Introduction
As ovarian cancer cells are only superficially
invasive and primarily disseminate within the peritoneal cavity,
ovarian carcinoma biology differs from that of hematogenously
metastasizing tumors. However, as ovarian carcinoma is only
temporarily chemosensitive, presents with rapidly proliferating
tumors that compress the visceral organs and has a cure rate of
only 30%, the disease is lethal (1). Conventional treatments, including
surgery, chemotherapy and radiation, are basically ineffective, as
surgical procedures are limited by disease staging, chemotherapy
may increase the risk of side-effects and radiotherapy may cause
serious local tissue injury(2–6).
Therefore, the identification of a safe and effective therapy for
the treatment of ovarian carcinoma is required.
Photodynamic therapy (PDT) is a novel technology for
the treatment of tumors; it is a minimally invasive therapeutic
modality that has been demonstrated to be effective in several
types of cancer and non-oncological conditions (7). The cationic porphyrin,
5,10,15,20-tetra-(N-methyl-4-pyridyl) porphine (TMPyP4),
is a novel type of synthetic water-soluble photosensitizer.
TMPyP4 binds to and stabilizes G-quadruplexes and has
been revealed to form G-quadruplexes in the promoter or regulatory
regions of important oncogenes, including c-myc, c-myb, c-fos and
c-Abl (8), as well as in the
single-stranded G-rich overhangs of telomeres in vitro
(9). Additionally, it has been
reported that the nuclear-mitochondrial shuttling of telomerase
reverse transcriptase and mitochondrial dysfunction are involved in
the arrest of cell proliferation that is induced by the
G-quadruplex ligand, TMPyP4 (10). These observations indicate that the
G-quadruplex structure presents a potential therapeutic target in
tumor cells. However, the precise effect of TMPyP4-PDT
against ovarian carcinoma cells and the underlying molecular
mechanisms have not yet been established.
In the current study, the apoptotic effect of
TMPyP4-PDT on tumor cells and the expression levels of
minichromosome maintenance protein-2 (MCM2) and carbonic anhydrase
(CA)-IX were investigated by analyzing the apoptotic rate of the
human ovarian carcinoma A2780 cell line in vitro in order to
highlight the clinical significance of TMPyP4-PDT in the
treatment of ovarian carcinoma patients.
Materials and methods
Cell lines and reagents
The human ovarian carcinoma A2780 cell line was
obtained from the Cancer Center Laboratory of Shandong University
(Jinan, Shandong, China) and cultured in RPMI-1640 (HyClone, Logan,
UT, USA) with 10% fetal bovine serum (FBS; HyClone), containing 2
mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin
(Shanghai Solarbio Bioscience and Technology Co., Ltd., Shanghai,
China) in an atmosphere of 5% CO2 and 100% humidity at
37°C.
TMPyP4 was purchased from Calbiochem (San
Diego, CA, USA) and was stored with minimal exposure to light. In
addition, suspension cultures were exposed to a single laser at the
energy densities of 0, 3, 6 and 12 J/cm2 by a 800-mW
power and 630-nm wavelength semiconductor laser (B100, Zhengzhou
Zhongxing Medical Equipment Co., Ltd., Henan, China).
The primary antibodies against anti-human MCM2
(rabbit polyclonal), anti-human CA-IX (rabbit monoclonal) and
anti-human GAPDH (rabbit monoclonal), and horseradish peroxidase
(HRP)-conjugated goat anti-rabbit and anti-mouse IgG were purchased
from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Cell viability
The cell viability was assessed using the 2-(2-m-
ethoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4disulfopheny)-2H-tetrazolium,
monosodium salt cell counting kit-8 (CCK-8) assay (Bestbio
Biotechnology, Shanghai, China). Fresh cells were seeded in 96-well
flat-bottomed tissue culture plates (Corning Inc., Corning, NY,
USA) at a concentration of 4×103 cells/well with
complete culture medium and incubated for 24 h. Following two
washes with phosphate-buffered saline (PBS), the cells were
incubated in 100 μl culture medium containing 3, 6, 15, 30 or 60 μM
TMPyP4 for 4 h. Next, cells at each concentration were
exposed to the single laser at an energy density of 0, 3, 6 and 12
J/cm2, respectively. Following irradiation, the cells
were incubated in fresh medium for an additional 24 h at 37°C prior
to the CCK-8 assay. A total of 10 μl CCK-8 and 100 μl RPMI-1640
culture medium was then added to each well, and following
incubation for 1 h at 37°C, the optical densities of the samples
were measured directly using a spectrophotometric microplate reader
(Beyotime Institute of Biotechnology, Haimen, China) at a
wavelength of 450 nm. Each experiment was performed in triplicate
and repeated five times.
