Introduction
Melanoma is a highly malignant type of tumor, with a
median survival rate of between six and 12 months, and a five-year
survival rate of <10% (1).
Primary malignant melanoma of the central nervous system (CNS)
accounts for 1% of all cases of melanoma. Primary spinal melanoma
is particularly rare and may be intra- or extradural, or may
possess intra- and extramural components (2). Spinal melanoma is primarily found in
the middle or lower thoracic spine. Since the first case of spinal
melanoma was reported by Hirschberg in 1906, <100 cases have
been reported (3). There are no
evidence-based guidelines regarding primary spinal melanoma, for
example specific incidence, treatment or prognosis guidelines. The
current report presents a case of primary spinal melanoma of the
thoracic spine, which presented unusual radiographic features at
the time of diagnosis. Magnetic resonance imaging (MRI) revealed
that the lesion exhibited typical signs of spinal melanoma and
enhanced MRI revealed a dural tail sign. Subsequent to total
resection, a six-month follow-up MRI scan demonstrated no tumor
recurrence. In the present case, the diagnosis of primary spinal
malignant melanoma was obtained through histological, radiological
and immunohistochemical analyses. The aim of the present case
report is to discuss the diagnosis, treatment and prognosis of this
rare condition.
Case report
A 57-year-old female was admitted to the Clinical
Medical College of Yangzhou University (Northern Jiangsu People’s
Hospital, Yangzhou, China) in December 2012 with a history of
bilateral lower-extremity numbness and back pain for one month. One
week prior to admission, the patient noticed a more marked tingling
sensation and progressive weakness in the lower extremities. Upon
admission to hospital, a neurological examination revealed
hypoesthesia below the T8 level and progressive weakness (grade IV)
of the bilateral lower extremity. The deep-tendon reflexes of the
lower extremity were weak and accompanied by uroschesis. The
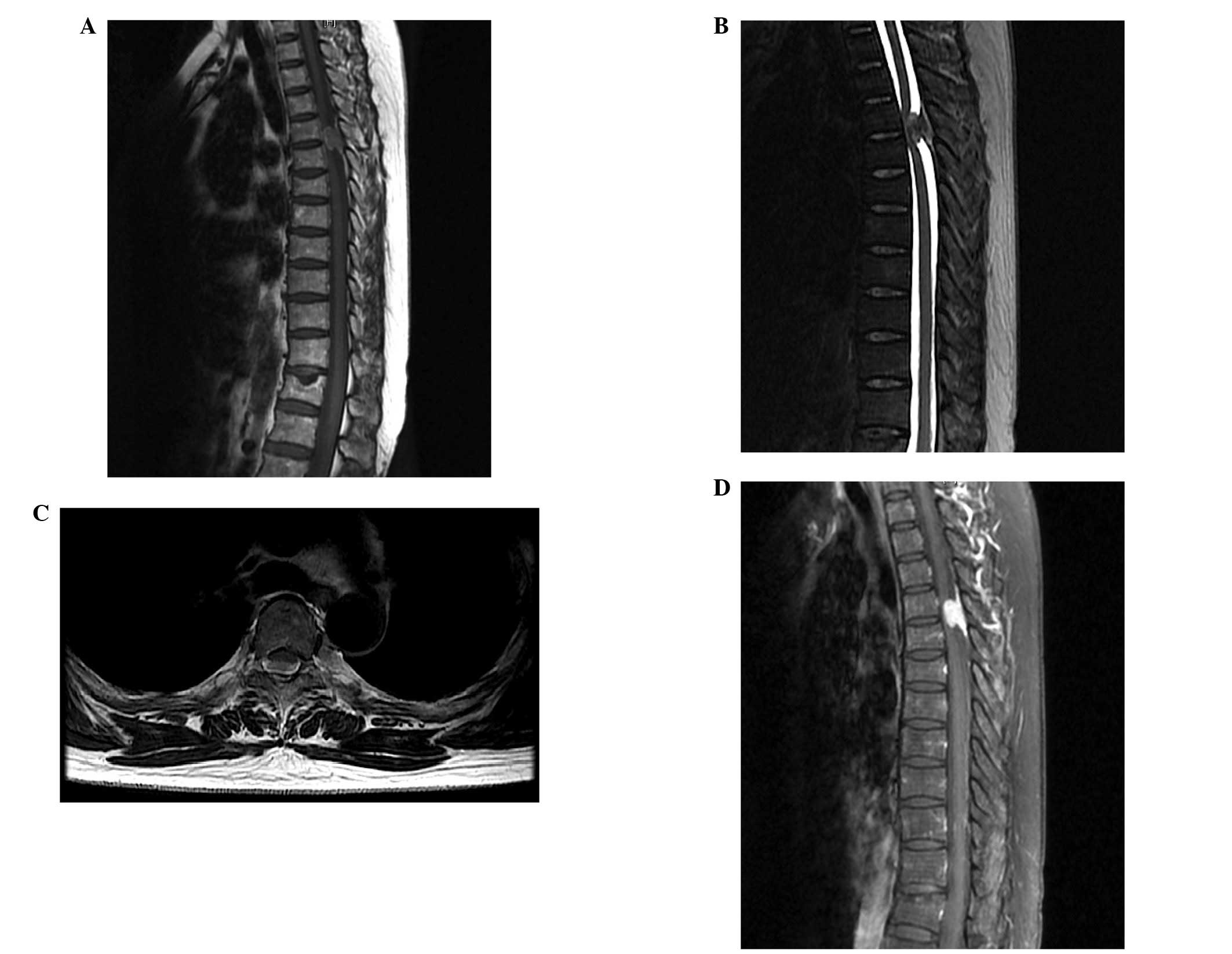
Babinski sign was positive on the two sides. MRI scans of the
thoracic spine revealed a space-occupying mass (size, 17×10 mm)
with an obvious dural tail sign at the T4–T5 level (Fig. 1). The margins of the mass were
indistinct and the adjacent dural exhibited local incrassation and
compression of the spinal cord. The lesion was initially diagnosed
as a complex spinal meningioma and surgery was performed on the
patient.
