells/ml, of which 50 μl was added to the upper
chamber. At 24 h post-incubation, the cells located on the inner
layer of the chamber were removed and the remaining cells were
fixed using 10% formalin. Giemsa stain was used to count the number
of invasive cells that had migrated through the membrane.
Cell counting kit (CCK)-8 cell
proliferation assay
The cells were digested with 0.25% pancreatic enzyme
(Wuhan Boster Biological Technology, Ltd.), which resulted in a
cell suspension containing 1.2×104 cells/ml. Next, the
cell suspension was seeded into 96-well plates (200 μl per well)
and separated into the following three groups: Control group,
lipopolysaccharide (1.0 μg/ml) intervention group and Toll-like
receptor-4 intervention group. After 24 h, CCK-8 (10 μl/well) was
added and the cells were incubated for another 2 h. The absorbance
at 450 nm was measured using a microplate reader and the
proliferation ability of the mesenchymal stem cells of different
mice were analyzed.
Tumorigenesis in nude mice
In total, 24 C57BL/6 nude mice were kept in a
biologically clean animal laboratory at a temperature of 23±1°C,
with a relative humidity of 55–60%. The mice were housed six per
cage in polycarbonate cages (8×13.5×8.1 cm in size). The dry
sawdust bedding was sterilized and replaced every five days, and
sterile distilled drinking water was provided. Animals were
randomized into intervention (n=12) and control (n=12) groups
following one week of acclimation to the same conditions.
MDA-MB-231BA-rfp cells in the exponential growth phase were
dissociated to prepare a single cell suspension, of which the cell
density was adjusted to 1×106 cells/ml, with a cell
viability of >95%. Briefly, 2 ml of the cell suspension was
injected into the caudal veins of the mice. The suspension included
MDA-MB-231BA-rfp cells transfected with CXCR4 siRNA for the
intervention group and MDA-MB-231BA-rfp cells without CXCR4 siRNA
transfection for the control group.
Micro-positron emission tomography (PET)
detection of bone metastasis in nude mice
The mice were deprived of food and water for 8 h and
anesthetized via inhalation of 2% isoflurane prior to microPET. At
40 min after the injection of the radioactive tracer,
fludeoxyglucose, into the tail vein, the mice were placed in a
prone position and imaging was performed for 10 min. Bone
metastasis was analyzed using ASIPro (Siemens Medical Solutions
USA, Inc., Knoxville, TN, USA) to determine the region of interest
(ROI), and the maximum standard uptake value (SUV) was used for the
statistical analysis. The ROI was evaluated by a researcher blinded
to the experimental schedule and groupings.
Statistical analysis
All data are presented as the mean ± standard
deviation and were analyzed using SPSS version 16.0 (SPSS, Inc.,
Chicago, IL, USA) by two-tailed t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
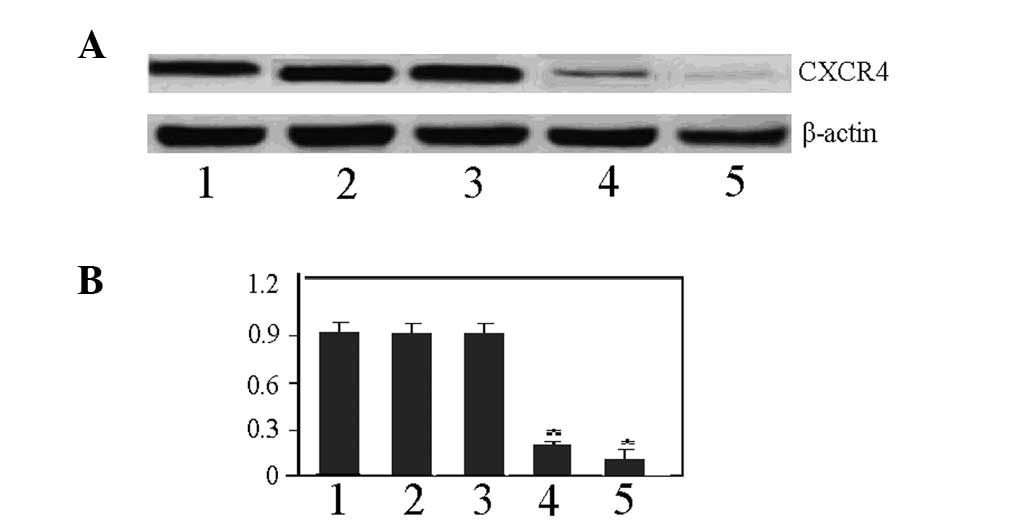
Interference effects of CXCR4 siRNA
The results of the western blot analysis revealed
that the expression of CXCR4 in the MDA-MB-231BA-rfp cell line was
significantly downregulated in the S1 and S2 groups compared with
the Con-B, Con-A and Sn groups at 24 h after the transfection with
100 nM CXCR4 siRNA. The interference efficiency was calculated
using the following formula: Interference efficiency =
(downregulation range of CXCR4 in control group - downregulation
range of CXCR4 in intervention group) / downregulation range in
control group. The results showed interference efficiencies of 83
and 92% in the S1 and S2 groups, respectively. Therefore, since the
S2 group exhibited a relatively higher interference efficacy than
the S1 group, the S2 group was selected to be used as the
CXCR4-specific interference sequence for the sequential study
(Fig. 1).