Morphological observations
Following treatment with TMPyP4 for 4 h,
the cells were incubated for an additional 24 h prior to
morphological analysis of the cells observed under an inverted
microscope (Olympus, Tokyo, Japan). The laser energy density was
set at 6 J/cm2 and the cells were stained using a
hematoxylin and eosin staining kit (Beyotime Institute of
Biotechnology).
Analysis of apoptotic cells
The apoptotic cells were identified using the
Annexin V-fluorescein isothiocyanate apoptosis detection kit
(Bestbio Biotechnology). The cells were then incubated for 24 h
following irradiation and three washes with PBS. The cell
concentration was then adjusted to 5×105 cells/ml. Next,
the apoptotic cells were analyzed using FACSCalibur (BD
Biosciences, San Jose, CA, USA), and the data was analyzed using
FlowJo 7.6.1 software (TreeStar, Inc., Ashland, OR, USA).
Western blot analysis
Following treatment with 3, 6 or 15 μM
TMPyP4 for 4 h and laser treatment, the cells were
incubated for an additional 48 h prior to the collection cells for
protein extraction. The examination of the expression levels of
MCM2 and CA-IX was then performed separately. The laser energy
density was set at 6 J/cm2 and total protein was
extracted with the radioimmunoprecipitation assay reagent in the
presence of phosphatase protease inhibitors (Beyotime Institute of
Biotechnology) and the bicinchoninic acid assay kit (Beyotime
Institute of Biotechnology) was used to measure the protein
concentration. The protein (50 μg) was separated by SDS-PAGE and
transferred onto a polyvinylidene fluoride membrane using wet
transfer apparatus (Bio-Rad, Hercules, CA, USA). The membranes were
then blocked with 5% skimmed milk and incubated overnight at 4°C
with the primary antibodies, followed by incubation with the
secondary antibodies labeled with HRP. Next, the protein bands were
visualized using an enhanced chemiluminescence kit (Millipore,
