Introduction
Tuberculous pleurisy and malignancy are two of the
most common causes of pleural effusions, but are difficult to
differentiate between in certain clinical situations. Increased
pleural permeability and exudates characterizing the inflammatory
process lead to the accumulation of pleural fluids and are believed
to be key events in tuberculous pleural effusions (TPEs) (1). Certain cytokines and chemokines
released by activated inflammatory cells, particularly T
lymphocytes and macrophages, are responsible for this process
(2). By contrast, malignant pleural
effusions (MPEs) are primarily caused by pleural metastasis or
lymphatic obstruction and are diagnosed by the presence of
malignant cells on the pleura or in the effusions (3). Cytokines, including interferon (IFN)-γ
and interleukin (IL)-1, -6, -16 and -17 have shown diagnostic,
differential and prognostic significance in TPEs and MPEs (1–3). These
findings indicate that the activation of lymphocytes and the
release of cytokines may have important roles in the development of
TPEs and MPEs.
IL-33 was initially identified as the nuclear factor
of high endothelial venules (HEVs) and was termed NF-HEV (4). In 2005, Schmitz et al (5) demonstrated that NF-HEV was a novel
member of the IL-1 cytokine superfamily, thus NF-HEV was termed
IL-33, which is also known as IL-F11 (5). The IL-33 gene is highly enriched in
the skin, lung, gastrointestinal tract and brain. IL-33 is
expressed by a variety of stromal cells, including epithelial
cells, alveolar epithelial cells, endothelial cells, fibroblasts
and eosinophilic cells, and its expression is stimulated in
response to inflammatory cytokine stimulation following injury or
infection (6,7). IL-33 is closely associated with asthma
and allergies, autoimmune rheumatic diseases, skin infections,
cancer, obesity, type 2 diabetes mellitus and cardiovascular system
diseases (8). The primary
biological activity of IL-33 is the induction of type 2 helper
(Th2) cell immunological responses through Th2 cytokines, including
IL-4, IL-5 and IL-13 (9). However,
accumulating evidence indicates that IL-33 is also capable of
modulating type 1 helper (Th1) cell cytokine responses.
Furthermore, IL-33 has been reported to induce potent cluster of
differentiation 8 (CD8)-positive T cell responses in response to
replicating, prototypic RNA and DNA viruses in mice (10). The binding of IL-33 to DNA, where it
acts as a nuclear factor, closely resembles the function of IL-1α
(11). The IL-33 precursor can bind
to nuclear factor κ-light-chain-enhancer of activated B cells
(NF-κB) p65, and IL-1β-induced tumor necrosis factor α is reduced
in cells overexpressing the IL-33 precursor (12). Thus, as a cytokine and multifaceted
immunomodulator, IL-33 has been proposed to have a role in the
pathogenesis of pleural inflammation and effusion. The present
study aimed to investigate the presence and potential diagnostic
value of IL-33 in TPE and MPE. The concentration of IL-33 in
pleural effusion and serum samples was detected in 23 patients with
TPE and 21 patients with MPE. The correlation between pleural and
serum IL-33 levels was analyzed and the diagnostic value of IL-33
was assessed using receiver operating characteristic (ROC)
curves.
Materials and methods
Study design
The present study was performed at the Union
Hospital Affiliated to Tongji Medical College (Huazhong University
of Science and Technology, Wuhan, China) and the Wuhan City
Tuberculosis Prevention and Control Institute (Wuhan, China). The
procedures were approved by the medical ethics committees of the
two hospitals. All patients and family members were fully informed
about the procedure and signed consent documents. Hospitalized
patients with TPE and MPE were screened according to inclusive and
exclusive criteria between December 2009 and June 2011. A total of
23 patients with TPE (17 male, female; age, 19–70 years; median
age, 45 years) and 21 patients with MPE (10 male, 11 female; age,
8–84 years; median age, 51 years) were selected for the present
study. The patients with MPE were suffering from lung cancer
(n=18), breast cancer (n=2) or lymphoma (n=1).