In December 2012 the patient underwent a T4–T5
laminectomy using a standard posterior thoracic midline approach.
The dural mater, which was black in color, was observed at the T4–5
region due to an intradural underlying mass. Upon opening the dura,
a black arachnoid that was covered in pigmentation was observed. A
vertical incision was made in the arachnoid and a black,
oval-shaped, hypervascular mass was found, which measured 15×12 mm.
The mass was indistinct, and was strongly adhered to the dura and
the arachnoid. The tumor compressed, although did not invade, the
spinal cord and was removed completely using a microsurgical
technique. Surgery indicated that the tumor was likely to be
malignant; therefore, a radical resection was performed on the
black-colored dura and arachnoid.
Standard histopathological examination of the tumor
samples (hematoxylin and eosin staining; magnification, ×100)
revealed cytologic atypia, mitotic activity and tumor cells with
cytoplasmic deposition. These cellular characteristics were highly
indicative of malignant melanoma. Immunohistochemically, the
neoplastic cells stained positive for S-100 protein and the
malignant melanoma monoclonal antibody, human melanoma black (HMB)
45. However, the neoplastic cells were negative for epithelial
membrane antigen (EMA) and neuron-specific enolase (NSE). The
proliferation rate, based on Ki-67 expression, was observed to be
high (10%). Thus, the histopathological and immunohistochemical
findings indicate that the tumor originated from a melanocyte.
Postoperative analyses, including clinical examination, full body
computed tomography, MRI, abdominal ultrasound and ocular
examination, revealed no lesions in the patient’s other organs.
A postoperative clinical examination of the patient
revealed no loss of motor capacity or decrease in motor strength.
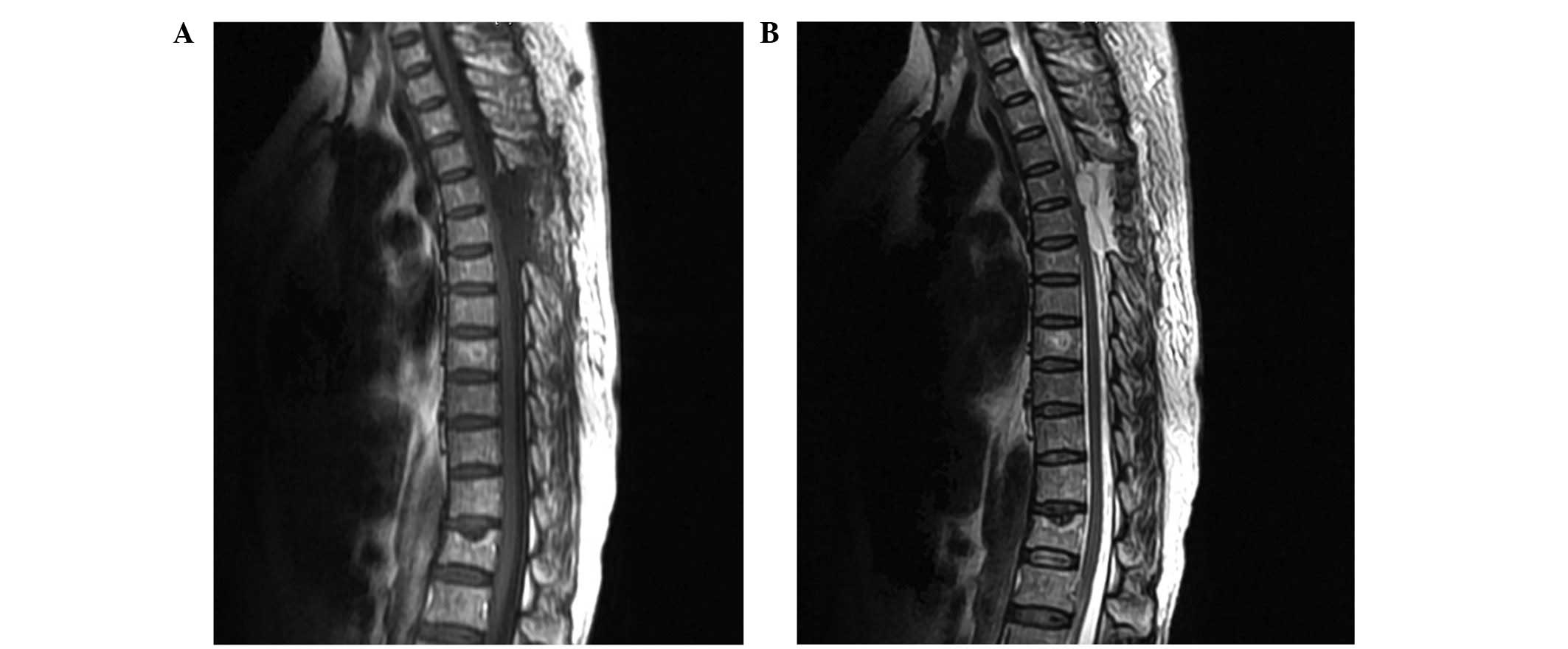
Repeated MRI scans of the thoracic spine, conducted one week after
surgery, demonstrated that the mass had been totally resected
(Fig. 2). The patient was
transferred to the oncology department for chemotherapy, and was
followed up by the medical oncology and neurosurgery
departments.
The present study was approved by the Ethics
Committee of the Clinical Medical College of Yangzhou University
(Yangzhou, China) and informed consent was obtained from the
patient.
Discussion
Primary spinal melanoma commonly arises from
melanoblasts along the neural crest and typically occurs in the
leptomeninges. More than 90% of spinal melanomas metastasize and
grow rapidly, which usually results in a fatal outcome within six
months (4). Due to the relative
rarity of primary spinal melanoma, at present, only 60 cases have
been identified in English studies (Table I). The mean age at presentation is
50 years (range, 15–80 years) and thoracic melanomas are the most
common type. Among all of the published cases of primary spinal
