Introduction
Metastases to the regional lymph nodes are the most
common first clinical manifestation of disease dissemination
following the excision of the primary tumor or in unknown primary
melanomas (1). Clinically detected
nodal metastases are classified as one subgroup (macrometastases)
in stage III disease according to the American Joint Committee on
Cancer (AJCC) classification version 7.0 (2009) (2,3).
However, it is a generally heterogeneous group of patients in terms
of prognosis following surgical therapy (2), without the possibility of
discrimination between patients with an aggressive and more
indolent course of disease based on classical pathological
features. The requirement for a fresh characterization of this
group of patients is clear to determine novel and reliable
prognostic and predictive factors, which may lead to individualized
therapeutic approaches.
The past decade has observed significant advances in
the understanding of the genetic changes that drive melanoma cells.
In addition, there is increasing evidence that melanoma is a
genetically complex disease which arises from the accumulation of
genetic abnormalities within melanocytes. The constitutive
hyperactivation of the RAS/RAF/MEK/ERK pathway has been identified
in the majority of melanomas as the critical player in the
regulation of cell proliferation, invasion and survival (4–8). This
genetic background is commonly achieved via oncogenic mutations in
the following two genes: v-raf murine sarcoma viral oncogene
homolog B1 (BRAF); or neuroblastoma RAS viral (v-ras)
oncogene homolog (NRAS). The occurrence of these activated
mutants is mutually exclusive (7),
suggesting functional redundancy. The reported frequency of
BRAF mutations varies between 40 and 70% in cutaneous
melanoma (5,6,9) and
these are most frequently detected in tumors occurring in skin that
is not chronically damaged by the sun (6). To date, >50 distinct mutations in
BRAF have been identified, however, ~90% of BRAF
mutants in melanoma are single-base transitions (T>A) at
position 1,799, leading to the substitution of glutamic acid for
valine at codon 600 of the BRAF protein (p.V600E) (5,10,11),
which leads to a 500-fold increase in its kinase activity. The
second most common mutation is p.V600K (16–20% of all BRAF
mutations), followed by p.V600D/p.V600R (12,13).
Mutated BRAF is important for melanogenesis, however,
BRAF p.V600E is not sufficient for the malignant
transformation of melanocytes (14)
and is an early oncogenic event also found at a high frequency in
benign nevi (15).
NRAS mutations are present in 15–30% of
melanomas of the skin (16,17), with codon 61 most commonly altered.
Although it has been demonstrated in experimental models that a
mutation in NRAS is capable of inducing melanoma in
Cdkn2a-deficient mice (4), NRAS
mutations occur in the congenital nevi at a similar frequency to
melanoma (18,19). Mutations of the two oncogenes
(BRAF and NRAS) have a well established and powerful
predictive role as validated targets in recently developed
molecular targeted therapy for melanoma. BRAF inhibitors, such as
vemurafenib and dabrafenib, demonstrate clinical benefit in
melanomas harboring the BRAF p.V600E mutation and MEK
inhibitors act in the presence of BRAF and NRAS
mutations (20,21). However, the prognostic role of
mutations in these genes requires further confirmation. One study
(12) has indicated that the
presence of a BRAF mutation markedly correlates with
inferior survival in a metastatic setting, however, this finding
was not paralleled by differences in disease-free survival (DFS)
from the time of the primary melanoma diagnosis. An additional
study has also implied that the presence of NRAS mutations
has a negative influence on survival in stage IV melanoma patients
(22). Such results for stage III
melanoma are contradictory or lacking, and assessment of the
prognosis for patients with regional nodal metastases continues to
depend on basic pathological features.
The aim of the current study was to determine the
BRAF and NRAS mutational status of nodal metastases
in a large homogeneous group of cutaneous melanoma patients (BRAF
inhibitor-naive patients with clinically detected regional nodal
metastases), and to correlate those results with the clinical data
and patient survival.
Materials and methods
Patient characteristics
Patients were considered eligible for the study if
they had been diagnosed with clinical stage III cutaneous melanoma
(stage IIIB according to the AJCC 2010 classification) (3), available tumor tissue and undergone
radical lymphadenectomy (LND) at the Department of Soft Tissue/Bone
Sarcoma and Melanoma at the Maria Sklodowska-Curie Memorial Cancer
Center and Institute of Oncology (CCIO; Warsaw, Poland) between May
1995 and November 2010, following pathological confirmation of
palpable regional nodal metastases without distant metastases.
Formalin-fixed, paraffin-embedded (FFPE) tumor samples (of melanoma
lymph node metastases exclusively) from the CCIO pathological
archives were selected for the study. The clinicopathological stage
of the patients was determined by pathological evaluation of the
primary lesion and dissected lymph nodes, as well as by physical
and routine imaging examination. There was access to the complete
clinical data, including the dates of the primary tumor excision,
LDN, disease relapse, final follow-up or mortality, for all
patients. The patient characteristics are summarized in Table I. Radical LNDs (axillary or
inguinal) were performed according to the technique described by
Karakousis (23). For the
ilioinguinal LND, the superficial and deep levels below the
inguinal ligament to the level of the aortic bifurcation combined
with obturator LND were routinely excised. The patients were not
treated with BRAF or MEK inhibitors. In accordance with the EORTC
18952 trial, 68 patients received interferon-α2b and 58 received
radiotherapy as adjuvant treatment following the LND [with no
significant influence on overall survival (OS) data as reported
previously] (24,25). Of the 324 consecutive patients who
underwent LND during the analyzed period of time, 250 cases with
sufficient data and pathological material were eligible for the
study.
 | Table IComparison between the patient
characteristics of BRAF-mutant and -wild-type clinical stage
III melanoma. |
Table I
Comparison between the patient
characteristics of BRAF-mutant and -wild-type clinical stage
III melanoma.