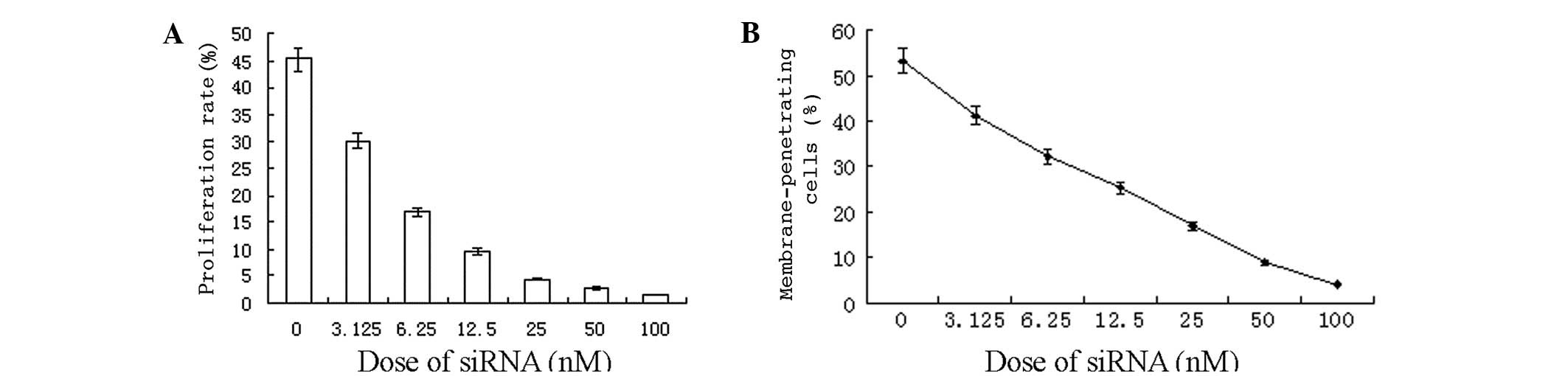
CXCR4 siRNA inhibition of cellular
proliferation and invasion
Based on the finding that the S2 group was found to
be the most effective in reducing CXCR4 expression, S2 was selected
as the CXCR4-specific sequence to proceed with in the study. The
CCK-8 proliferation assay revealed that the proliferation rate of
the S2-transfected cells was decreased with increasing
concentrations of the transfection reagent (0, 3.125, 6.25, 12.5,
25, 50 and 100 nM; Fig. 2A). At 48
h after the transfection of the breast cancer cells with the
varying S2 concentrations, the results of the Transwell migration
assay also indicated that the number of cancer cells that had
migrated through the filter membrane was substantially decreased
with increasing siRNA concentration (Fig. 2B).