Billerica, MA, USA) and the protein levels were detected using the
chemiluminescence reader, ImageQuant™ LAS4000 (GE Healthcare,
Pittsburgh, PA, USA), and analyzed by ImageJ software (US National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Student’s two-tailed t-test was used to determine the statistical
differences between the treatment and control groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of TMPyP4-PDT on cell
growth
The results showed that treatment with PDT alone at
various laser energy densities did not significantly inhibit the
growth of the A2780 cells (P>0.05). However, treatment with
TMPyP4 alone at doses of 3, 6, 15, 30 or 60 μM
significantly inhibited the growth of the cells (P<0.05) in a
dose-dependent manner. Furthermore, treatment with
TMPyP4-PDT at doses of 3, 6, 15, 30 or 60 μM also
significantly inhibited the growth of the cells, and these
inhibitory effects were observed to be in a laser energy- and
dose-dependent manner (P<0.01 and P<0.01, respectively)
(Fig. 1).
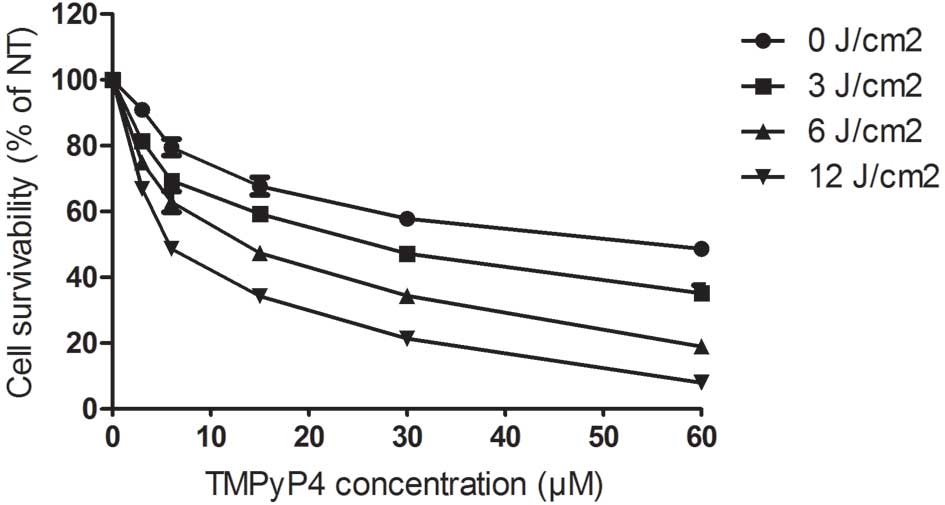 | Figure 1Effects of TMPyP4-PDT on
the cell growth of A2780 cells. In the TMPyP4-PDT group,
the cells were incubated with 3, 6, 15, 30 or 60 μM
TMPyP4 and then exposed to single laser energy at
densities of 0, 3, 6 and 12 J/cm2 with a semiconductor
laser. TMPyP4,
5,10,15,20-tetra-(N-methyl-4-pyridyl)porphine; PDT, photodynamic
therapy. |
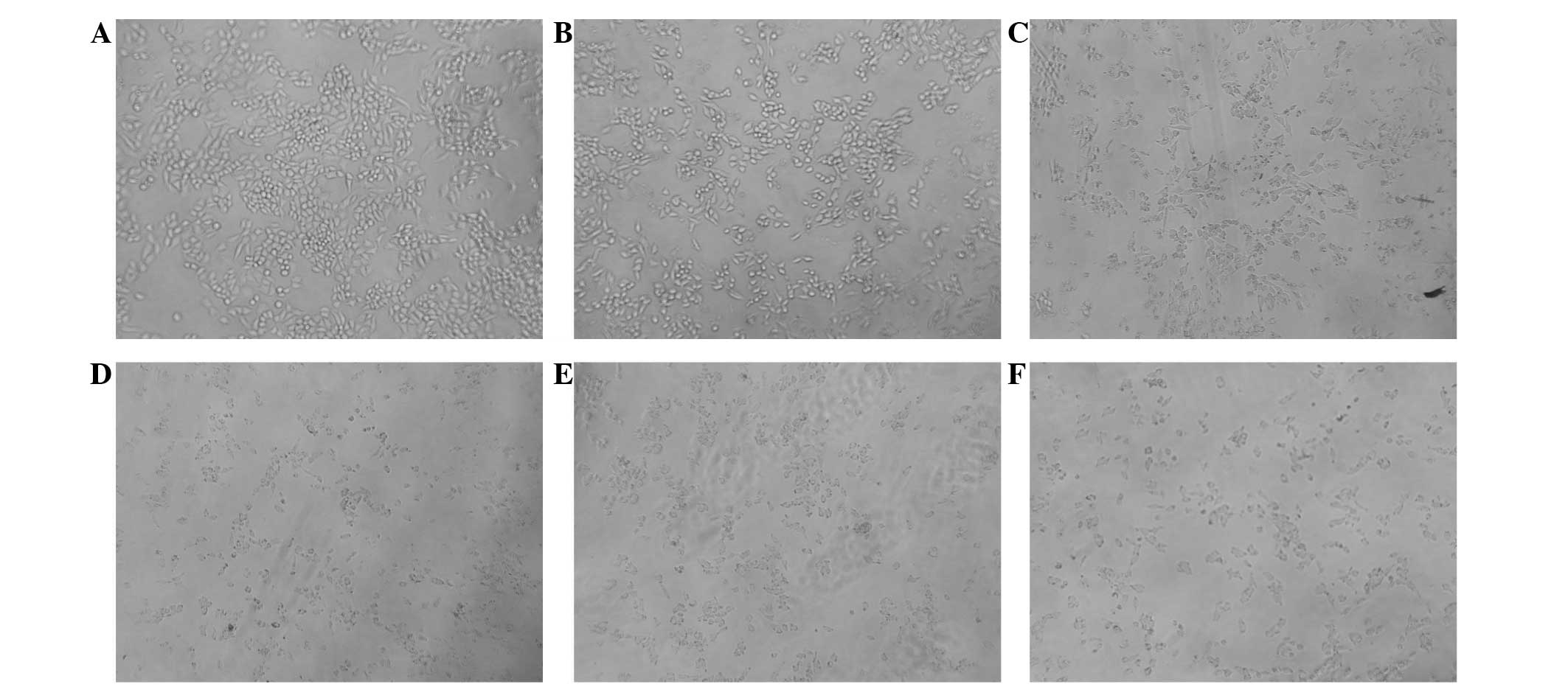
Effects of TMPyP4-PDT on cell
morphology
The cells in the blank control were observed to be
polygonal or spindle-shaped. The cells treated with 3 μM
TMPyP4 were partially round in shape, whereas cells
treated with 6 μM TMPyP4 were in a poor state and
adherent cells were sparse. Furthermore, with increasing
TMPyP4 concentration, the cells became increasingly
round in shape and the number of adherent cells was significantly
reduced. In addition, apoptotic bodies were clearly visible
following treatment with 6 μM TMPyP4 (Fig. 2).