| Patient
characteristics | n (%)(n=250) | BRAF-mutant,
n (%) (n=154) | BRAF
wild-type, n (%) (n=96) | P-value |
|---|
| Median age,
years | 54 | 52 | 60 | P<0.005 |
| Age, years |
| 0–40 | 44 (17.6) | 26 (16.9) | 18 (18.8) | P=0.008 |
| >40–60 | 118 (47.2) | 84 (54.5) | 34 (35.4) | |
| >60 | 88 (35.2) | 44 (28.6) | 44 (45.8) | |
| Gender |
| Female | 128 (51.2) | 76 (49.4) | 52 (54.2) | N.S. |
| Male | 122 (48.8) | 78 (50.6) | 44 (45.8) | |
| Primary tumor
site |
| Upper
extremity | 26 (10.4) | 14 (9.1) | 12 (12.5) | N.S. |
| Lower
extremity | 97 (38.8) | 57 (37.0) | 40 (41.7) | |
| Trunk | 92 (36.8) | 58 (37.7) | 34 (35.4) | |
| Unknown
primary | 35 (14.0) | 25 (16.2) | 10 (10.4) | |
| Lymph nodal
basin |
| Axillary | 122 (48.8) | 78 (50.6) | 44 (45.8) | N.S. |
| Inguinal | 128 (51.2) | 76 (49.4) | 52 (55.2) | |
| Primary melanoma
Breslow thickness, mm |
| ≤1.00 | 8 (4.2) | 6 (5.3) | 2 (2.6) | N.S. |
| 1.01–2.00 | 34 (17.8) | 24 (21.2) | 10 (12.8) | |
| 2.01–4.00 | 60 (31.4) | 37 (32.7) | 23 (29.5) | |
| >4.00 | 89 (17.8) | 46 (40.8) | 43 (55.1) | |
| Data not
availablea | 59 | 41 | 18 | |
| Median primary
melanoma Breslow thickness, mm | 3.9 | 3.75 | 4.9 | N.S. |
| Ulceration of
primary melanoma |
| No | 69 (36.3) | 38 (33.9) | 31 (39.7) | N.S. |
| Yes | 121 (63.7) | 74 (66.1) | 47 (60.3) | |
| Data not
availablea | 60 | 42 | 18 | |
| Metastatic nodes,
n |
| 1 | 64 (25.6) | 40 (25.9) | 24 (25.0) | N.S. |
| 2–3 | 72 (28.8) | 46 (29.9) | 26 (27.1) | |
| ≥4 | 114 (45.6) | 68 (44.2) | 46 (47.9) | |
| Median | 3 | 3 | 3 | |
| Extracapsular
extension of nodal metastases |
| No | 114 (45.6) | 74 (48.1) | 40 (41.7) | N.S. |
| Yes | 136 (54.4) | 80 (51.9) | 56 (58.3) | |
The study was approved by the local bioethics
committee of Maria Sklodowska-Curie Memorial Cancer Center and
Institute of Oncology (no. 3/2012) according to the Good Clinical
Practice Guidelines. Patients provided written informed
consent.
The patients had not undergone any other preliminary
selection and only patients who met all the aforementioned
conditions were enrolled in the study. All patients were followed
closely with a median follow-up time of 53 months for survivors
(range, 4–186 months). Postoperative follow-up consisted of
physical examination and routine imaging investigations (chest
X-ray, ultrasound of the abdominal cavity and computed tomography
imaging, if metastases were suspected). Routinely, surveillance was
recommended every three months for the first two years, every four
months in year three, every six months for years four and five, and
annually thereafter.
Mutational testing
For the purpose of the study, all lymph node FFPE
samples of each patient were revived by a pathologist in order to
select blocks (one per patient) with the highest tumor content and
best possible material quality. Insufficient tumor content, massive
necrosis, blood spills or calcification, as well as no
amplification of DNA, were accounted for as the main excluding
criteria. In total, 250 paraffin blocks were selected; 220 samples
had a tumor content of >90%, and no samples had a tumor content
of <10%. Samples were excised from the whole block surface. The
genomic DNA was isolated using the Sherlock AX DNA kit (A&A
Biotechnology, Gdynia, Poland) and amplified in the following
standard polymerase chain reaction (PCR) conditions performed at a
final volume of 37.5 μl, containing 50 ng of genomic DNA, 1 unit of
Maxima Hot Start Taq DNA polymerase (Thermo Fisher Scientific,
Waltham, MA, USA), 0.2 mM of each dNTP, 0.2 M of each primer, 1.5
mM MgCl2 and 1× buffer. The amplification was performed
as follows: One cycle at 95°C for 4 min; 35 cycles at 94°C for 30
sec, 58°C for 30 sec and 72°C for 30 sec; and a final extension
step at 72°C for 7 min with primers designed in-house for
BRAF exons 11 and 15, and NRAS exons 1 and 2. The
products were bidirectly sequenced using the BigDye Terminator
Cycle sequencing kit and ABI Prism 3100 Genetic Analyzer (both
Applied Biosystems, Carlsbad, CA, USA). In order to identify
mutations, the sequences were then compared between BRAF
(GenBank ref.: NM_004333.4) and NRAS (GenBank ref.:
NM_002524.4).
Statistical analysis
All statistical analyses were performed using the R
2.15.1 statistical software (R Core Team, 2012; http://www.R-project.org). Contingency tables were
analyzed using the χ2 test. The non-parametric
Mann-Whitney U test was applied for comparisons of two groups with
a non-normal distribution.
For survival analysis, the Kaplan-Meier estimator
was used with log-rank tests for bivariate comparisons. OS time for
the assessment of the prognostic value of clinical, pathological
and molecular parameters was calculated from the date of the
primary tumor excision or LND, to the date of the most recent
follow-up (censored data) or mortality (as in the melanoma AJCC
staging system) (2,26). DFS was calculated from the date of
the therapeutic LND to the date of the most recent follow-up or
disease recurrence.