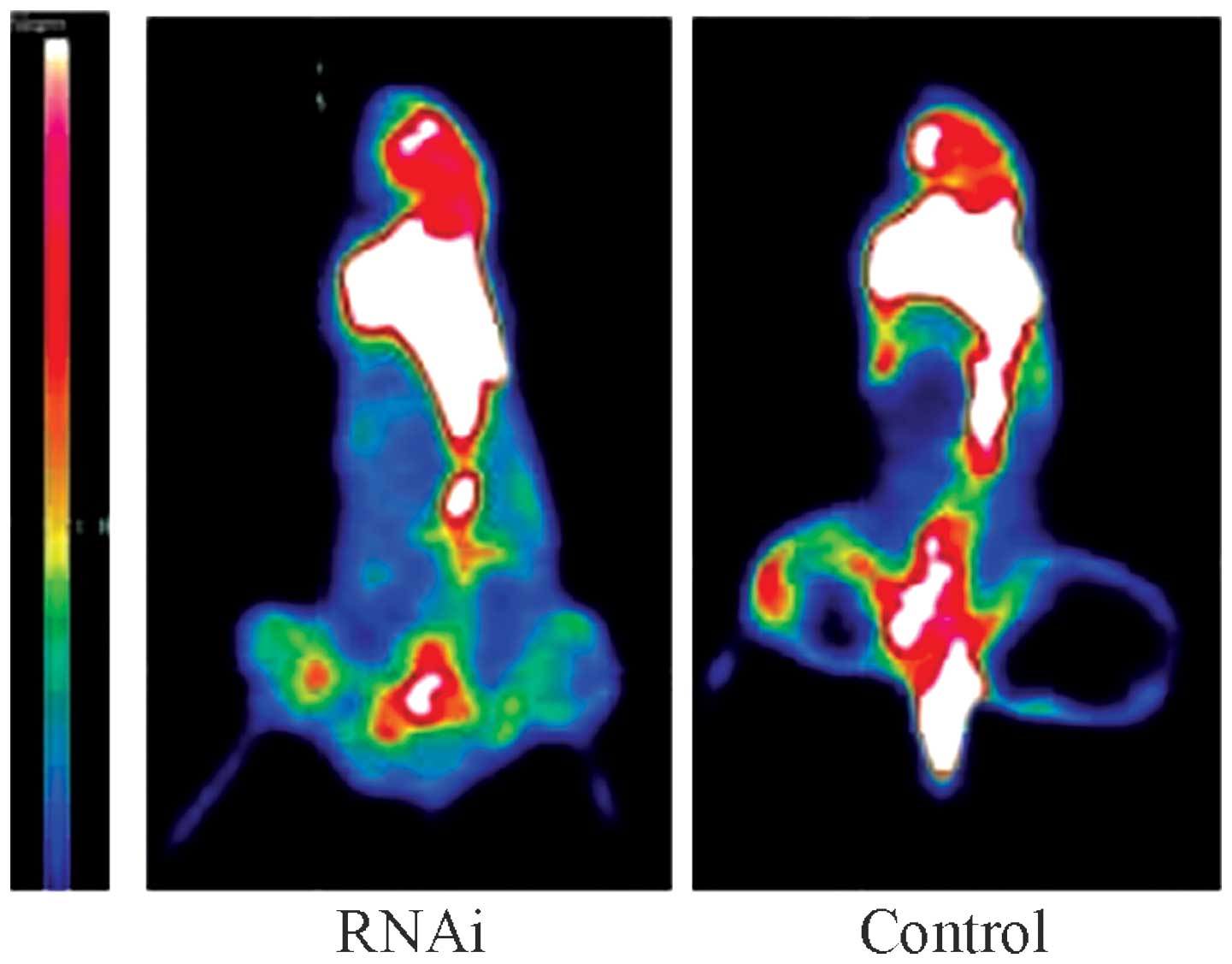
CXCR4 RNAi inhibition of bone metastasis
in nude mice
The nude mice in the intervention group were
injected with MDA-MB-231BA-rfp cells that had been transfected with
100 nM S2 for 48 h (n=8), and the control group were injected with
an equal amount of non-transfected MDA-MB-231BA-rfp cells (n=8).
MicroPET analysis revealed that the nude mice in the control group
exhibited a mild breakdown of cortical bone in the lower
extremities four weeks after tumor cell injection. By contrast, it
was not until the sixth week after tumor cell injection that
distinct bone invasion, indicating the emergence of bone
metastasis, was identified in the interference group. Direct
observation of the microPET images six weeks after the injections
revealed that the bright white region in the lower extremities,
ribs and spine was much larger in the control group than in the
interference group (Fig. 3).
