Introduction
Malignant melanoma is a cancer with an increasing
incidence and mortality rate. Furthermore, it is not sensitive to
radiotherapy or chemotherapy, and thus presents a problem with
regard to clinical treatment. Therefore, a number of studies have
focused on the development of efficient and sensitive anticancer
drugs. Sansalvamide A, a cyclic depsipeptide derived from a marine
fungus (Fusarium), has been found to exhibit significant
antiproliferative effects in the National Cancer Institute’s 60
cancer cell line panel (1,2). In recent years, the synthesis of
sansalvamide A derivatives have received increasing attention.
Novel sansalvamide A derivatives show improved anticancer abilities
(3), suggesting that these novel
compounds may prove to be valuable therapeutic agents. In the
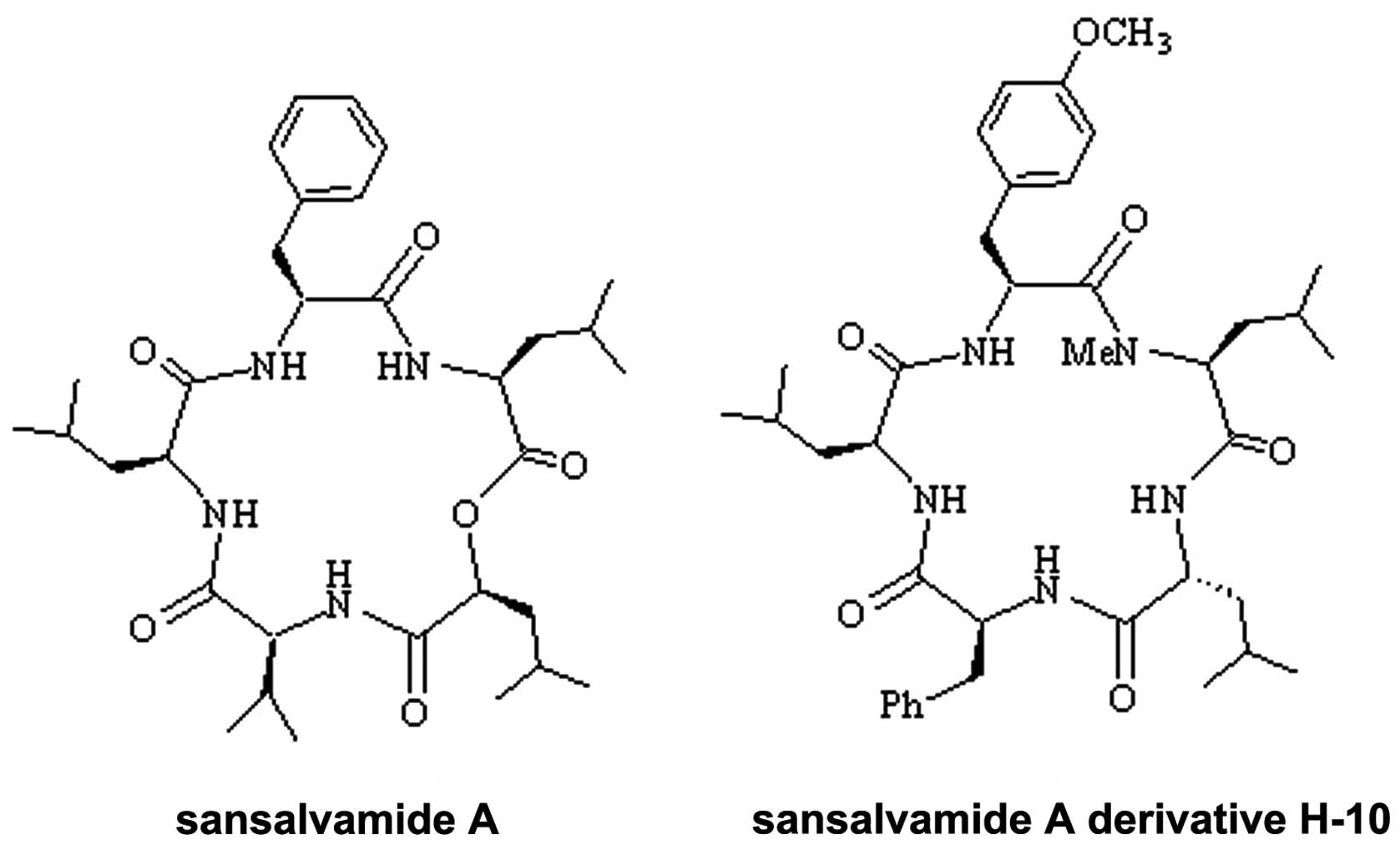
present study, a novel sansalvamide A derivative, H-10, a cyclic
pentapeptide (molecular formula,
C38H55N5O6; molecular
weight, 677.8732; Fig. 1), was
investigated. Furthermore, this study focused on the effects of
H-10 on the growth and apotosis of rat malignant melanoma B16
cells. The results may provide a basis for future sutides of this
novel compound.