melanoma, including the present case, the patients have presented
non-specific and progressive symptoms of myelopathy. These symptoms
mimic those of other intraspinal mass lesions, which occupy similar
locations and demonstrate similar growth patterns, including spinal
meningioma, meningeal melanocytomas and metastatic melanoma.
 | Table ISummary of the 60 cases of primary
spinal cord melanoma reported in English studies. |
Table I
Summary of the 60 cases of primary
spinal cord melanoma reported in English studies.
| Case | First author
(ref) | Year | Location | Age (years)
/Gender | Laminectomy | Adjuvant
treatement | Metastasis | Survival duration
(months) | Condition of
follow-up end point | Duration symptom
(months) |
|---|
| 1 | Yu (8) | 2012 | C2–C6 | 48/M | Y | Y | N | 2 | Alive | 6 |
| 2 | Yan (9) | 2012 | L2–L4 | 44/F | Y | N | N | NR | NR | 24 |
| 3 | Fuld (10) | 2011 | C2 | 62/M | Y | Y | N | 11 | Alive | NR |
| 4 | Vij (11) | 2010 | C1–C2 | 40/M | Y | N | N | 12 | Alive | 9 |
| 5 | Lee (12) | 2010 | C1–C6 | 39/M | Y | Y | N | 14 | Alive | 11 |
| 6 | Kolasa (13) | 2010 | T10 | 57/F | Y | Y | N | 12 | Alive | 2 |
| 7 | Kim (14) | 2010 | T4 | 34/F | Y | N | N | 36 | Alive | 12 |
| 8 | Kwang (15) | 2010 | T7–T8 | 68/F | Y | Y | N | 6 | Alive | |
| 9 | Kounin (16) | 2005 | C2–C4 | 41/F | Y | N | N | 3 | Alive | 9 |
| 10 | Kwon (17) | 2004 | C6–C7 | 45/F | Y | Y | N | 8 | Alive | 48 |
| 11 | Tosaka (18) | 2001 | CSF | 20/M | N | NR | Brainstem;
leptomeningeal | 5 | Succumbed | 7 |
| 12 | Farrokh (19) | 2001 | T12-L1 | 80/F | Y | N | N | 9 | Alive | NR |
| 13 | Bidzinski (20) | 2000 | C6–C7 | 36/M | Y | Y | N | 48 | Alive | 8 |
| 14 | Brat (21) | 1999 | T10 | 71/F | Y | N | N | 14 | Alive | NR |
| 15 | | | C1 | 52/M | Y | Y | N | 16 | Alive | NR |
| 16 | | | C4 | 20/F | Y | N | N | 20 | Alive | NR |
| 17 | | | C4 | 57/F | Y | Y | N | 8 | Succumbed | NR |
| 18 | Salame (22) | 1998 | T9–T10 | 76/F | Y | Y | N | 15 | Alive | 6 |
| 19 | Francois (23) | 1998 | T8 | 62/M | Y | N | NR | 28 | Alive | 18 |
| 20 | Salpietro (24) | 1998 | C3 | 62/M | Y | Y | Brain | 15 | Succumbed | 1 |
| 21 | Magni (25) | 1996 | T | 64/M | Y | Y | N | 18 | Alive | 24 |
| 22 | Yamasaki (26) | 1989 | T7–T8 | 31/M | Y | Y | N | 23 | Alive | 6 |
| 23 | Schneider (27) | 1987 | L3–L4 | 68/F | Y | N | Cerebellum;
frontal | 10 | Alive | NR |
| 24 | Larson (28) | 1987 | T6–T8 | 73/M | Y | Y | Leptomeningeal
dissemination | 84 | Alive | 6 |
| 25 | | | T9 | 63/M | Y | Y | N | 156 | Succumbed | 96 |
| 26 | | | T9–T11 | 67/F | Y | Y | N | Short period | Alive | 18 |
| 27 | | | C1–C3 | 57/F | Y | Y | N | 30 | Succumbed | 3 |
| 28 | | | T9–T10 | 69/F | Y | N | N | 45 | Succumbed | 24 |
| 29 | Ozden (29) | 1984 | T7–T10 | 30/F | Y | Y | N | 16 | Alive | NR |
| 30 | | | C1–C6 | 15/F | Y | N | N | 18 | Alive | NR |
| 31 | Holaday (30) | 1968 | S2 | 20/F | Y | N | NR | 12 | Succumbed | 3 |
| 32 | Clifford (31) | 1968 | C3–C5 | 64/M | Y | N | N | 24 | Succumbed | 4 |