The following clinical, pathological and molecular
parameters were tested as potential factors affecting patient
survival: Gender, age (≤40, >40–60 and >60 years), primary
tumor Breslow thickness (≤1.00, 1.01–2.00, 2.01–4.00 and >4.00
mm), presence of ulceration of the primary lesion, primary tumor
level of invasion (II/III vs. IV/V), localization of LND (inguinal
vs. axillary), number of lymph nodes with metastases (1, 2–3 or
≥4), presence of extracapsular invasion in the involved lymph
nodes, BRAF status [BRAF-mutated vs. wild-type (WT);
and p.V600E mutation vs. other BRAF mutations and vs.
wild-type] and NRAS status (NRAS-mutated vs. WT).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Mutational status and correlation with
clinicopathological features
BRAF mutations were detected in 154 of 250
(61.6%) melanoma nodal metastases and were predominantly p.V600E
mutations (Table II). The
NRAS gene was altered in 42 (43.8%) of the BRAF-WT
samples. Mutations in BRAF and NRAS were mutually
exclusive and 54 samples did not harbor any.
 | Table IIOncogenic BRAF and NRAS
mutations within the study group. |
Table II
Oncogenic BRAF and NRAS
mutations within the study group.
| Mutation | n (%) | Exon |
|---|
| BRAF | 154 (61.6) | 15 |
| Codon 600 | 151 (98) | 15 |
|
p.V600Ea | 141 (91.6) | 15 |
| p.V600K | 9 (5.8) | 15 |
| p.V600D | 1 (0.7) | 15 |
| Other | 3 (2.0) | 15 |
| p.E586K | 1 (0.7) | 15 |
|
p.V600_K601delinsE | 1 (0.7) | 15 |
| p.G469E | 1 (0.7) | 11 |
| NRAS | 42 (16.8) | 2 |
| Codon 61 | 40 (95.2) | 2 |
|
p.Q61Rb | 25 (59.5) | 2 |
| p.Q61K | 11 (26.2) | 2 |
| p.Q61L | 3 (7.1) | 2 |
| p.Q61H | 1 (2.4) | 2 |
| Codon 13 | 2 (4.8) | 1 |