Materials and methods
Materials
RPMI 1640, trypsin-EDTA solution and fetal bovine
serum (FBS) were purchased from Gibco-BRL (Carlsbad, CA, USA). H-10
cells were provided by the Hebei Province Key Laboratory of
Molecular Chemistry for Drug (Shijiazhuang, China). Sulforhodamine
B (SRB) was purchased from Tokyo Chemical Industry Co., Ltd
(Tokoyo, Japan), and the bicinchoninic acid (BCA) kit was purchased
from Shanghai Generay Biotechnology Co., Ltd. (Shanghai, China).
The polyvinylidene fluoride (PVDF) membranes were purchased from
Shanghai Generay Biotech Co., Ltd. The antibody against GAPDH
(polyclonal rabbit anti-mouse) was purchased from Hangzhou Goodhere
Biotechnology Co., Ltd. (Hangzhou, China). The antibodies against
caspase-8, -9 and -3 (all polyclonal rabbit anti-mouse) were
purchased from Bioworld Technology, Inc. (Minneapolis, MN, USA).
The secondary fluorescence antibody (polyclonal goat anti-rabbit)
was purchased from Nanjing Gene Biotech Co., Ltd. (Nanjing, China)
The B16 cell line was stored at the Research Center of the Fourth
Hospital of Hebei Medical University (Shijiazhuang, China).
Cell culture
The cells were cultured in RPMI 1640 medium with 10%
heat-inactivated FBS and 100 μg/ml penicillin and streptomycin. The
cell line was grown in 25-cm2 flasks in a humidified
atmosphere of 5% CO2 at 37°C, and the media were changed
every second or third day. At 80–90% confluence, the cells were
digested with trypsin-EDTA and plated in 25-cm2 flasks
with media changes every second or third day, on 24- or 96-well
plates.
Concentration-dependent effect of H-10 on
B16 cell growth inhibition
H-10 was dissolved in dimethyl sulfoxide (DMSO) and
diluted with serum-free medium to prepare solutions of 1,000, 500,
100, 10 and 1 μM. Single cell suspensions of B16 cells were
prepared and adjusted to the indicated concentration. The cells
were then inoculated in 96-well plates (90 μl per well), with
~2,000 cells/well. Following 4 h of cell adherence, 10 μl H-10 was
added to each well to form final concentrations of 100, 50, 10, 1
and 0.1 μM. Each group was placed into three wells, while a 1% DMSO
group was simultaneously prepared as the control. The SRB
colorimetric method was used to calculate the percentage growth of
the B16 cells treated with the various concentrations of H-10 for
48 h.
SRB colorimetric method
Following treatment with H-10 for 48 h, the cells
were fixed with trichloroacetic acid (TCA) and the intracellular
protein was stained with SRB. A total of 100 μl Tris base was then
added to each well, and the dissolved SRB was detected using a
microplate reader (Thermo Fisher Scientific, Vienna, Austria),
whereby the values indirectly reflected the numbers of living
cells. The medium in the 96-well plates was discarded and 100 μl
TCA was added at a temperature of 4°C for 30 min. Next, the TCA was
discarded and the cells were washed three times in distilled water,
prior to being dried at room temperature for 1 h. A total of 100 μl
0.4% SRB was then added and the cells were agitated for 20 min.
Next, the dye solution was discarded, and the cells were washed
three times with 1% acetic acid and dried at room temperature for
>6 h. Finally, 100 μl Tris base was added and cells were
agitated for 5 min. The optical density was recorded at a
wavelength of 490 nm using a microplate reader.
Time-dependent effect of H-10 on B16 cell
growth inhibition
At 80–90% confluence, the cells were harvested with
trypsin, and serum-free medium was used to produce a single-cell
suspension. The cells were then seeded in 24-well plates at the
concentration of 20,000 cells/well. After 24 h, the wells were
replaced with fresh medium, including FBS. Next, the wells were
treated with 50 μM H-10 and the cell numbers were counted following
24, 48, 72, 96, 120 and 144 h. A control group was prepared
simultaneously and a growth curve was generated.