| 33 | Kiel (32) | 1961 | C4–C6 | 33/F | Y | NR | Cerebral;
leptomeningeal | 19 | Succumbed | 25 |
| 35 | Hirano (33) | 1960 | T | 42/F | Y | NR | NR | NR | Alive | 1 |
| 36 | Zimmerman (34) | 1958 | D9–D10 | 42/M | Y | N | N | 4 | Succumbed | 8 |
| 37 | Gibson (35) | 1957 | T | 51/F | N | Y | Leptomeningeal
dissemination | NR | Alive | NR |
| 38 | De Roca (36) | 1954 | T | 50/F | Y | Y | NR | NR | Alive | 6 |
| 39 | Perino (37) | 1953 | T | 40/M | Y | Y | NR | NR | Alive | NR |
| 40 | King (38) | 1952 | L | 53/M | Y | N | Dura mater; base
brain | NR | Alive | 12 |
| 41 | De Assis (39) | 1951 | L | 26/M | Y | Y | NR | NR | Succumbed | 7 |
| 42 | King (40) | 1951 | L | 47/M | Y | N | Brain;
leptomeningeal | NR | NR | 2 |
| 43 | Forbes (41) | 1950 | T | 57/M | Y | N | Leptomeningeal
dissemination | NR | Alive | NR |
| 44 | Kissel (42) | 1950 | C | 25/F | Y | N | NR | NR | NR | 2 |
| 45 | Castaner (43) | 1950 | L | 52/F | Y | NR | NR | NR | NR | 12 |
| 46 | Mackay (44) | 1942 | C | 32/F | N | NR | Cervical, spinal
cord | NR | Alive | 10 |
| 47 | Garcin (45) | 1941 | L | 52/M | Y | Y | NR | NR | NR | 3 |
| 48 | DaCosta (46) | 1939 | T | 55/F | Y | N | NR | NR | NR | 24 |
| 49 | Schnitker (47) | 1938 | D9–D10 | 49/F | Y | Y | Lung, liver,
uterus, leptomeningeal | 6 | Succumbed | 30 |
| 50 | Van Bogaert
(48) | 1933 | T 6 | 38/M | Y | NR | NR | NR | NR | 6 |
| 51 | De Blasi (49) | 1930 | T | 71/F | Y | NR | NR | NR | Alive | 6 |
| 52 | Bell (50) | 1930 | C | 48/F | Y | NR | NR | NR | NR | 3 |
| 53 | Prussak (51) | 1929 | T | 29/M | Y | NR | NR | NR | NR | 3 |
| 54 | Ringertz (52) | 1926 | T | 61/F | Y | NR | Brain not
examined | NR | NR | 6 |
| 55 | Schmid (53) | 1926 | T | 71/M | N | NR | Cerebral, dura
mater | NR | NR | 14 |
| 56 | Koelichen (54) | 1916 | T | 25/M | Y | NR | NR | NR | NR | 1.5 |
| 57 | Lindbom (55) | 1912 | C1–C3 | 45/F | N | NR | NR | NR | NR | 2 |
| 58 | Kawashima (56) | 1910 | C | 26/F | N | NR | Leptomeningeal
dissemination | NR | NR | 7 |
| 59 | Esser (57) | 1907 | T1–T2 | 32/M | Y | NR | NR | NR | NR | 0.5 |
| 60 | Boit (58) | 1907 | T8–T11 | 51/M | N | NR | Liver, spleen | NR | NR | 11 |
| 61 | Hirschberg
(59) | 1906 | T | 67/F | N | NR | NR | NR | NR | 3 |
With regard to radiological examination, MRI scans
are commonly used to identify different spinal lesions. The typical
pattern of spinal melanoma observed using MRI, includes signal
hyperintensity on T1-weighted images and signal isointensity or
hypointensity on T2-weighted images. These signal characteristics
are inconsistent as the MRI signal depends on the presence of
melanin, intratumoral hemorrhages and fat deposits, which
complicates the majority of spinal melanoma images. MRI scanning
aids diagnosis, however, it does not specifically differentiate
between primary melanoma and other malignant lesions. The signal
characteristics of MRI may easily lead to an erroneous diagnosis.