Semi-quantitative analysis was performed based on the SUV ratios,
which revealed that the SUVmax in the interference group was
9.38±0.54 versus 2.13±0.21 in the control group (P<0.01),
indicating that the onset and degree of MDA-MB-231SA-rfp cell bone
metastasis may be significantly inhibited by CXCR4 RNAi.
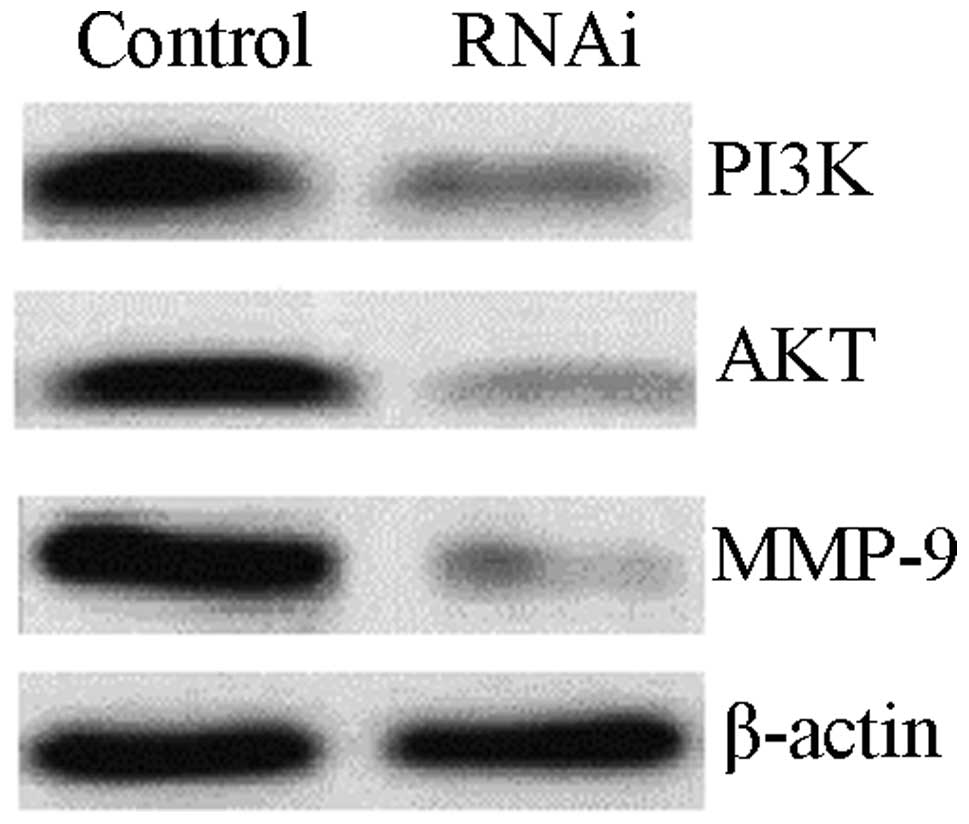
CXCR4 RNAi downregulates MMP-9 via
blockade of the PI3K/AKT signaling pathway
To investigate the potential mechanism of CXCR4 RNAi
controlling breast cancer metastasis to the bone, western blot
analysis was used to analyze the expression of PI3K/AKT/MMP-9
following the silencing of CXCR4 by RNAi. As a result, the
expression of PI3K/AKT/MMP-9 was reduced by CXCR4 RNAi, with the
levels of PI3K, AKT and MMP-9 far lower than those in the control
group (0.32±0.06 vs. 0.89±0.12 for PI3K; 0.16±0.03 vs. 0.86±0.10
for AKT; and 0.12±0.02 vs. 1.12±0.16 for MMP-9; P<0.01; Fig. 4).