Flow cytometric analysis of apoptotic
cell death
At 80–90% confluence, the cells were treated with 50
μM H-10 for 24 h, while a control group was prepared. The treated
and untreated cells were then harvested, washed with
phosphate-buffered saline (PBS) and fixed with 70% ethanol for 24
h. Next, the cells were centrifuged at 300 × g for 5 min and the
pellet was resuspended in PBS containing 50 μg/ml propidium iodide
and 10 μg/ml RNase A. The cells with <2N DNA content were
classified as apoptotic cells.
Detection of caspase-8, -9 and -3
expression by western blot analysis
The cells at 80–90% confluence were treated with 10,
30 and 50 μM H-10 for 24 h, and a control group was prepared. The
cellular protein was extracted by radioimmunoprecipitation assay
lysis buffer and the concentration was measured using the BCA kit.
A total of 50 μg protein was electrophoretically separated on a 10%
polyacrylamide gel. The proteins were then transferred to a PVDF
membrane under the conditions of 90 V and 200 mA for 60 min. Next,
the membranes were incubated in antibody dilution solution (rabbit
anti-mouse caspase-3, -8, -9 and GAPDH; 1:500) overnight at 4°C.
The blots were then incubated with the secondary fluorescence
antibody (1:5,000) for 2 h in the dark, and the results were
detected using the Odyssey infrared imager (LI-COR, Inc., Lincoln,
NE, USA).
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard error of the mean and were
analyzed by t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
H-10 exhibits a concentration-dependent
effect on B16 cell growth
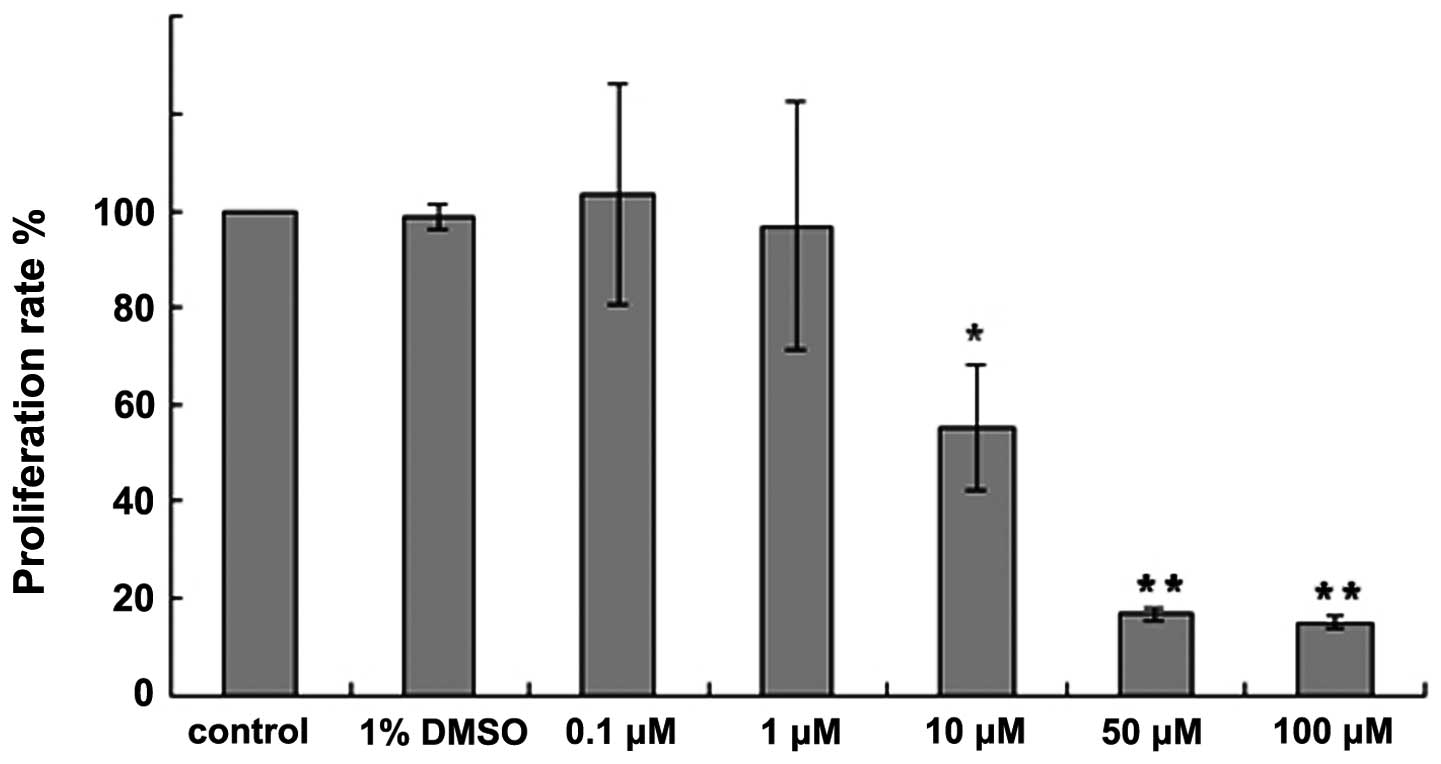
Compared with the control group, no significant
difference was identified in the proliferation rate of the 1% DMSO
group (P>0.05). Following the treatment of the B16 cells with
the various concentrations of H-10 (0.1, 1, 10, 50 and 100 μM) for
48 h, the proliferation rate of the B16 cells was found to
gradually decrease. Furthermore, compared with control group, the
proliferation rate of the B16 cells treated with 100, 50 and 10 μM
H-10 was found to significantly decrease (P<0.01 for 100 μM;
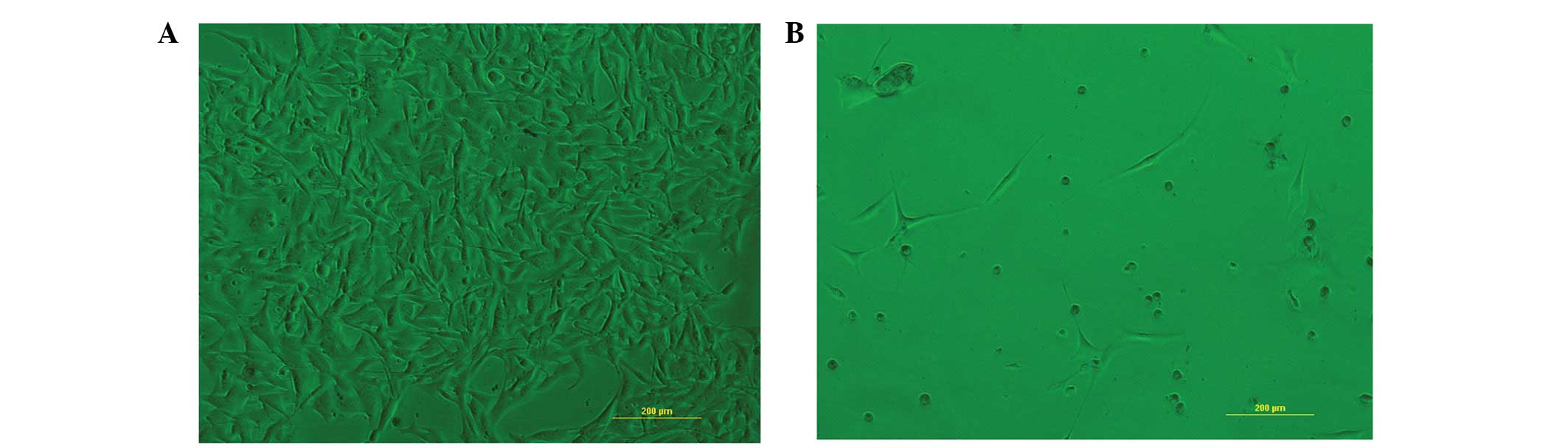
P<0.01 for 50 μM; and P<0.05 for 10 μM; Fig. 2). The morphological changes were
observed under light microscopy (Fig.
3). B16 cells treated with 50 μM H-10 for 48 h exhibited marked
morphological changes, including decreased cell density, separation
of the adjacent cells, rounding of the cells and cell
shrinkage.