Diagnostic criteria
TPE was diagnosed as pleural effusions meeting any
of the following criteria and the exclusion of other causes: (i)
The detection of acid-fast bacilli in the pleural effusion
examination and/or granuloma-like alterations in the pleural biopsy
samples, and the exclusion of pleurisy from other causes; (ii) the
identification of exudate pleural effusions, assessed using Light’s
criteria (13). Light’s criteria
suggest that a pleural effusion is an exudate if lymphocytes are
the major cell type in the pleural effusion, the pleural effusion
has an adenosine deaminase (ADA) concentration >40 U/l, the
tuberculin test is positive, the pleural effusion is absorbed and
if the clinical symptoms are reduced with anti-tuberculosis
treatment.
The diagnosis of MPE should be made only with the
pathological diagnosis of a primary malignancy, radiological or
clinical evidence of pleural effusions and cytological/pathological
diagnosis of metastatic tumor cells. Moreover, imaging results
should be in accordance with primary bronchogenic lung cancer
complicated by pleural effusion. Transbronchial or thoracoscopic
lung biopsy should pathologically diagnose lung cancer, and the
cast-off cells from the pleural effusion should be detected as
metastatic tumor cells.
Exclusion criteria
Patients with any of the following criteria were
excluded from the present study: (i) Invasive pleural cavity
inspection and/or treatment or chest trauma three months prior to
admission; (ii) treatment with anticancer or antituberculosis
therapy and the use of glucocorticoid, non-steroidal
anti-inflammatory drugs or immunosuppressants; (iii) the detection
of metastatic tumor cells in the pleural effusion; or (iv) no