| p.G13D | 1 (2.4) | 1 |
| p.G13R | 1 (2.4) | 1 |
All ‘weak’ sequence peak (<30% of WT signal)
mutations were resequenced from the point of PCR reaction and 110
randomly selected samples were reanalyzed from the point of
paraffin block dissection, in an independently validated PCR-based
in-house diagnostic test, resulting in complete confirmation of the
results. Based on our recent study, we estimate that the
sensitivity of the approach, concerning V600E mutations, was
>98% (27).
Among the clinicopathological features (Table I), the presence of BRAF
mutations was found to correlate with a younger age of patients
(median age, 52 years for BRAF-mutated and 60 years for
BRAF-WT; P<0.01). The opposite correlation was observed
for NRAS-mutants versus NRAS-WT (median age, 61 years
for NRAS-mutants and 53 years for NRAS-WT; P=0.05;
data not shown).
Survival analysis
Detailed OS data (from the date of the primary tumor
excision and LND) are presented in Table IIIA and B. The five-year OS rates
for the entire group, calculated from the date of the primary tumor
excision and LND, were 43.6 and 32.6%, respectively, and the median
survival was 45.5 and 24.3 months, respectively. No correlation was
identified between BRAF mutational status and OS (calculated
from the date of the LND and primary tumor excision) and the
prognosis did not differ between BRAF-mutated (P=0.73) and
BRAF-WT (P=0.87) melanomas, however, a trend for an improved
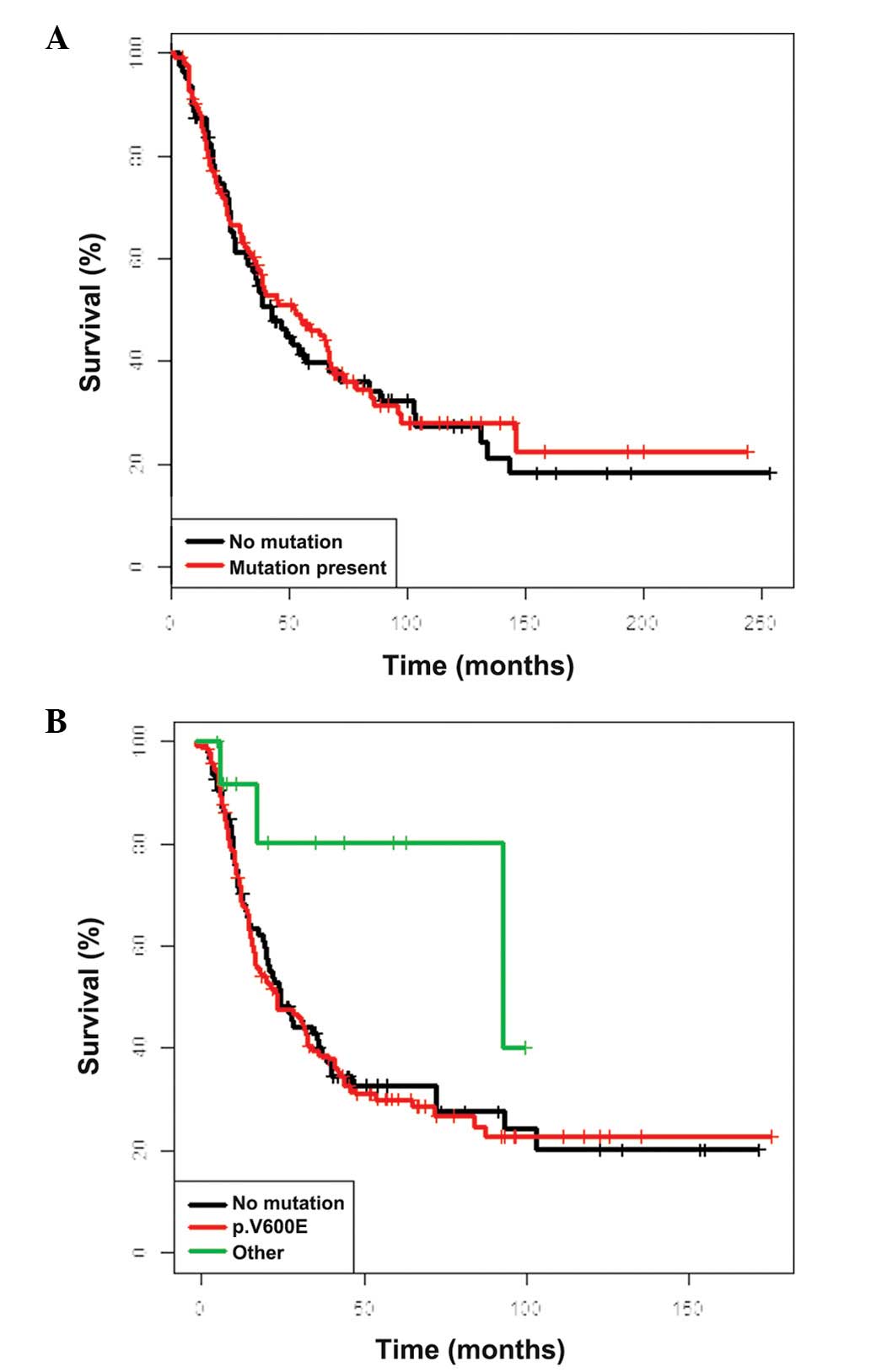
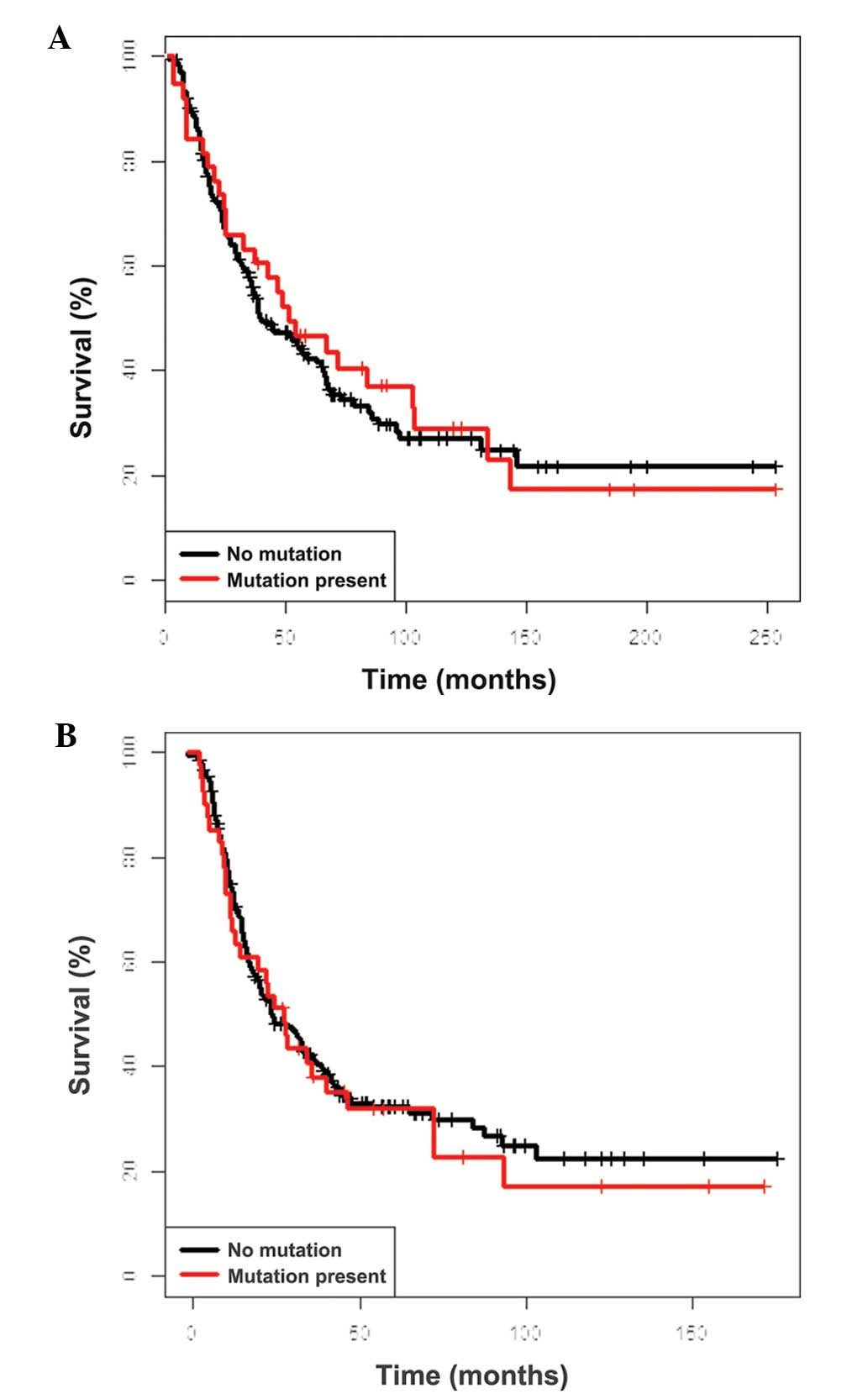
OS was identified for non-V600E mutants (Fig. 1). Similarly, NRAS mutational
status had no impact on survival (Fig.