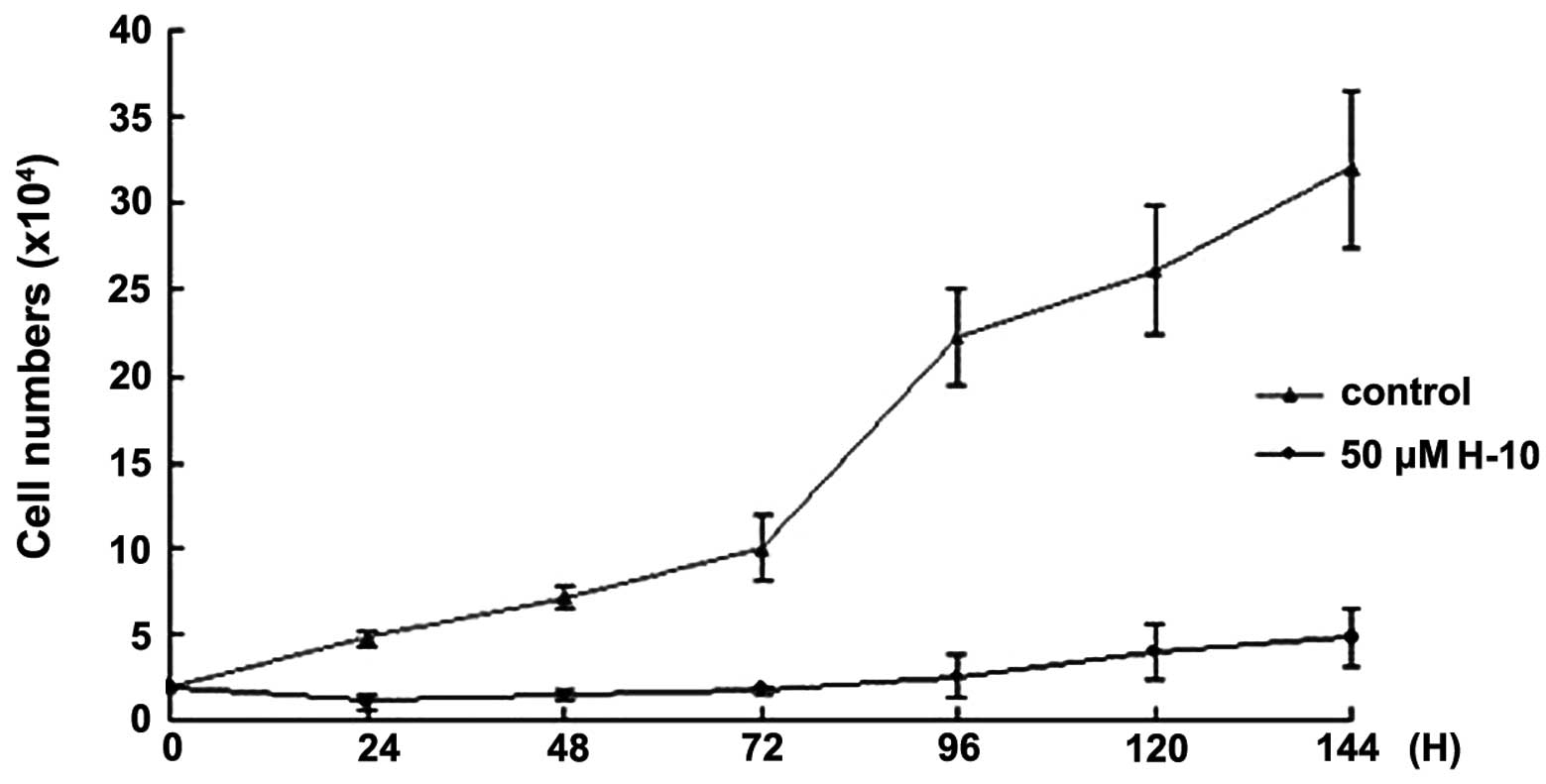
H-10 exhibits a time-dependent effect on
B16 cell growth
The time-dependent effect of H-10 on cell
proliferation was measured by cell number. Following treatment with
50 μM H-10 for 24, 48, 72, 96, 120 and 144 h, the B16 cell numbers
were counted and compared with the control group. The results
indicated that H-10 inhibited the growth of the B16 cell line in a
time-dependent manner (Fig. 4).
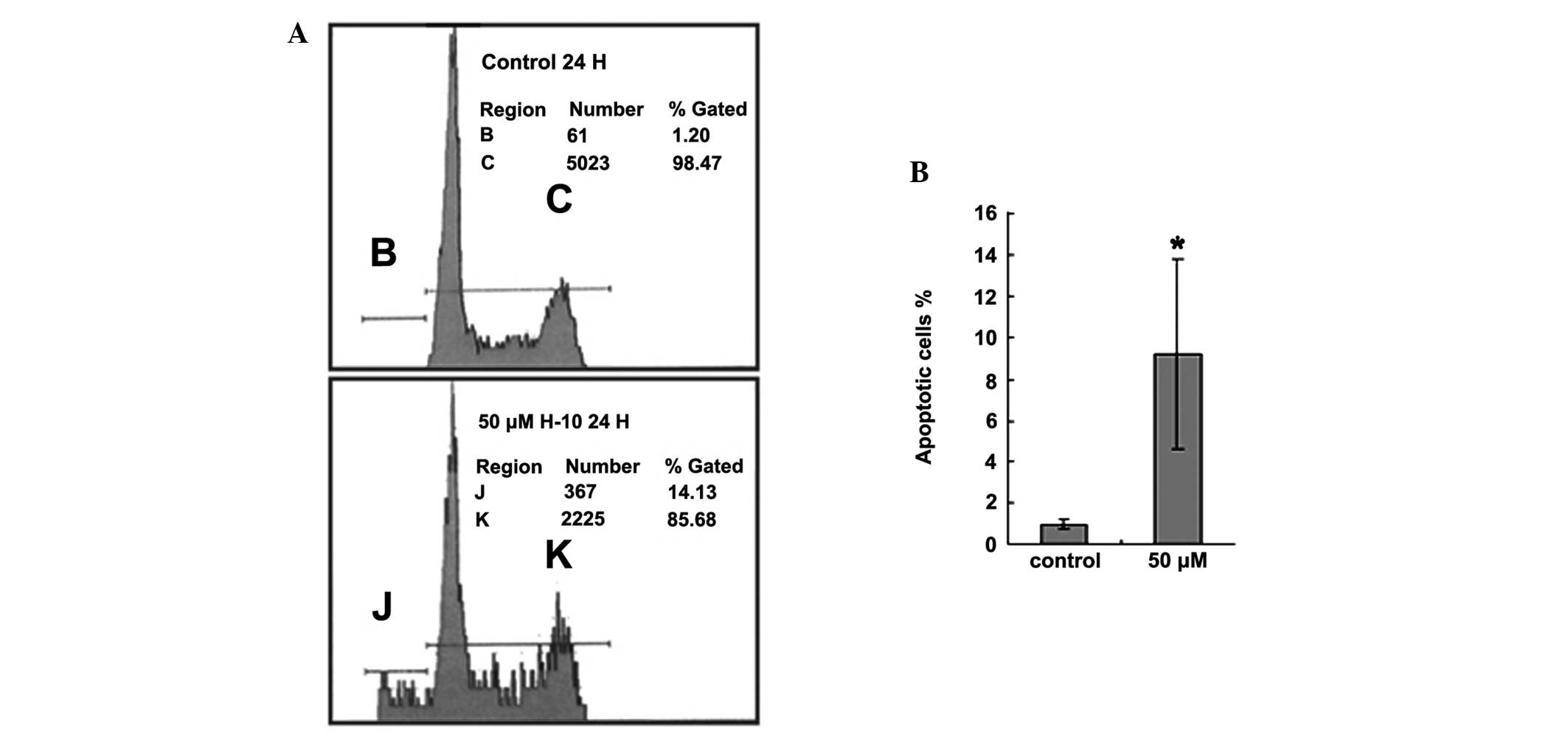
H-10 induces the apoptosis of B16
cells
Flow cytometry analysis of the cell samples
demonstrated the ability of H-10 to induce apoptosis. Apoptotic
cells were defined as those with subdiploid DNA content and were
presented as the percentage of all counted cells per sample. The
proportion of apoptotic cells in the untreated B16 cell group was
0.96±0.22%. However, in the group treated with 50 μM H-10 for 24 h,
the percentage of apoptotic cells was 9.21±4.62% and this
difference was found to be statistically significant (P<0.05;
Fig. 5).
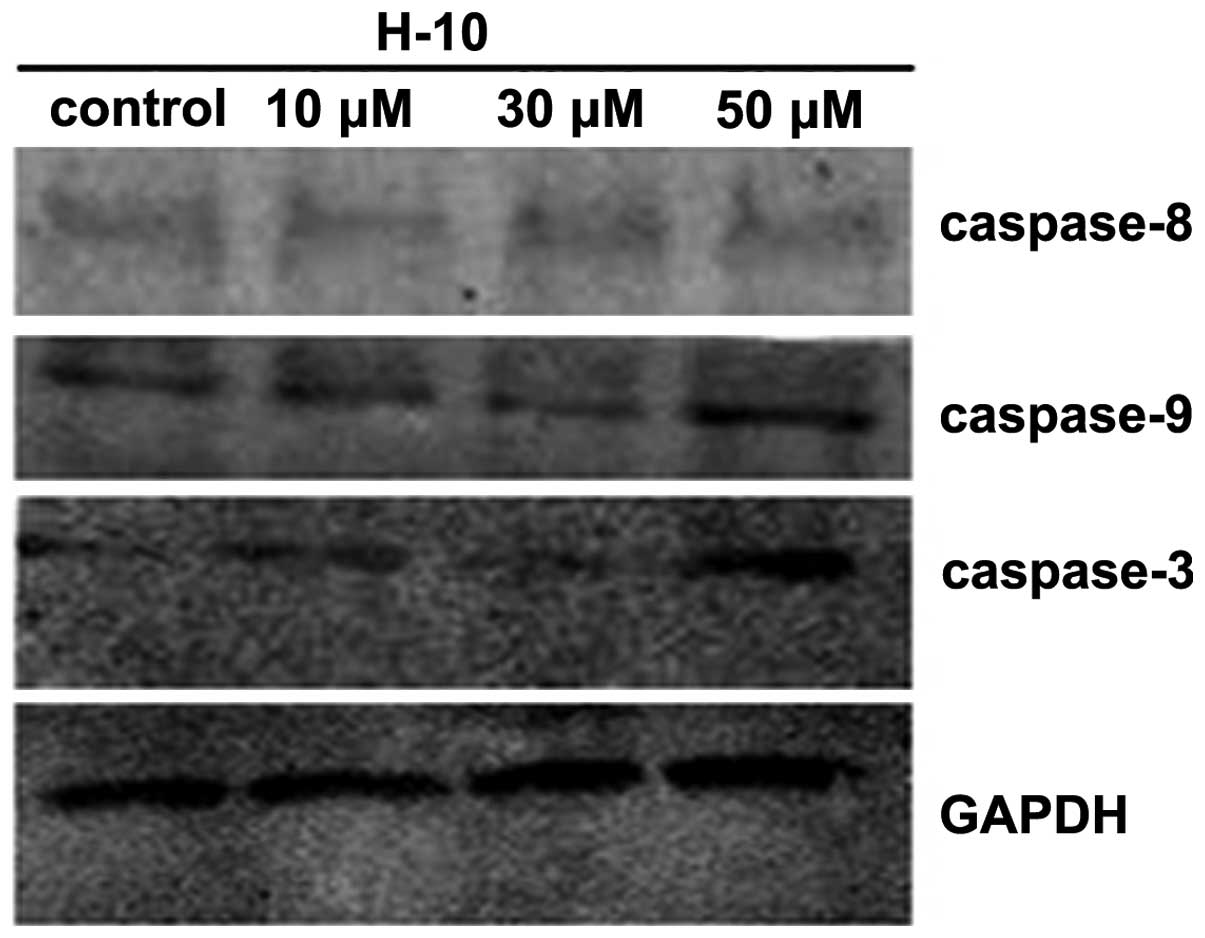
Caspase-3 plays a key role in cell apoptosis and is
significant in its initiation. In the present study, the expression
of caspase-3 was analyzed by western blot analysis, which further
confirmed that H-10 induced apoptosis. Following treatment with 10,