diagnosis of pleural effusion.
Sample collection and IL-33
measurement
Pleural effusion samples were obtained using a
standard thoracentesis procedure within 24 h of the patients being
hospitalized. Serum samples (20 ml) were obtained from venous blood
collected at the same time as the pleural effusion samples. Pleural
effusion and serum samples were centrifuged with 500 U/ml heparin
at 200 × g for 5 min. The supernatants were collected and frozen at
−80°C until required. IL-33 concentration was detected using an
enzyme-linked immunosorbent assay kit (Biolegend Inc., Chicago, IL,
USA) according to the manufacturer’s instructions. All samples were
analyzed twice.
Statistical analysis
Data are presented as the mean ± standard deviation.
The differences between the serum and pleural IL-33 levels, and
between the IL-33 levels in the TPE and MPE groups were compared
using independent sample t-tests for normally distributed data and
Mann-Whitney U or Kruskal-Wallis rank sum tests for non-normally
distributed data. Correlations were determined using Spearman’s
rank correlation analysis. The diagnostic accuracies of serum or
pleural IL-33 levels for discriminating between TPE and MPE were
investigated using ROC curve analysis. Data were analyzed using
SPSS version 5.0 (SPSS, Inc., Chicago, IL, USA) and SigmaPlot 10.0
(Systat Software, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
IL-33 concentrations in pleural
effusions
Results showed that IL-33 was present in the
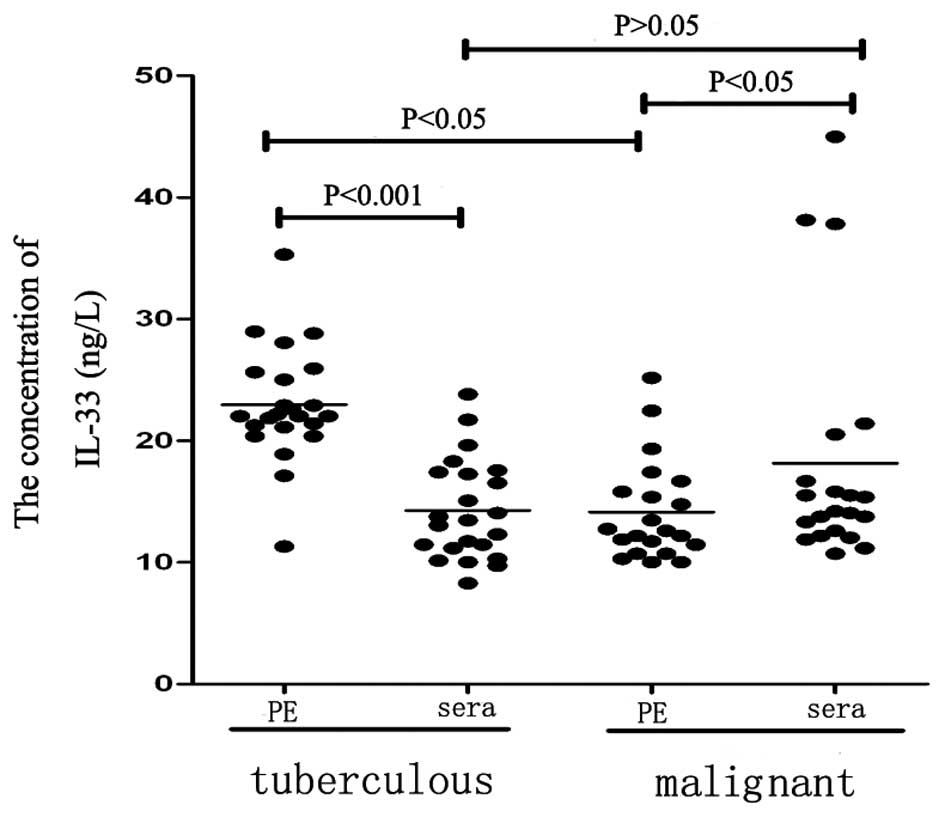
patients with TPE and MPE. The IL-33 concentration in the pleural
effusion (22.96±0.98 ng/l) was significantly higher than that in
the corresponding concentration of serum (14.27±0.86 ng/l;
P<0.01; Table I; Fig. 1) in the patients with TPE.