It is important for surgeons to make an accurate diagnosis and be
aware of the limitations of the diagnostic value of MRI. In the
present case report, enhanced MRI revealed an obvious dural tail
sign, which is a classic characteristic of meningioma. However,
T1-weighted images with hyperintensity and T2-weighted images with
hypointensity are typical features for melanoma, and atypical for
meningioma. Therefore, it is difficult to exclude the diagnosis of
spinal meningioma prior to surgery, as intratumoral bleeding may
result in an uneven hyperintensive signal in T1-weighted images. In
the present case report, the final diagnosis of the patient
required further investigation using methods other than MRI.
Histologically, melanin-containing tumors, including
melanocytosis, melanocytoma, malignant melanoma and meningeal
melanomatosis, exhibit spindle or epithelioid cells arranged in
sheets, bundles, nests or whorls containing variable quantities of
melanin pigment in the cytoplasm (5). Accurate pathological diagnoses are
important as the histological distinction, clinical course and
prognosis vary for different melanin-containing tumors.
Furthermore, appropriate case-specific therapy, involving surgery,
and radio- and chemotherapy should be planned on the basis of a
specific diagnosis. The differential diagnosis between malignant
melanoma and meningeal melanocytoma require consideration, as the
two originate from melanocytes. In the present case report, the
presence of the histological characteristics of tumor necrosis,
cytologic atypia and high mitotic activity resulted in the initial
diagnosis of a malignant melanoma. Therefore, distinguishing
between malignant melanoma and melanotic meningioma or metastatic
carcinoma is important. Immunohistochemical analysis facilitates
the differentiation between these different melanin-containing
tumors. Positive staining of the anti-melanoma antibody, HMB45 and
the S-100 protein indicates that cells are of melanocytic origin. A
negative reaction for EMA eliminates the possibility of a mass
being a melanotic meningioma of the spinal cord, and a negative
reaction for EMA and NSE exclude metastatic carcinoma of
melanocytic origin. Thus, in order to accurately diagnose primary
spinal malignant melanoma, it is important to combine histological,
radiological and immunohistochemical analyses.
In the present case, complete surgical resection was
recommended in order to obtain a curative outcome. Local control
rates have been reported to be four-fold higher if complete
resection is achieved (6).
Intraoperatively, the differentiation between various
melanin-containing tumors is often difficult. Certain typical
features, including dura mater attachment, an indistinct mass and a
dark, black color may indicate that the tumor has originated from
leptomeningeal melanocytes. Spinal meningioma may mimic this
appearance, when the lesion presents within a large volume of
hematoma. Therefore, pathological and immunohistochemical analyses
of the resected specimen are required to provide a specific
diagnosis. The selection of an appropriate individual therapy, for
example radio- and/or chemotherapy, is based on all of these
findings. A previous study reported that Gamma Knife therapy
improved the clinical outcome and reduced the complication rate in
metastatic CNS melanoma (7).
However, the efficiency and long-term survival rate of Gamma Knife
therapy requires further investigation to confirm these findings.
Despite treatment strategies involving total resection and adjuvant
therapy, the prognosis of patients with primary spinal melanoma
remains particularly poor. Therefore, close follow-up studies are
required, even in cases of complete surgical resection.