Discussion
CXCR4 is a highly conserved G protein-coupled
receptor of the chemokine receptor family that mediates chemotactic
activity and is a receptor specific to CXC ligand 12 (CXCL12)
(7). Previous studies have
demonstrated that CXCR4 is the most common chemokine receptor
expressed in tumor cells, and that it plays a predominant role in
the migration and invasion of tumors (8–11). In
addition, a recent study has revealed that CXCR4 is vital for the
migration, invasion, treatment and prognosis of breast cancer
(12,13). Furthermore, Gil et al
(14) reported that a virus-coated
CXCR4 antagonist is effective in the treatment of primary or
metastatic breast cancer, functioning by disrupting the internal
environment for tumor cell growth and inhibiting the
vascularization and expression of CXCL12 and vascular endothelial
growth factor (VEGF). Additionally, Ling et al (15) reported that the CXCR4 antagonist,
AMD3465, inhibits the growth and migration of breast cancer by
partially blocking signal transducer and activator of transcription
3 signaling, which has an impact on tumor and immune cells in the
internal tumor environment.
In the present study, the effect of CXCR4 on the
bone metastasis of breast cancer by targeting the downregulation of
CXCR4 using RNAi techniques was observed (Fig. 1). Firstly, the CCK-8 cell
proliferation and Transwell chamber assays were used to detect the
oncological characteristics of the breast cancer cells prior to and
following CXCR4 suppression. The observations revealed that CXCR4
siRNA significantly inhibits the proliferation and invasion of
breast cancer cells (Fig. 2).
Therefore, CXCR4 is key in the growth and proliferation of breast
cancer cells, indicating that the control of its activity may
significantly reduce the proliferation of breast cancer cells in
vitro. In addition, as CXCR4 is involved in the motility and
chemotaxis of breast cancer cells, the suppression of CXCR4
expression may significantly reduce the migration of breast cancer
cells to distant organs (16).
Furthermore, Wendel et al (17) reported that CXCR4/CXCL12 is
significant in the migration of breast cancer cells by affecting
the adhesiveness, morphology and migration of the cells and the
regulation of the expression of the protein family in the
extracellular matrix.
Based on the results of the in vitro
experiment, an in vivo experiment was conducted to
investigate the effect of CXCR4 inhibition on breast cancer bone
metastasis. The mouse model of breast cancer was simulated by
injecting MDA-MB-231BA-rfp cells transfected with CXCR4 RNAi into
the tail vein. As a result, the onset of the bone metastasis of
breast cancer cells was prolonged and the metastasis was attenuated
with the interference of CXCR4, which tentatively confirmed that
CXCR4 RNAi inhibits the spread of breast cancer cells to the bone.
A previous cohort study indicated that the detection of CXCR4
expression is of great value in predicting the bone metastasis of
breast cancer (18). To exclude
non-bone metastasis, the present study used MDA-MB-231BA-rfp cells,
which are breast cancer cells with a high bone-specific metastatic
potential, in order to establish the bone metastasis model.
The mechanism of breast cancer cell metastasis to
the bone is complicated, however, the CXCR4/stromal cell-derived
factor-1 axis has a vital regulatory function (19). To study the effect of CXCR4 in the
regulation of the PI3K/AKT signaling pathway, CXCR4 was inhibited
in the current study. As a result, the inhibition of CXCR4 had an
impact on the activity of PI3K/AKT. Ping et al (20) also reported that the vascularization
of glioma cells is attenuated by the downregulation of CXCR4 using
the CXCR4 antagonist, AMD3100, or RNAi, and the reduction of VEGF
expression via the inhibition of the PI3K/AKT signaling pathway.
However, Zheng et al (21)
reported that CXCR4 mediates the endothelial progenitor cells via
the PI3K/AKT signaling pathway. To further investigate the effect
of CXCR4 on the regulation of downstream cytokines, its impact on
the expression of MMP-9 was observed in the present study. The
results showed that the expression of MMP-9 was reduced with the
interference of CXCR4. Consequently, we hypothesize that the
CXCR4/PI3K/AKT/MMP-9 signaling pathway is involved in the bone
metastasis of breast cancer.
In conclusion, the preliminary in vitro
experiment revealed that the proliferation and invasion of breast
cancer cells is inhibited with the interference of CXCR4. The
construction of in vivo models of breast cancer metastasis
to the bone further confirmed the inhibition of bone metastasis as
a result of CXCR4 interference and the involvement of
CXCR4/PI3K/AKT/MMP-9 signaling in bone metastasis. The present
study provides supporting evidence for the mechanism of the
metastasis of breast cancer to the bone.
References
|
1
|
Chen J, Zhu S, Xie XZ, et al: Analysis of
clinicopathological factors associated with bone metastasis in
breast cancer. J Huazhong Univ Sci Technolog Med Sci. 33:122–125.