2). The factors exhibiting a negative impact on OS were: Male
gender (P<0.001), >1 metastatic lymph node (P<0.01) and
extracapsular extension of nodal metastases (P<0.001).
 | Table IIIOverall survival according to the
molecular features of nodal metastases and significant features of
primary tumor and nodal metastases (calculated from the date of A,
the primary tumor excision and B, the lymph node dissection). |
Table III
Overall survival according to the
molecular features of nodal metastases and significant features of
primary tumor and nodal metastases (calculated from the date of A,
the primary tumor excision and B, the lymph node dissection).
| A, Primary tumor
excision |
|---|
|
|---|
| Features | Median survival
(95% CI) | Five-year OS rate
(95% CI) | P-value |
|---|
| Total group | 45.5
(36.9–65.5) | 43.6
(37.0–51.4) | |
| BRAF
status |
| Wild-type | 42.7 (32.9–72) | 39.8
(29.9–52.9) | 0.736 |
| Mutated | 53 (36.8–67.9) | 46.1
(37.7–56.4) | |
| BRAF codon
600 status |
| Wild-type | 42.7 (32.9–72) | 39.8
(29.9–52.9) | 0.098 |
| p.V600E
mutants | 40.2 (34–66.2) | 42.6
(34.1–53.3) | |
| Non-V600E
mutants | 96
(96.0–145.1) | 90.9
(75.4–100) | |
| NRAS
status |
| Wild-type | 40.2 (36–64.9) | 42.5
(35.1–51.4) | 0.710 |
| Mutated | 51.9
(32.9–134.1) | 46.8
(33.2–65.9) | |
| Ulceration of
melanoma |
| No | 67.4
(38.8–103.8) | 55.2
(42.8–71.1) | 0.08 |
| Yes | 38.6
(29.8–55.6) | 36.7
(28.4–47.4) | |
| Gender |
| Female | 66.8 (52–97.6) | 52.6
(43.8–63.1) | <0.001 |
| Male | 29.8
(23.6–44.8) | 32.3
(23.3–44.7) | |
| Age, years |
| ≤40 | 72
(42.8–102.3) | 57.6
(42.9–77.4) | 0.33 |
| <40–60 | 40.2
(29.8–66.2) | 41.1
(32.1–52.6) | |
| ≥60 | 39.3
(27.6–83.9) | 39.7
(28.9–54.6) | |
| Metastatic lymph
nodes, n |
| 1 | 64.9
(52.7–105.0) | 54.1
(42.0–69.8) | 0.01 |
| 2–3 | 54.3
(29.2–104) | 46.8
(35.7–61.3) | |
| ≥4 | 32.6 (24.8–47) | 33.0
(23.7–46.1) | |
| Extracapsular
extension of nodal metastases |
| No | 67.4
(54.3–102.5) | 55.3
(45.9–66.6) | <0.0001 |
| Yes | 32.6
(24.3–40.2) | 30.5
(22.2–41.8) | |
|
| B, Lymph node
dissection |
|
| Features | Median survival
(95% confidence interval) | Five-year OS rate
(95% confidence interval) | P-value |
|
| Total group | 24.3
(20.0–34.2) | 32.6
(26.8–39.7) | |
| BRAF
status |
| Wild-type | 24.4
(19.7–38.3) | 32.6
(23.7–44.9) | 0.867 |
| Mutated | 23.5
(16.8–38.8) | 32.9
(25.8–42.1) | |
| BRAF codon
600 status |
| Wild-type | 24.4
(19.7–38.3) | 32.6
(23.7–44.9) | 0.13 |
| p.V600E
mutants | 23.4
(16.4–32.7) | 30.0
(22.9–39.3) | |
| Non-V600E
mutants | 83 (82.0–93.0) | 80.2
(58.7–100) | |
| NRAS
status |
| Wild-type | 23.5
(19.7–36.1) | 32.3
(26.0–40.3) | 0.697 |
| Mutated | 27.4
(14.2–72.1) | 32.0
(20.1–50.9) | |
| Ulceration of
melanoma |
| No | 38.8
(27.5–93.0) | 37.6
(25.7–55.0) | 0.08 |
| Yes | 21.7
(16.4–31.2) | 27.2
(19.8–37.3) | |
| Gender |
| Female | 36.1
(28.1–64.8) | 41.0
(32.9–51.1) | <0.001 |
| Male | 16.8
(13.9–23.8) | 22.6
(15.3–33.4) | |
| Age, years |
| ≤40 | 32.4
(23.0–44.8) | 34.2
(21.9–53.4) | 0.59 |
| <40–60 | 23.4
(16.6–38.3) | 31.5
(23.8–41.6) | |
| ≥60 | 20.7
(14.6–40.9) | 34.9
(25.1–48.5) | |
| Metastatic lymph
nodes |
| 1 | 42 (31.2–75.3) | 37.2
(26.0–53.1) | 0.005 |
| 2–3 | 23 (14.5–72.1) | 40.0
(29.8–53.7) | |
| ≥4 | 19.2
(14.3–24.5) | 24.1
(16.6–34.9) | |
| Extracapsular
extension of nodal metastases |
| No | 40.9
(31.2–83.8) | 41.7
(33.0–52.5) | <0.0001 |
| Yes | 17.6
(14.2–23.4) | 23.4
(16.4–33.4) | |
The interval between the diagnosis of the initial
melanoma to regional nodal metastasis was not significantly
different between the BRAF-mutant and -WT patients (median,
10 months; P=0.29) or between NRAS-mutant and -WT patients
(median, 10 months; P=0.34).
The five-year DFS rate (from the date of the LND)
was 24.6% in the entire group [95% confidence interval (CI),
19.4–31.3] and the median DFS was 12.1 months (95% CI, 9.1–14.8).