The efficacy of radio- and chemotherapy remains
controversial in the treatment of melanoma. While melanoma is a
radiotherapy-resistant tumor, patients benefit from surgical
resection, which has been reported to significantly alleviate the
symptoms that result from its compressive effect. Surgical
resection has also been reported to reduce the growth rate of
melanoma (60). Furthermore,
Hamilton et al (61)
reported preoperative radiotherapy in a patient with spinal
melanoma and obtained satisfactory clinical outcomes. The
radiotherapy dose depended on the tumor size, location, compression
symptoms and patient tolerance; however, a dose of 12–24 Gy was
recommended by the majority of the doctors. Attitudes towards
adjuvant treatment vary worldwide. A study in the USA reported that
high-dose interferon treatment improved patient prognosis, although
it resulted in severe side-effects (62). A meta-analysis showed that chemo-
and biological therapy were capable of reducing the recurrence rate
and increasing survival by only 3% after five years (63). Previous studies have shown that
treatment with chemotherapy and/or novel monoclonal antibodies, for
example using ipilimumab, overcomes cytotoxic T-lymphocyte antigen
4-mediated T cell suppression and improves overall survival
(64,65). The patient in the present case was
treated with chemotherapy subsequent to surgery and no tumor
recurrence was observed at the six-month follow-up.
In conclusion, the clinical features of primary
spinal melanoma are complex and may be easily misdiagnosed as other
spinal lesions. In the current report, a case of primary malignant
melanoma of the thoracic spine is presented. Primary malignant
melanoma is a particularly rare and aggressive tumor, therefore,
total resection is recommended. An accurate diagnosis based on
histological and immunohistochemical analyses of the resected
tissue, is critical for selecting the appropriate therapy to
enhance patient outcome. Unlike the majority of cases of primary
intradural melanoma, the present case exhibited unusual
radiological features, including a dural tail sign that mimicked a
spinal meningioma. Thus, the present case report illustrates the
importance for neurosurgeons to analyze radiological data carefully
to increase the accuracy of their initial diagnosis. The diagnostic
potential of malignant melanoma requires consideration at the time
of surgery to establish the need for aggressive surgical resection.
Thus, early complete surgical resection followed by individualized
radio- or chemotherapy may enhance patient outcome. Furthermore, a
meta-analysis focuses on the best treatment strategy for this
disease and aids with the diagnosis and treatment of primary spinal
melanoma.
Acknowledgements
The authors would like to thank Dr Guangyu Lu from
Ruprecht Karl University of Heidelberg (Heidelberg, Germany) for
the editorial assistance.
References
|
1
|
Balch CM, Buzaid AC, Soong SJ, et al:
Final version of the American Joint Committee on cancer staging
system for cutaneous melanoma. J Clin Oncol. 19:3635–3648.
2001.
|
|
2
|
Ganiüsmen O, Özer FD, Mete M, Özdemir N
and Bayol Ü: Slow progression and benign course of a primary malign
melanoma of a lumbar nerve root. Clin Neurol Neurosurg.
114:166–168. 2012.
|
|
3
|
Fuld AD, Speck ME, Harris BT, et al:
Primary melanoma of the spinal cord: a case report, molecular
footprint, and review of the literature. J Clin Oncol.
29:e499–e502. 2011.
|
|
4
|
Lee CH, Moon KY, Chung CK, et al: Primary
intradural extramedullary melanoma of the cervical spinal cord:
case report. Spine (Phila Pa 1976). 35:E303–E307. 2010.
|
|
5
|
Hayward RD: Malignant melanoma and the
central nervous system. A guide for classification based on the
clinical findings. J Neurol Neurosurg Psychiatry. 39:526–530.
1976.
|
|
6
|
Bhatia S, Tykodi SS and Thompson JA:
Treatment of metastatic melanoma: an overview. Oncology (Williston
Park). 23:488–496. 2009.
|
|
7
|
Yu J, Zhao DD, Chen S, Zhang JM and Xu J:
Primary melanoma of the cervical spine with cerebral metastases:
case report and review of the literature. J Int Med Res.
40:1207–1215. 2012.
|
|
8
|
Yu J, Zhao DD, Chen S, et al: Primary
melanoma of the cervical spine with cerebral metastases: case
report and review of the literature. J Int Med Res. 40:1207–1215.
2012.
|
|
9
|
Yan L, Chang Z, Liu Y, et al: Primary
spinal melanoma: a case report and literature review. Chin Med J
(Engl). 125:4138–4141. 2012.
|
|
10
|
Fuld AD, Speck ME, Harris BJ, et al:
Primary melanoma of the spinal cord: a case report, molecular
footprint, and review of the literature. J Clin Oncol.
29:e499–e502. 2011.
|
|
11
|
Vij M, Jaiswal S, Jaiswal AK and Behari S:
Primary spinal melanoma of the cervical leptomeninges: report of a
case with brief review of literature. Neurol India. 58:781–783.