Furthermore, the concentration of IL-33 in the pleural effusion
(12.60±5.15 ng/l) was significantly lower than that in the
corresponding serum concentration (14.20±6.22 ng/l; P<0.05;
Table I) in the patients with MPE.
The concentration of IL-33 in the pleural effusion of the patients
with TPE (22.96±0.98 ng/l) was significantly higher than that in
the patients with MPE (12.60±5.15 ng/l; P<0.01); however, the
concentration of serum IL-33 in the patients with TPE was not
significantly different to that in the patients with MPE
(P>0.05). Statistically significant differences were identified
in the pleural effusion/serum gradient and ratio between TPE and
MPE (P<0.01).
 | Table IIL-33 expression in the pleural
effusion and serum and their differences and ratios in patients
with tuberculous pleural effusion and malignant pleural
effusion. |
Table I
IL-33 expression in the pleural
effusion and serum and their differences and ratios in patients
with tuberculous pleural effusion and malignant pleural
effusion.
| IL-33 | Tuberculous | Malignant | P-value |
|---|
| Pleural effusion,
ng/l | 22.92±0.98 | 12.60±5.15 | <0.01 |
| Serum, ng/l | 14.27±0.86 | 14.20±6.22 | >0.05 |
| Pleural
effusion-serum gradient, ng/l | 8.69±0.82 | −1.31±6.56 | <0.01 |
| Pleural
effusion-serum ratio, ng/l | 1.68±0.77 | 0.86±0.53 | <0.01 |
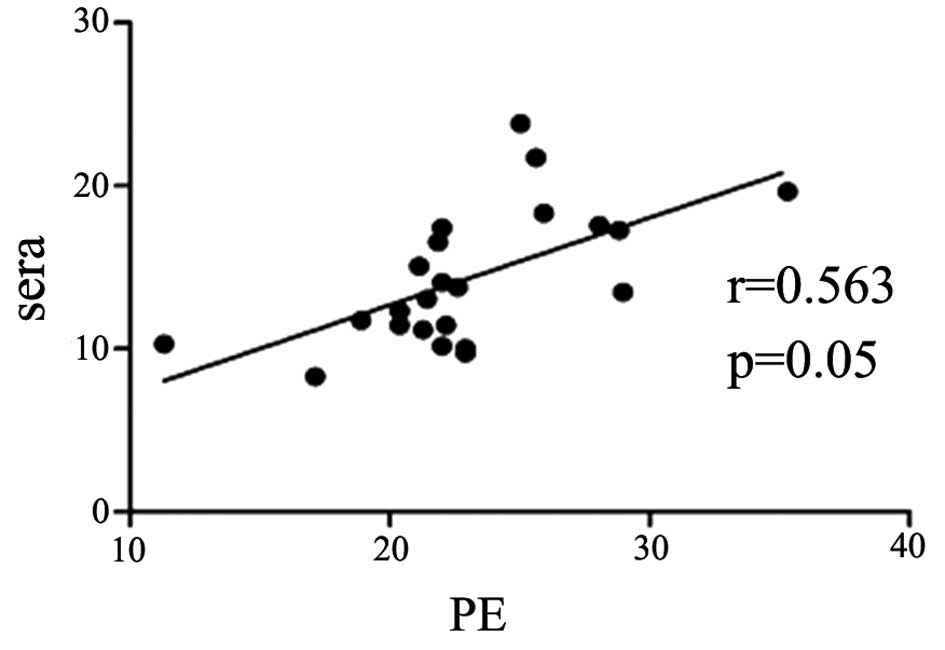
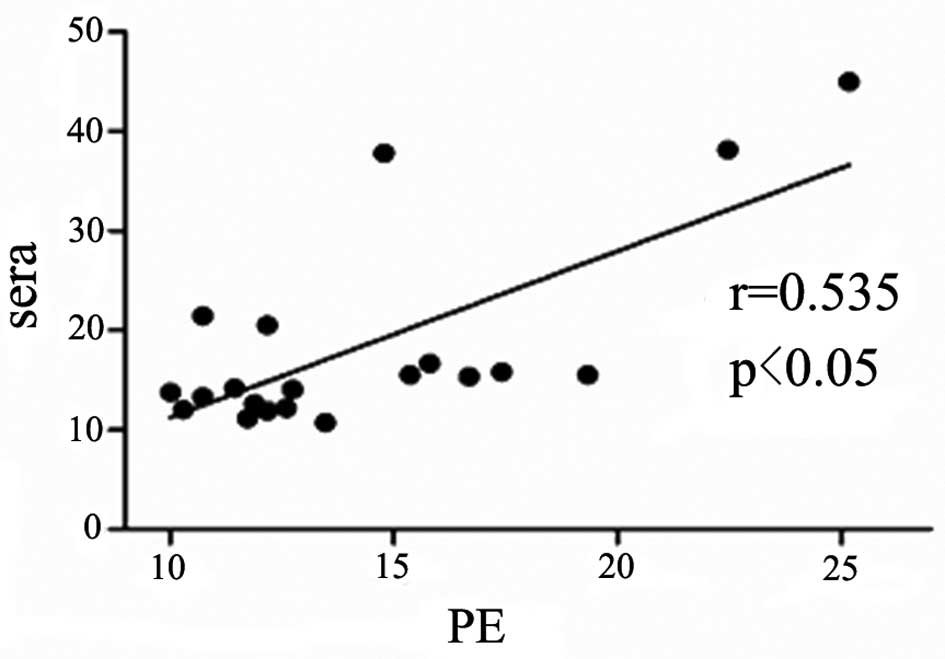
Serum IL-33 level is correlated with
pleural IL-33 level in the patients with TPE and MPE
The concentration of IL-33 in the pleural effusions
was positively correlated with that in the serum samples in the
patients with TPE and MPE (r=0.56, P=0.05; and r=0.54, P<0.05;
Figs. 2 and 3, respectively).