Similar to the OS, no differences were identified in DFS in
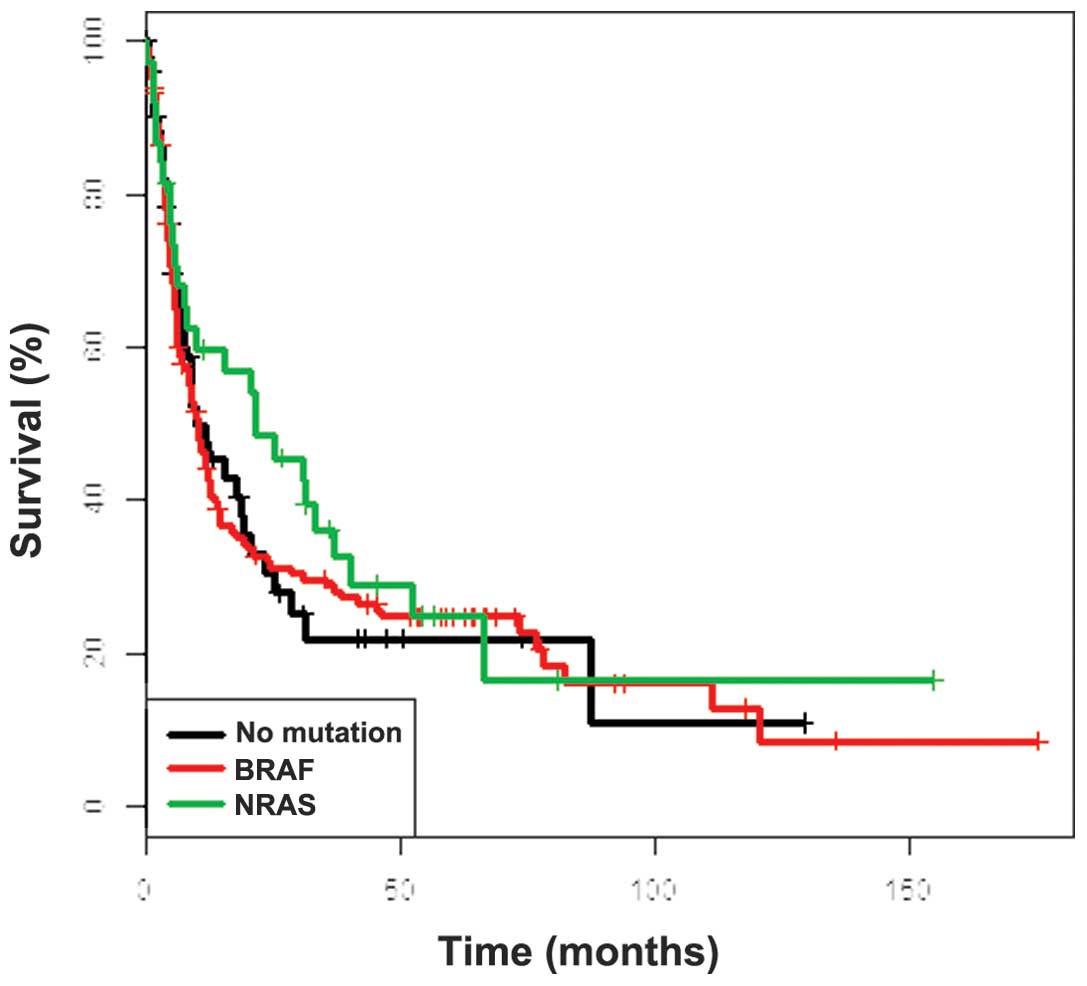
relation to BRAF and NRAS mutational status (Fig. 3), while the clinicopathological
factors exhibited similar prognostic significance (data not shown).
A total of 181 patients (72%) experienced disease relapse during
follow-up. In addition, 135 patients had distant metastases as the
first site of recurrent disease; the rates of disease relapse did
not differ between the BRAF-mutant and -WT patients (75 vs.
68%, respectively; P=0.36) or between the NRAS-mutant and
-WT patients (71 vs. 73%; P=1.00). In terms of distant metastases,
the first relapse site showed a trend towards the presence of brain
metastases in BRAF-mutated versus -WT patients (19 vs. 9%,
respectively).
Discussion
Employment of individualized therapeutic strategies
in the treatment of melanoma requires the identification of
reliable prognostic and predictive markers. The present study has
expanded the detailed molecular analysis of clinical stage III
melanoma by the characterization of BRAF and NRAS
mutations in a homogeneous group of patients with regional nodal
macrometastases. The distribution of BRAF and NRAS
mutations in this cohort was similar to that in previously reported
studies (particularly consistent with stage IV melanoma) with
mutually exclusive BRAF-mutants found in 62% and
NRAS-mutants in 17% of cases (5,12,16,28).
These findings confirm that these mutations are early oncogenic
events that remain stable throughout disease progression (19,29).
Direct sequencing (considered a gold standard in mutation
screening) was used as it is the most sensitive method for the
detection of rare and/or undefined mutations (30). The tissue material from metastatic
nodes was analyzed exclusively, which may be important in the
context of future adjuvant treatment.
The results of the current study confirmed former
clinical associations with tumor mutational status, but also
differed from the observations in stage IV melanoma concerning the
role of BRAF or NRAS mutations as a prognostic marker
following complete surgical resection of the metastatic regional
lymph nodes. The patients’ age at diagnosis of stage III melanoma
was significantly lower in tumors with BRAF mutations in
contrast to patients with NRAS mutations, who were on
average older. This is consistent with the observation that
BRAF-mutated melanomas more frequently affect younger
individuals with lower cumulative ultraviolet exposure (16,31,32).
Several studies have reported no influence of
BRAF or NRAS mutations on patient survival from the
time of diagnosis (12,16,33,34) in
earlier stages of the disease. However, the mutations may impact
survival in stage IV disease (12,22,35).