2010.
|
|
12
|
Lee CH, Moon KY, Chung CK, et al: Primary
intradural extramedullary melanoma of the cervical spinal cord:
case report. Spine (Phila Pa 1976). 35:E303–E307. 2010.
|
|
13
|
Kolasa M, Jesionek-Kupnicka D, Kordek R
and Kolasa P: Primary spinal cord melanoma - a case report. Folia
Neuropathol. 48:212–216. 2010.
|
|
14
|
Kim MS, Yoon DH and Shin DA: Primary
spinal cord melanoma. J Korean Neurosurg Soc. 48:157–161. 2010.
|
|
15
|
Jo KW, Kim SR, Kim SD and Park IS: Primary
thoracic epidural melanoma: a case report. Asian Spine J. 4:48–51.
2010.
|
|
16
|
Kounin GK, Romansky KV, Traykov LD, et al:
Primary spinal melanoma with bilateral papilledema. Clin Neurol
Neurosurg. 107:525–527. 2005.
|
|
17
|
Kwon SC, Rhim SC, Lee DH, et al: Primary
malignant melanoma of the cervical spinal nerve root. Yonsei Med J.
45:345–348. 2004.
|
|
18
|
Tosaka M, Tamura M, Oriuchi N, et al:
Cerebrospinal fluid immunocytochemical analysis and neuroimaging in
the diagnosis of primary leptomeningeal melanoma. Case report. J
Neurosurg. 94:528–532. 2001.
|
|
19
|
Farrokh D, Fransen P and Faverly D: MR
findings of a primary intramedullary malignant melanoma: case
report and literature review. AJNR Am J Neuroradiol. 22:1864–1866.
2001.
|
|
20
|
Bidziński J, Kroh H, Leszczyk C and
Bojarski P: Primary intraspinal cervical melanoma. Acta Neurochir
(Wien). 142:1069–1070. 2000.
|
|
21
|
Brat DJ, Giannini C, Scheithauer BW and
Burger PC: Primary melanocytic neoplasms of the central nervous
systems. Am J Surg Pathol. 23:745–754. 1999.
|
|
22
|
Salame K, Merimsky O, Yosipov J, et al:
Primary intramedullary spinal melanoma: diagnostic and treatment
problems. J Neurooncol. 36:79–83. 1998.
|
|
23
|
François P, Lioret E and Jan M: Primary
spinal melanoma: case report. Br J Neurosurg. 12:179–182. 1998.
|
|
24
|
Salpietro FM, Alafaci C, Gervasio O, et
al: Primary cervical melanoma with brain metastases. Case report
and review of the literature. J Neurosurg. 89:659–666. 1998.
|
|
25
|
Magni C, Yapo P, Mocaer J, et al: Primary
intramedullary melanoma. Apropos of a case. J Neuroradiol.
23:41–45. 1996.(In French).
|
|
26
|
Yamasaki T, Kikuchi H, Yamashita J, et al:
Primary spinal intramedullary malignant melanoma: case report.
Neurosurgery. 25:117–121. 1989.
|
|
27
|
Schmidt P, Neuen JE, Blanke M, et al:
Primary malignant melanoblastosis of the meninges. Clinical,
cytologic and neuropathologic findings in a case. Acta Cytol.
32:713–718. 1988.
|
|
28
|
Larson TC, Houser OW, Onofrio BM, et al:
Primary spinal melanoma. J Neurosurg. 66:47–49. 1987.
|
|
29
|
Ozden B, Barlas O and Hacihanefioğlu U:
Primary dural melanomas: report of two cases and review of the
literature. Neurosurgery. 15:104–107. 1984.
|
|
30
|
Holaday WJ and Evans EB: Spinal
(meningeal) melanoma. A case report. J Bone Joint Surg Am.
50:738–742. 1968.
|
|
31
|
Clifford JH, McClintock HG and Lubchenco
AE: Primary spinal cord malignant melanoma: case report. J
Neurosurg. 29:410–413. 1968.
|
|
32
|
Kiel FW, Starr LB and Hansen JL: Primary
melanoma of the spinal cord. J Neurosurg. 18:616–629. 1961.
|
|
33
|
Hirano A and Carton CA: Primary malignant
melanoma of the spinal cord. J Neurosurg. 17:935–944. 1960.
|
|
34
|
Zimmerman HM and Adams RD: Seminar on
diseases of nervous tissue and muscle. In: Presented at 24th
seminar of American Society of Clinical Pathologists; Chicago.
1958
|
|
35
|
Gibson JB, Burrows D and Weir WP: Primary
melanoma of the meninges. J Pathol Bacteriol. 74:419–438. 1957.
|
|
36
|
Roca de VR, Elizalde AC and Coma FA: A CNS
melanoma: the practice of three clinical cases. Med Clín.
22:304–311. 1954.
|
|
37
|
Ruben PF: Spinal melanoma. Neurocirugia.
9:81953.(In Undetermined Language).
|
|
38
|
King AB, Chambers JW and Garey J: Primary
malignant melanoma of the spinal cord. AMA Arch Neurol Psychiatry.