2013.
|
|
2
|
Chu QD, Holm NT, Madumere P, et al:
Chemokine receptor CXCR4 overexpression predicts recurrence for
hormone receptor-positive, node-negative breast cancer patients.
Surgery. 149:193–199. 2011.
|
|
3
|
Hiller D and Chu QD: CXCR4 and axillary
lymph nodes: review of a potential biomarker for breast cancer
metastasis. Int J Breast Cancer. 2011:4209812011.
|
|
4
|
Hiller DJ, Li BD and Chu QD: CXCR4 as a
predictive marker for locally advanced breast cancer
post-neoadjuvant therapy. J Surg Res. 166:14–18. 2011.
|
|
5
|
Andre F, Xia W, Conforti R, et al: CXCR4
expression in early breast cancer and risk of distant recurrence.
Oncologist. 14:1182–1188. 2009.
|
|
6
|
Rhodes LV, Short SP, Neel NF, et al:
Cytokine receptor CXCR4 mediates estrogen-independent
tumorigenesis, metastasis, and resistance to endocrine therapy in
human breast cancer. Cancer Res. 71:603–613. 2011.
|
|
7
|
Weiss ID and Jacobson O: Molecular imaging
of chemokine receptor CXCR4. Theranostics. 3:76–84. 2013.
|
|
8
|
Albert S, Riveiro ME, Halimi C, et al:
Focus on the role of the CXCL12/CXCR4 chemokine axis in head and
neck squamous cell carcinoma. Head Neck. 35:1819–1828. 2013.
|
|
9
|
Luker KE and Luker GD: Functions of CXCL12
and CXCR4 in breast cancer. Cancer Lett. 238:30–41. 2006.
|
|
10
|
Peled A and Tavor S: Role of CXCR4 in the
pathogenesis of acute myeloid leukemia. Theranostics. 3:34–39.
2013.
|
|
11
|
Katsumoto K and Kume S: The role of
CXCL12-CXCR4 signaling pathway in pancreatic development.
Theranostics. 3:11–17. 2013.
|
|
12
|
Mukherjee D and Zhao J: The Role of
chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer
Res. 3:46–57. 2013.
|
|
13
|
Parker CC, Kim RH, Li BD and Chu QD: The
chemokine receptor CXCR4 as a novel independent prognostic marker
for node-positive breast cancer patients. J Surg Oncol.
106:393–398. 2012.
|
|
14
|
Gil M, Seshadri M, Komorowski MP, et al:
Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy
disrupts tumor vasculature and inhibits breast cancer metastases.
Proc Natl Acad Sci USA. 110:E1291–E1300. 2013.
|
|
15
|
Ling X, Spaeth E, Chen Y, et al: The CXCR4
antagonist AMD3465 regulates oncogenic signaling and invasiveness
in vitro and prevents breast cancer growth and metastasis in vivo.
PLoS One. 8:e584262013.
|
|
16
|
Hernandez L, Magalhaes MA, Coniglio SJ, et
al: Opposing roles of CXCR4 and CXCR7 in breast cancer metastasis.
Breast Cancer Res. 13:R1282011.
|
|
17
|
Wendel C, Hemping-Bovenkerk A, Krasnyanska
J, et al: CXCR4/CXCL12 participate in extravasation of
metastasizing breast cancer cells within the liver in a rat model.
PLoS One. 7:e300462012.
|
|
18
|
Sacanna E, Ibrahim T, Gaudio M, et al: The
role of CXCR4 in the prediction of bone metastases from breast
cancer: a pilot study. Oncology. 80:225–231. 2011.
|
|
19
|
Wang J, Loberg R and Taichman RS: The
pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis.
Cancer Metastasis Rev. 25:573–587. 2006.
|
|
20
|
Ping YF, Yao XH, Jiang JY, et al: The
chemokine CXCL12 and its receptor CXCR4 promote glioma stem
cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT
signalling. J Pathol. 224:344–354. 2011.
|
|
21
|
Zheng H, Fu G, Dai T and Huang H:
Migration of endothelial progenitor cells mediated by stromal
cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal
transduction pathway. J Cardiovasc Pharmacol. 50:274–280. 2007.
|