The only exception is the study by Moreau et al (36), which approached BRAF
mutational analysis in a heterogenous group of 105 stage III
cutaneous melanoma patients and showed a negative prognostic value
of BRAF mutations. This study had a significantly lower
number of cases compared with the cohort of the current study, as
well as a shorter follow-up and unusually poor survival
(particularly if the authors included the group of patients
following positive sentinel node biopsy). The present study showed
no difference in patient survival from the primary tumor diagnosis
and date of the LND, based on BRAF or NRAS mutational
status. This evidence highlights the role of different genes in
melanoma with regional and distant metastases. From the perspective
of planned trials with targeted drugs distributed in the adjuvant
setting, it is important to note that: i) The genetic abnormalities
analyzed in the present study have not altered the natural course
of the disease; and ii) the probability of mortality in this group
of patients, following conceivably curative surgery, without
effective adjuvant therapy may be >60%. The survival in this
group of patients is almost identical to that reported in the
largest cohort used for validation of the AJCC staging system
(3). It appears counter-intuitive
that positive BRAF status may be associated with a negative
prognosis, if the presence of BRAF mutations in melanoma
closely correlates with a younger age of patients, a
well-documented positive prognostic factor in stage I–III melanoma
(2,37–39).
For non-V600E BRAF-mutants, a trend was identified in the
current study for a further improved prognosis, which may be
associated with a different molecular pathogenesis of this subgroup
(40). There remains a requirement
for reliable molecular prognostic markers in melanoma (at least in
the high-risk stage III), however, in the current study, only
established clinicopathological features confirmed their prognostic
significance.
In conclusion, the present study represents the
largest and most comprehensive molecular evaluation of clinical
stage III melanoma undergoing radical LND. The BRAF and
NRAS genotype distribution in the nodal metastases of
cutaneous melanomas is identical to that observed in stage IV
melanoma, with BRAF p.V600E as the most frequent mutation
harbored by melanoma cell metastases in lymph nodes. It cannot be
confirmed that the BRAF and NRAS mutations are
associated with a more aggressive course of disease, as has been
observed in a series of patients with distant metastases (12,22).
In the current study, BRAF and NRAS mutational status
was not identified as a prognostic marker in stage III melanoma
patients with macroscopic nodal involvement, but had a neutral role
in terms of patient survival, which may be of importance for
potential adjuvant therapy. BRAF and NRAS status also
had no impact on the disease-free interval from the diagnosis of
the primary melanoma to nodal metastases.
Acknowledgements
The authors would like to thank Dr Daniel Rabczenko
for statistical advice and Dr Anna Wilczynska for proofreading the
manuscript. The study was supported by the Polish National Science
Centre (grant no. 2011/03/B/NZ5/04513). The preliminary study was
presented as a poster presentation during the Annual Meeting of the
American Society of Clinical Oncology (June 2012; Chicago, IL,
USA).
References
|
1
|
Leiter U, Meier F, Schittek B and Garbe C:
The natural course of cutaneous melanoma. J Surg Oncol. 86:172–178.
2004.
|
|
2
|
Balch CM, Gershenwald JE, Soong SJ, et al:
Multivariate analysis of prognostic factors among 2,313 patients
with stage III melanoma: comparison of nodal micrometastases versus
macrometastases. J Clin Oncol. 28:2452–2459. 2010.
|
|
3
|
Balch CM, Gershenwald JE, Soong SJ, et al:
Final Version of 2009 AJCC Melanoma Staging and Classification. J
Clin Oncol. 27:6199–6206. 2009.
|
|
4
|
Hocker TL, Singh MK and Tsao H: Melanoma
genetics and therapeutic approaches in the 21st century: moving
from the benchside to the bedside. J Invest Dermatol.
128:2575–2595. 2008.
|
|
5
|
Davies H, Bignell GR, Cox C, et al:
Mutations of the BRAF gene in human cancer. Nature. 417:949–954.
2002.
|
|
6
|
Curtin JA, Fridlyand J, Kageshita T, et
al: Distinct sets of genetic alterations in melanoma. N Engl J Med.
353:2135–2147. 2005.
|
|
7
|
Garrido MC and Bastian BC: KIT as a
therapeutic target in melanoma. J Invest Dermatol. 130:20–27.
2010.
|
|
8
|
Kumar R, Angelini S, Snellman E and
Hemminki K: BRAF mutations are common somatic events in melanocytic
nevi. J Invest Dermatol. 122:342–348. 2004.
|
|
9
|
Gorden A, Osman I, Gai W, et al: Analysis
of BRAF and N-RAS mutations in metastatic melanoma tissues. Cancer
Res. 63:3955–3957. 2003.
|
|
10
|
Satyamoorthy K, Li G and Gerrero MR:
Constitutive mitogen-activated protein kinase activation in
melanoma is mediated by both BRAF mutations and autocrine growth
factor stimulation. Cancer Res. 63:756–759. 2003.
|
|
11
|
Gray-Schopfer VC, da Rocha Dias S and
Marais R: The role of B-RAF in melanoma. Cancer Metastasis Rev.
24:165–183. 2005.
|
|
12
|
Long GV, Menzies AM, Nagrial AM, et al:
Prognostic and clinicopathologic associations of oncogenic BRAF in
metastatic melanoma. J Clin Oncol. 29:1239–1246. 2011.
|
|
13
|
Rubinstein JC, Sznol M, Pavlick AC, et al:
Incidence of the V600K mutation among melanoma patients with BRAF
mutations, and potential therapeutic response to the specific BRAF
inhibitor PLX4032. J Transl Med. 8:672010.
|
|
14
|
Michaloglou C, Vredeveld LC, Soengas MS,
et al: BRAFE600-associated senescence-like cell cycle arrest of
human naevi. Nature. 436:720–724. 2005.
|
|
15
|
Pollock PM, Harper UL, Hansen KS, et al:
High frequency of BRAF mutations in nevi. Nat Genet. 33:19–20.