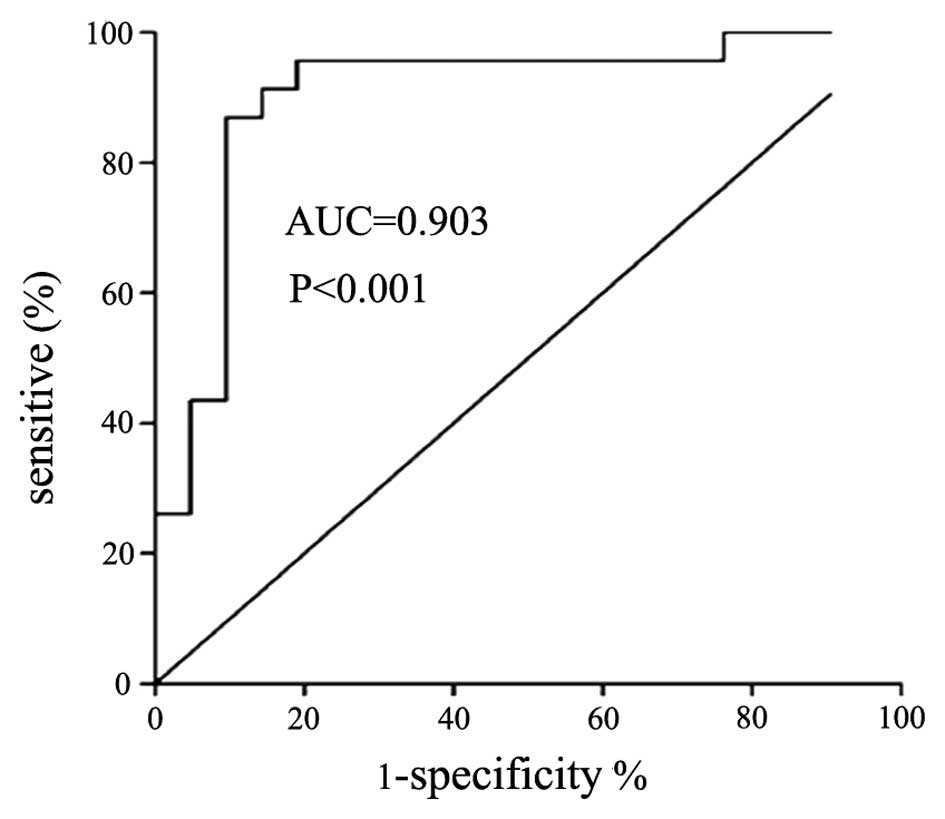
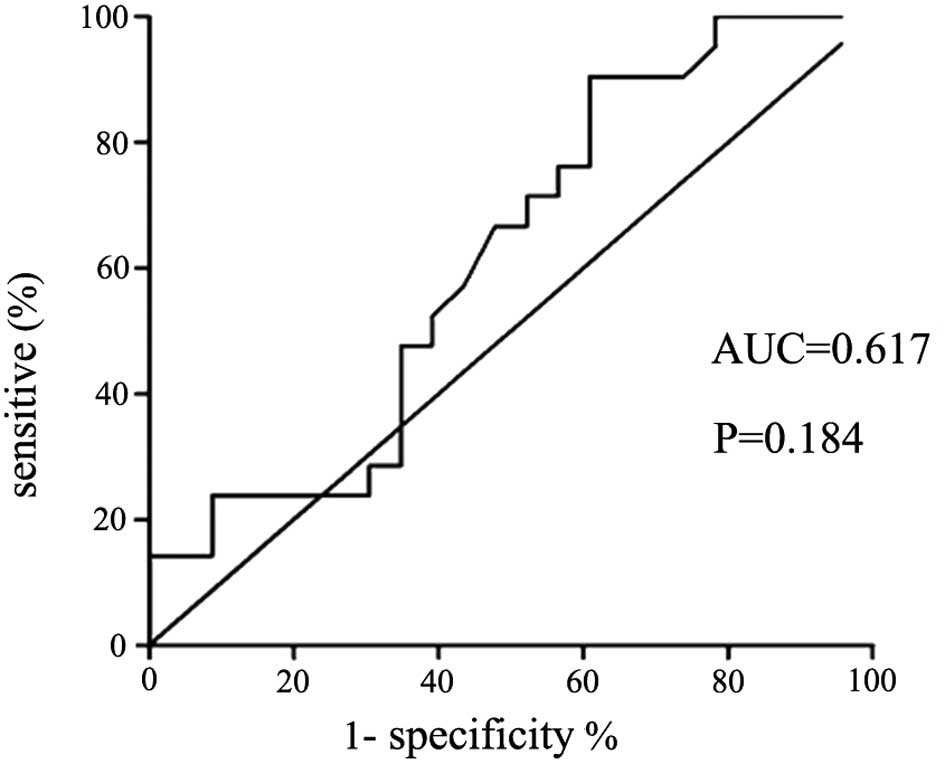
IL-33 level distinguishes between
patients with TPE and those with MPE
The cut-off value of pleural IL-33 for the diagnosis
of TPE was 19.86 ng/l, therefore patients with IL-33 concentrations
higher than this threshold had a high probability of being
diagnosed with TPE. The value for the area under the corresponding
ROC curve (AUC) was 0.903, the 95% confidence interval was 0.80–1,
the corresponding sensitivity and specificity were 86.96 and 90.48%
(P<0.01), the positive likelihood ratio (+LR) was 9.13 and the
negative likelihood ratio (−LR) was 0.13. The concentrations of
pleural IL-33, but not serum IL-33, differentiates TPE and MPE
(AUC=0.903 and P<0.001 for TPE; and AUC=0.617 and P=0.184 for
MPE).