30 and 50 μM H-10, the expression of caspase-3 in the B16 cells was
found to increase with the concentration (Fig. 6).
H-10 induces apoptosis of B16 cells via a
mitochondrial pathway
The results of the western blot analysis revealed an
ascendant trend in the expression of caspase-3 and -9. However, no
significant difference was identified in the expression of
caspase-8 among the control and test groups (Fig. 6). These results indicated that H-10
induces the apoptosis of B16 cells via a mitochondrial pathway.
Discussion
Malignant melanoma is a cancer with an increasing
incidence, high malignancy and poor prognosis. This cancer is
characterized by its strong resistance and high metastasis and
mortality rates. At present, no effective methods or drugs have
been identified for treatment, and thus, novel methods are
desperately required. Sansalvamide A has been found to exhibit
marked antitumor effects by the National Cancer Institute’s 60
cancer cell line panel (1).
Following numerous years of study, a variety of sansalvamide A
derivatives have been synthesized and demonstrated to exhibit
evident antitumor activity and improved stability. Compared with
the linear peptide, sansalvamide and its derivatives cyclic
structures may resist attack by exopeptidases and exhibit increased
stability (4–6). Furthermore, sansalvamide A and its
analogues are lipophilic, and thus exhibit rapid membrane
absorption (7). Due to a specific
cyclic peptide structure, the bioactive mechanism of sansalvamide A
and its derivatives is extremely complicated. Under the action of
different enzymes, the products of cyclic peptides are more complex
than linear peptides. However, the amino acid sequences are
different, and thus, the cyclic peptide is converted into various
linear peptides, which may perform different functions. Compared
with normal cells, malignant tumor cells exhibit increased
reproductive activity and more complex enzyme and ligand-receptor
signal transduction systems to maintain their specific biological
behavior, which present as potential targets for sansalvamide A and
its derivatives (8). Further study
is required to explain the effects of these compounds on the
specific signaling pathways.
Malignant melanoma cells exhibit enhanced survival
and proliferation capabilities. One of the most important reasons
for this is their antiapoptosis ability, which is the predominant
problem for clinical chemotherapy drug tolerance. Therefore, the
identification of an effective drug has become the focus of
melanoma treatment. The results of the present study revealed that
the proliferation rate of B16 cells is significantly inhibited by
various concentrations of H-10 (0.1, 1, 10, 50 and 100 μM).