2003.
|
|
16
|
Edlundh-Rose E, Egyházi S, Omholt K,
Mansson-Brahme E, Platz A, Hansson J and Lundeberg J: NRAS and BRAF
mutations in melanoma tumours in relation to clinical
characteristics: a study based on mutation screening by
pyrosequencing. Melanoma Res. 16:471–478. 2005.
|
|
17
|
Goel VK, Lazar AJ, Warneke CL, Redston MS
and Haluska FG: Examination of mutations in BRAF, NRAS, and PTEN in
primary cutaneous melanoma. J Invest Dermatol. 126:154–160.
2006.
|
|
18
|
Poynter JN, Elder JT, Fullen DR, et al:
BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma
Res. 16:267–273. 2006.
|
|
19
|
Omholt K, Platz A, Kanter L, Ringborg U
and Hansson J: NRAS and BRAF mutations arise early during melanoma
pathogenesis and are preserved throughout tumor progression. Clin
Cancer Res. 9:6483–6488. 2003.
|
|
20
|
Chapman PB, Hauschild A, Robert C, et al:
Improved survival with vemurafenib in melanoma with BRAF V600E
mutation. N Engl J Med. 364:2507–2516. 2011.
|
|
21
|
Hauschild A, Grob JJ, Demidov LV, et al:
Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre,
open-label, phase 3 randomised controlled trial. Lancet.
380:358–365. 2012.
|
|
22
|
Jakob JA, Bassett RL Jr, Ng CS, et al:
NRAS mutation status is an independent prognostic factor in
metastatic melanoma. Cancer. 118:4014–4023. 2012.
|
|
23
|
Karakousis CP: Therapeutic node
dissections in malignant melanoma. Semin Surg Oncol. 14:291–301.
1998.
|
|
24
|
Eggermont AMM, Suciu S, MacKie R, et al:
Post-surgery adjuvant therapy with intermediate doses of interferon
alfa 2b versus observation in patients with stage IIb/III melanoma
(EORTC 18952): randomized controlled trial. Lancet. 366:1189–1196.
2005.
|
|
25
|
Nowecki ZI, Rutkowski P and Michej W: The
survival benefit to patients with positive sentinel node melanoma
after completion lymph node dissection may be limited to the
subgroup with a primary lesion Breslow thickness greater than 1.0
and less than or equal to 4 mm (pT2-pT3). Ann Surg Oncol.
5:2223–2234. 2008.
|
|
26
|
Berd D, Mastrangelo MJ and Sato T:
Calculation of survival of patients with stage III melanoma. J Clin
Oncol. 23:94272005.
|
|
27
|
Gos A, Jurkowska M, Siedlecki JA, Michej
W, Wiater K, Switaj T, Kosela H and Rutkowski P: Comparison between
two widely used laboratory methods in BRAF V600 mutation
detection rate in FFPE clinical samples of stage III cutaneous
melanoma metastases to the lymph nodes. In: 17th
ECCO-38thESMO-32ndESTRO European Cancer Congress; 2013, Abstract
P465.
|
|
28
|
Lee JH, Choi JW and Kim YS: Frequencies of
BRAF and NRAS mutations are different in histological types and
sites of origin of cutaneous melanoma: a meta-analysis. Br J
Dermatol. 164:776–784. 2011.
|
|
29
|
Colombino M, Capone M, Lissia A, et al:
BRAF/NRAS Mutation Frequencies Among Primary Tumors and Metastases
in Patients With Melanoma. J Clin Oncol. 30:2522–2529. 2012.
|
|
30
|
Pont-Kingdon G, Gedge F,
Wooderchak-Donahue W, et al: Design and analytical validation of
clinical DNA sequencing assays. Arch Pathol Lab Med. 136:41–46.
2012.
|
|
31
|
Liu W, Kelly JW, Trivett M, et al:
Distinct clinical and pathological features are associated with the
BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest
Dermatol. 127:900–905. 2007.
|
|
32
|
Viros A, Fridlyand J, Bauer J,
Lasithiotakis K, Garbe C, Pinkel D and Bastian BC: Improving
melanoma classification by integrating genetic and morphologic
features. PLoS Med. 5:e1202008.
|
|
33
|
Ellerhorst JA, Greene VR, Ekmekcioglu S,
et al: Clinical Correlates of NRAS and BRAF Mutations in Primary
Human Melanoma. Clin Cancer Res. 17:229–235. 2011.
|
|
34
|
Akslen LA, Angelini S, Straume O, Bachmann
IM, Molven A, Hemminki K and Kumar R: BRAF and NRAS mutations are
frequent in nodular melanoma but are not associated with tumor cell
proliferation or patient survival. J Invest Dermatol. 125:312–317.
2005.
|
|
35
|
Houben R, Becker JC, Kappel A, et al:
Constitutive activation of the Ras-Raf signaling pathway in
metastatic melanoma is associated with poor prognosis. J Carcinog.
3:62004.
|
|
36
|
Moreau S, Saiag P, Aegerter P, et al:
Prognostic Value of BRAFV600 Mutations in Melanoma Patients After
Resection of Metastatic Lymph Nodes. Ann Surg Oncol. 19:4314–4321.
2012.
|
|
37
|
Rutkowski P, Nowecki ZI, Zdzienicki M, et
al: Cutaneous melanoma with nodal metastases in elderly people. Int
J Dermatol. 49:907–913. 2010.
|
|
38
|
Kretschmer L, Starz H, Thoms KM, et al:
Age as a key factor influencing metastasizing patterns and
disease-specific survival after sentinel lymph node biopsy for
cutaneous melanoma. Int J Cancer. 129:1435–1442. 2011.
|
|
39
|
Macdonald JB, Dueck AC, Gray RJ, Wasif N,
Swanson DL, Sekulic A and Pockaj BA: Malignant Melanoma in the
Elderly: Different Regional Disease and Poorer Prognosis. J Cancer.
2:538–543. 2011.
|
|
40
|
Menzies AM, Haydu LE, Visintin L, et al:
Distinguishing clinicopathologic features of patients with V600E
and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res.
18:3242–3249. 2012.
|