Discussion
The present study aimed to investigate the
concentration and potential diagnostic significance of IL-33 in TPE
and MPE. The level of IL-33 in pleural effusion and serum samples
obtained from patients with MPE and TPE were analyzed. The present
study identified that the level of IL-33 was increased in the
pleural effusions and sera of the patients with MPE and TPE.
Furthermore, the pleural IL-33 level was found to be higher than
the serum IL-33 level in the patients with TPE. Moreover, the
concentration of pleural IL-33 in the patients with TPE was
observed to be significantly higher than that in the patients with
MPE, which indicated that IL-33 may serve as a biomarker for
distinguishing between TPE and MPE. The findings of the present
study are consistent with those of the study by Lee et al
(14), which found that pleural and
serum IL-33 levels were higher in patients with TPE compared with
those patients with other types of pleural effusion, and that
pleural IL-33 is of diagnostic significance in distinguishing
between TPE and other types of pleural effusion.
IL-33 belongs to the IL-1 subfamily and was formerly
termed IL-1F11. Neutrophil proteinase 3, neutrophil elastase and
cathepsin G are capable of truncating precursor IL-33, generating
IL-33 with different N-termini and varying levels of activity
(15). Thus, through processing the
IL-33 precursor, neutrophil enzymes generate active IL-33 isoforms
with varying levels of activity. Neutrophils are activated in
various stages of pleural tuberculosis infection, and thus may
contribute to the elevated level of IL-33 in pleural effusions
(16). However, it has yet to be
elucidated which cells in the pleura or effusions produce the most
IL-33 precursor, and whether the IL-33 gene is overexpressed in
TPE.
Though binding with its receptor IL-33Rα, IL-33
exerts immunoregulatory capabilities, including inducing Th2 immune
responses, and Th1 responses in certain situations (9–12,17).
The cytokine balance is primarily Th2-dominant in MPE, while Th1
responses dominate TPE (18). In
situations other than MPE or TPE, IL-33 induces the production of
IFN-γ, which enhances the Th1 responses (19–21).
IFN-γ directly regulates the innate immunity against tuberculosis,
therefore IL-33 may modulate and enhance host immunity through the
induction of IFN-γ in tuberculous pleurisy (22–25).
IL-33 may also regulate the functional status of macrophages, which
may regulate infectious and tumor immunity (26). Furthermore, IL-33 has been reported
to be expressed in endothelial cells in normal organs, but not in
tumor tissues (27,28). IL-33 may have a significant role in
tumor angiogenesis and immunosurveillence. IL-33 has been found to
enhance the activity of CD8+ T cells, NK cells and other
tumor killer cells, therefore IL-33 may also have a role in tumor
immunity or immunotherapy (10,11,20).
These hypotheses require further investigation.
In the present study, the pleural effusion-serum
IL-33 gradient and the pleural effusion-serum IL-33 ratio were
observed to be significantly increased in the patients with TPE
compared with those with MPE. This may be due to the sharper
elevation of IL-33 in the TPE patients compared with the MPE
patients, and may reflect the hyperinflamed pleural cavity
following tuberculosis infection (16). This also indicates that pleural
IL-33 may be a better candidate than serum IL-33 for the diagnosis
of TPE, consistent with the overall findings of the present study
(Fig. 4 and 5). There are various other biomarkers
specific for TPE, including IFN-γ and ADA, which have been
standardized as diagnostic criteria for TPE. Lee et al
(14) compared the diagnostic
accuracy of pleural IL-33, IFN-γ and ADA, which yielded AUCs of
0.74, 0.97 and 0.95, respectively. The diagnostic significance of
IL-33 may increase if combined with other parameters.