68:266–275. 1952.
|
|
39
|
De Assis and De Luccia: Primary melanoma
due to cauda equina. Rev Paul Med. 39:388–389. 1951.(In
Undertermined Language).
|
|
40
|
King AB and Propst HD: Melanomas of the
central nervous system: description of a primary spinal cord
melanoma. Guthrie Clin Bull. 21:19–29. 1951.
|
|
41
|
Forbes W and Maloney AF: Primary
melanomatosis of the leptomeninx. J Pathol Bacteriol. 62:403–409.
1950.
|
|
42
|
Kissel P, Rousseaux R, Beau A, et al:
Primary meningeal melanoma with bulbo-cervical localization. Rev
Neurol (Paris). 82:385–389. 1950.(In Undetermined Language).
|
|
43
|
Castaner AE, Oliveras de la Riva C and
Barraquer-Bordas L: Primitive melanoma of the cauda equina.
Monatsschr Psychiatr Neurol. 120:227–236. 1950.
|
|
44
|
Mackay FH and Hurteau EF: Primary melanoma
of the central nervous system. J Nerv Ment Dis. 96:369–377.
1942.
|
|
45
|
Garcin R, Petit DD and Bertrand I:
Mélanoblastome primitif de la queue de cheval. Rev neurol.
73:255–257. 1941.
|
|
46
|
Da Costa DG and Love JG: Primary
melano-epithelioma of the spinal cord. Proc Staff Meet Mayo Clin.
14:628–631. 1939.
|
|
47
|
Schnitker MT and Ayer D: The primary
melanomas of the leptomeninges. A clinico-pathologic study with a
review of the literature and the report of an additional case. J
Nerv Ment Dis. 87:45–73. 1938.
|
|
48
|
Van Bogaert and Verbrugge J: A
meningoblastoma inclusions in spinal melanoma. J Beige Neurol
Psychiat. 33:813–817. 1933.
|
|
49
|
De Blasi: A primitive melanoma of spinal
cord. Pathologica. 22:606–613. 1930.
|
|
50
|
Bell FG: Primary melano-sarcoma of the
spinal arachnoid. J Coll Surg Australasia. 3:2791930.
|
|
51
|
Prussak B and Mackiewicz J: A case of
spinal cord chromatophoroma. Rev Neurol. 2:2321929.
|
|
52
|
Ringertz N: Two cases of pigmented tumor
of the spinal cord. Rev Neurol. 1:451–461. 1926.
|
|
53
|
Schmid HJ: A case of primary melanoma of
the spinal cord. Frankfurt Z Path. 33:372–379. 1926.
|
|
54
|
Koelichen J: Spinal cord chromatophoroma.
Ztschr Fd Ges Neurol Psychiat. 31:174–183. 1916.
|
|
55
|
Lindbom O: A case of chromatophoroma
originate from spinal dural. Hygiea. 74:198–218. 1912.
|
|
56
|
Kawashima K: About a sarcoma of the dura
mater and its dissemination in the meningeal space with diffuse
pigmentation of the leptomeninges. Virchows Arch. 201:297–311.
1910.
|
|
57
|
Esser: About a rare spinal cord of
chromatophorom (Chromatophorom). Dtsch Z Nervenheilk. 32:118–123.
1907.
|
|
58
|
Boit H: A case of chromatophoroma
originate from spinal dural. Contribution to the knowledge of the
pial chromatophoroma. Frank Z Pathol. 1:248–266. 1907.
|
|
59
|
Hirschberg A: Spinal cord of
chromatophoroma. A contribution to the knowledge of the primary
chromatophorome the central nervous system. Virchows Arch.
186:229–240. 1906.
|
|
60
|
Katalinic D, Anic B, Stern-Padovan R, et
al: Low back pain as the presenting sign in a patient with primary
extradural melanoma of the thoracic spine - a metastatic disease 17
years after complete surgical resection. World J Surg Oncol.
17:1502011.
|
|
61
|
Hamilton AJ, Lulu BA, Fosmire H, Stea B
and Cassady JR: Preliminary clinical experience with linear
accelerator-based spinal stereotactic radiosurgery. Neurosurgery.
36:311–319. 1995.
|
|
62
|
O’Day SJ, Atkins MB, Boasberg P, et al:
Phase II multicenter trial of maintenance biotherapy after
induction concurrent Biochemotherapy for patients with metastatic
melanoma. J Clin Oncol. 27:6207–6212. 2009.
|
|
63
|
Wheatley K, Ives N, Eggermont A, et al:
Interferon-α as adjuvant therapy for melanoma: an individual
patient data meta-analysis of randomised trials. J Clin Oncol.
25:85262007.
|
|
64
|
Hodi FS, O’Day SJ, McDermott DF, et al:
Improved survival with ipilimumab in patients with metastatic
melanoma. N Engl J Med. 363:711–723. 2010.
|
|
65
|
Robert C, Thomas L, Bondarenko I, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011.
|