Following the treatment of the B16 cells with 50 μM H-10 for 48 h,
the cell proliferation rate was only 16.7%. In addition, the
time-dependent test confirmed that H-10 exhibited a long-lasting
suppressive effect on the B16 cell line.
Caspase-3 is the key enzyme in the execution of
apoptosis, playing a significant role in the process. The
initiation of cell apoptosis predominantly occurs via two routes,
the exogenous death receptor and endogenous mitochondrial pathways
(9,10). The exogenous pathway is initiated by
death receptors, which then activate caspase-8. The endogenous
pathway is initiated by ultraviolet radiation, the disappearance of
growth factors or trophic factors, or various other stimulation
factors. These lead to the release of cytochrome c into the
cytoplasm from the mitochondria, and the subsequent activation of
caspase-9. Procaspase-3 is subsequently hydrolyzed and activated by
caspase-8 or -9. In the present study, the expression of caspase-3,
-8 and -9 was detected following treatment with the various
concentrations of H-10 (10, 30 and 50 μM), and the results of
western blot analysis revealed an ascendant trend in the expression
of caspase-3 and -9. However, no significant difference was
identified in caspase-8 expression among the control and test
groups. These results support the hypothesis that H-10 may inhibit
the growth of B16 cells via mitochondrial pathway-induced
apoptosis.
In conclusion, the results of the present study
indicate that H-10 may induce the apoptosis of B16 cells.
Considering the chemoresistance exhibited by melanoma towards
conventional chemotherapy drugs, this novel compound may provide
promising improvements in the therapeutic approach to melanoma
treatment.
Acknowledgements
The authors would like to express appreciation for
the financial assistance received from the National Basic Research
Program of China (grant nos. 2011CB512007 and 2012CB723501) and the
National Natural Science Foundation of China (grant no.
30873139).
References
|
1
|
Liu S, Gu W, Lo D, Ding XZ, Ujiki M,
Adrian TE, Soff GA and Silverman RB: N-methylsansalvamide a peptide
analogues. Potent new antitumor agents. J Med Chem. 48:3630–3638.
2005.
|
|
2
|
Belofsky GN, Jensen PR and Fenical W:
Sansalvamide: a new cytotoxic cyclic depsipeptide produced by a
marine fungus of the genus Fusarium. Tetrahedron Lett.
40:2913–2916. 1999.
|
|
3
|
Pan PS, Vasko RC, Lapera SA, Johnson VA,
Seller RP, Lin CC, Pan CM, Davis MR, Ardi VC and McAlpine SR: A
comprehensive study of Sansalvamide A derivatives: The
structure-activity relationships of 78 derivatives in two
pancreatic cancer cell lines. Bioorg Med Chem. 17:5806–5825.
2009.
|
|
4
|
Styers TJ, Kekec A, Rodriguez R, Brown JD,
Cajica J, Pan PS, Parry E, Carroll CL, Medina I, Corral R, et al:
Synthesis of Sansalvamide A derivatives and their cytotoxicity in
the MSS colon cancer cell line HT-29. Bioorg Med Chem.
14:5625–5631. 2006.
|
|
5
|
Rodriguez RA, Pan PS, Pan CM, Ravula S,
Lapera S, Singh EK, Styers TJ, Brown JD, Cajica J, Parry E, et al:
Synthesis of second-generation sansalvamide A derivatives: novel
templates as potential antitumor agents. J Org Chem. 72:1980–2002.
2007.
|
|
6
|
Otrubova K, Lushington G, Vander Velde D,
McGuire KL and McAlpine SR: Comprehensive study of sansalvamide A
derivatives and their structure-activity relationships against
drug-resistant colon cancer cell lines. J Med Chem. 51:530–544.
2008.
|
|
7
|
Ujiki MB, Milam B, Ding XZ, Roginsky AB,
Salabat MR, Talamonti MS, Bell RH, Gu W, Silverman RB and Adrian
TE: A novel peptide sansalvamide analogue inhibits pancreatic
cancer cell growth through G0/G1 cell-cycle arrest. Biochem Biophys
Res Commun. 340:1224–1228. 2006.
|
|
8
|
Kunicki JB, Petersen MN, Alexander LD, et
al: Synthesis and evaluation of biotinylated sansalvamide A analogs
and their modulation of Hsp90. Bioorg Med Chem Lett. 21:4716–4719.
2011.
|
|
9
|
Poter AG and Jänicke RU: Emerging roles of
caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
|
|
10
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas Path
of apoptosis. Cell. 94:491–501. 1998.
|