There are several limitations to the present study.
Firstly, the reliability of this study is limited by its sample
size. Compared with the study by Lee et al (14), the present study showed a higher AUC
of IL-33 in differential diagnosis (0.903 vs. 0.74). Thus a larger
sample size is required for a more accurate assessment of IL-33 in
distinguishing between TPE and MPE. Secondly, the present study
does not provide direct evidence of IL-33-producing cells and
whether the IL-33 gene is overexpressed in TPE. Immunological
methods, including flow cytometry, western blot analysis and
immunostaining, may be used in future investigations to identify
the IL-33-producing cells. Thirdly, the concentrations of IL-33
have not been followed in the treatment of MPE or TPE, which may
provide evidence to the correlation between IL-33 and the
pathobiology of MPE and TPE.
In conclusion, the present study showed that the
pleural IL-33 level is significantly elevated in patients with TPE
and may serve as a novel biomarker to differentiate between TPE and
MPE. Further investigations are required to identify the specific
mechanisms and effects of IL-33 release in TPE and MPE.
Acknowledgements
The present study was supported by grants from the
Hubei Provincial Natural and Scientific Foundation (grant no.
2009cbd399) and the Science and Technology Persons Project of the
Bureau of Public Health of Hubei Province (grant no. QJX2010-7).
The authors would like to thank Zheng Wang from the Department of
Respiratory and Critical Medicine, The People’s Hospital of
Zhengzhou University (Zhengzhou, China), for advice and revisions
to the manuscript.
References
|
1
|
Hua CC, Chang LC, Chen YC and Chang SC:
Proinflammatory cytokines and fibrinolytic enzymes in tuberculous
and malignant pleural effusions. Chest. 116:1292–1296. 1999.
|
|
2
|
Wong CF, Yew WW, Leung SK, Chan CY, Hui M,
Yeang C and Cheng AF: Assay of pleural fluid interleukin-6, tumour
necrosis factor-alpha and interferon-gamma in the diagnosis and
outcome correlation of tuberculous effusion. Respir Med.
97:1289–1295. 2003.
|
|
3
|
Qin XJ, Shi HZ, Huang ZX, Kang LF, Mo WN
and Wu C: Interleukin-16 in tuberculous and malignant pleural
effusions. Eur Respir J. 25:605–611. 2005.
|
|
4
|
Carriere V, Roussel L, Ortega N, et al:
IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a
chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci
USA. 104:282–287. 2007.
|
|
5
|
Schmitz J, Owyang A, Oldham E, et al:
IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type2-associated
cytokines. Immunity. 23:479–490. 2005.
|
|
6
|
Yasuda K, Muto T, Kawagoe T, et al:
Contribution of IL-33-activated type II innate lymphoid cells to
pulmonary eosinophilia in intestinal nematode-infected mice. Proc
Natl Acad Sci USA. 109:3451–3456. 2012.
|
|
7
|
van der Veerdonk FL and Netea MG: New
insights in the immunobiology of IL-1 family members. Front
Immunol. 4:1672013.
|
|
8
|
Milovanovic M, Volarevic V, Radosavljevic
G, Jovanovic I, Pejnovic N, Arsenijevic N and Lukic ML: IL-33/ST2
axis in inflammation and immunopathology. Immunol Res. 52:89–99.
2012.
|
|
9
|
Tjota MY, Williams JW, Lu T, et al:
IL-33-dependent induction of allergic lung inflammation by FcγRIII
signaling. J Clin Invest. 123:2287–2297. 2013.
|
|
10
|
Bonilla WV, Fröhlich A, Senn K, et al: The
alarmin interleukin-33 drives protective antiviral CD8+
T cell responses. Science. 335:984–989. 2012.
|
|
11
|
Cohen I, Rider P, Carmi Y, et al:
Differential release of chromatin-bound IL-1alpha discriminates
between necrotic and apoptotic cell death by the ability to induce
sterile inflammation. Proc Natl Acad Sci USA. 107:2574–2579.
2010.
|
|
12
|
Ali S, Mohs A, Thomas M, et al: The dual
function cytokine IL-33 interacts with the transcription factor
NF-κB to dampen NF-κB-stimulated gene transcription. J Immunol.
187:1609–1616. 2011.
|
|
13
|
Light RW, Macgregor MI, Luchsinger PC and
Ball WC Jr: Pleural effusions: the diagnostic separation of
transudates and exudates. Ann Intern Med. 77:507–513. 1972.
|
|
14
|
Lee KS, Kim HR, Kwak S, et al: Association
between elevated pleural interleukin-33 levels and tuberculous
pleurisy. Ann Lab Med. 33:45–51. 2013.
|
|
15
|
Lefrançais E, Roga S, Gautier V,
Gonzalez-de-Peredo A, Monsarrat B, Girard JP and Cayrol C: IL-33 is
processed into mature bioactive forms by neutrophil elastase and
cathepsin G. Proc Natl Acad Sci USA. 109:1673–1678. 2012.
|
|
16
|
Barnes PF, Fong SJ, Brennan PJ, Twomey PE,
Mazumder A and Modlin RL: Local production of tumor necrosis factor
and IFN-gamma in tuberculous pleuritis. J Immunol. 145:149–154.
1990.
|
|
17
|
Oboki K, Ohno T, Kajiwara N, Saito H and
Nakae S: IL-33 and IL-33 receptors in host defense and diseases.
Allergol Int. 59:143–160. 2010.
|
|
18
|
Okamoto M, Hasegawa Y, Hara T, Hashimoto
N, Imaizumi K, Shimokata K and Kawabe T: T-helper type 1/T-helper
type 2 balance in malignant pleural effusions compared to
tuberculous pleural effusions. Chest. 128:4030–4035. 2005.
|
|
19
|
Smithgall MD, Comeau MR, Yoon BR, Kaufman
D, Armitage R and Smith DE: IL-33 amplifies both Th1- and Th2-type
responses through its activity on human basophils,
allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol.
20:1019–1030. 2008.
|
|
20
|
Bourgeois E, Van LP, Samson M, et al: The
pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK
cells to induce IFN-gamma production. Eur J Immunol. 39:1046–1055.
2009.
|
|
21
|
Espinassous Q, Garcia-de-Paco E,
Garcia-Verdugo I, et al: IL-33 enhances lipopolysaccharide-induced
inflammatory cytokine production from mouse macrophages by
regulating lipopolysaccharide receptor complex. J Immunol.
183:1446–1455. 2009.
|
|
22
|
Feng CG, Kaviratne M, Rothfuchs AG, et al:
NK cell-derived IFN-gamma differentially regulates innate
resistance and neutrophil response in T cell-deficient hosts
infected with Mycobacterium tuberculosis. J Immunol.
177:7086–7093. 2006.
|
|
23
|
Schierloh P, Yokobori N, Alemán M, et al:
Mycobacterium tuberculosis-induced gamma interferon
production by natural killer cells requires cross talk with
antigen-presenting cells involving Toll-like receptors 2 and 4 and
the mannose receptor in tuberculous pleurisy. Infect Immun.
75:5325–5337. 2007.
|
|
24
|
Caramori G, Lasagna L, Casalini AG, et al:
Immune response to Mycobacterium tuberculosis infection in
the parietal pleura of patients with tuberculous pleurisy. PLoS
One. 6:e226372011.
|
|
25
|
Miller AM: Role of IL-33 in inflammation
and disease. J Inflamm (Lond). 8:222011.
|
|
26
|
Joshi AD, Oak SR, Hartigan AJ, et al:
Interleukin-33 contributes to both M1 and M2 chemokine marker
expression in human macrophages. BMC Immunol. 11:522010.
|
|
27
|
Choi YS, Choi HJ, Min JK, et al:
Interleukin-33 induces angiogenesis and vascular permeability
through ST2/TRAF6-mediated endothelial nitric oxide production.
Blood. 114:3117–3126. 2009.
|
|
28
|
Jovanovic I, Radosavljevic G, Mitrovic M,
et al: ST2 deletion enhances innate and acquired immunity to murine
mammary carcinoma. Eur J Immunol. 41:1902–1912. 2011.
|