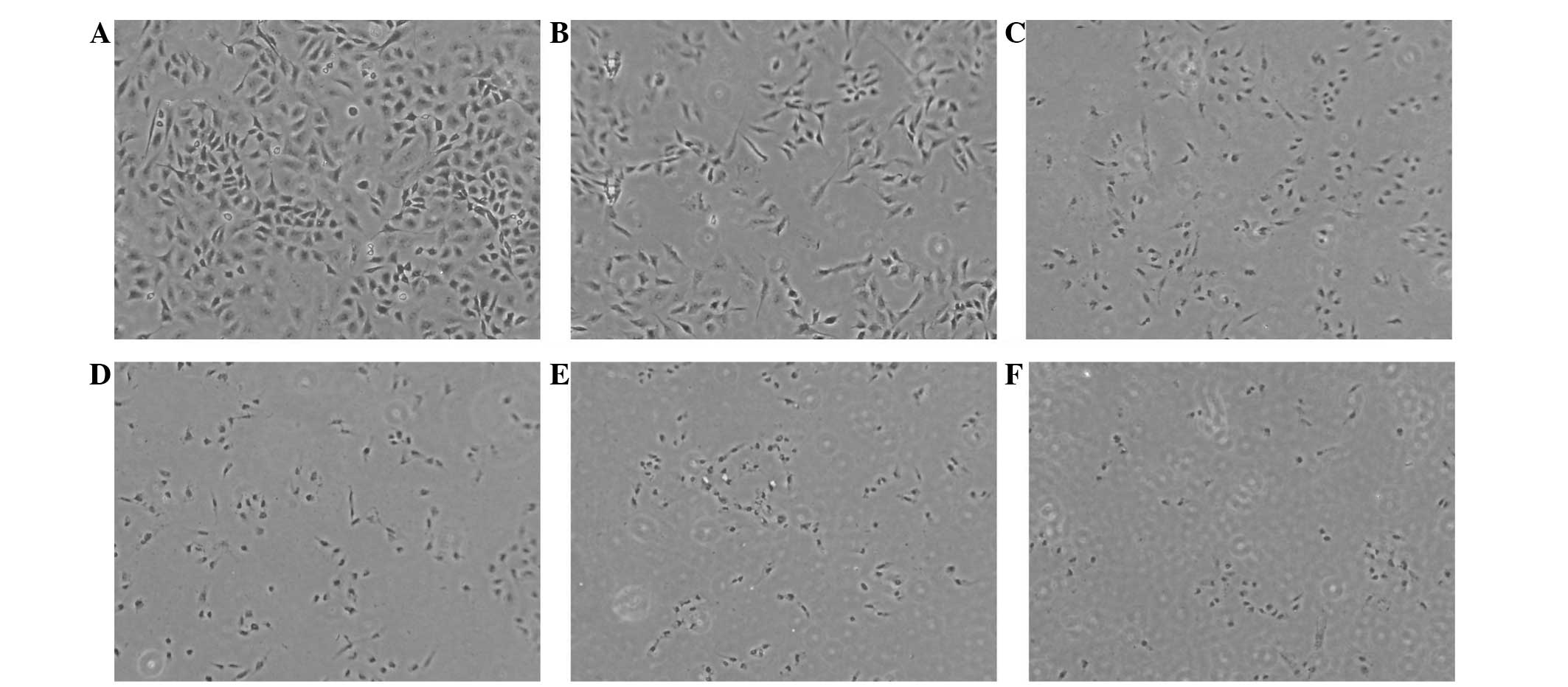
Under the conditions of the laser energy density set
at 6 J/cm2, the blank control group morphology was more
consistent with a high nuclear cytoplasm ratio. Furthermore, with
increasing TMPyP4-PDT concentration, the cell morphology
of the TMPyP4-PDT group became increasingly irregular
and nuclear pyknosis or fragments were observed (Fig. 3).
Effects of TMPyP4-PDT on cell
apoptosis
Propidium iodide and Annexin V double-staining were
used to detect the changes in apoptotic cells treated with
TMPyP4-PDT, and the apoptosis-inducing effects of
TMPyP4-PDT were assessed using FlowJo software. Under
the conditions of the laser energy density set at 6
J/cm2, the effects of TMPyP4-PDT on the
apoptosis of the A2780 cells was examined following treatment at
doses of 3, 6, 15, 30, or 60 μM. The cells that were treated with
the various doses of TMPyP4 for 24 h showed a
significant increase in the number of apoptotic bodies compared
with the negative control group, and the apoptotic cell percentage
increased in a dose-dependent manner. The cell apoptosis rates were
1.0±0.10, 14.7±2.22, 32.3±1.69, 52.2±1.47, 56.3+1.23 and 80.3±3.14%
for doses of 3, 6, 15, 30, or 60 μM TMPyP4, respectively
(Fig. 4).
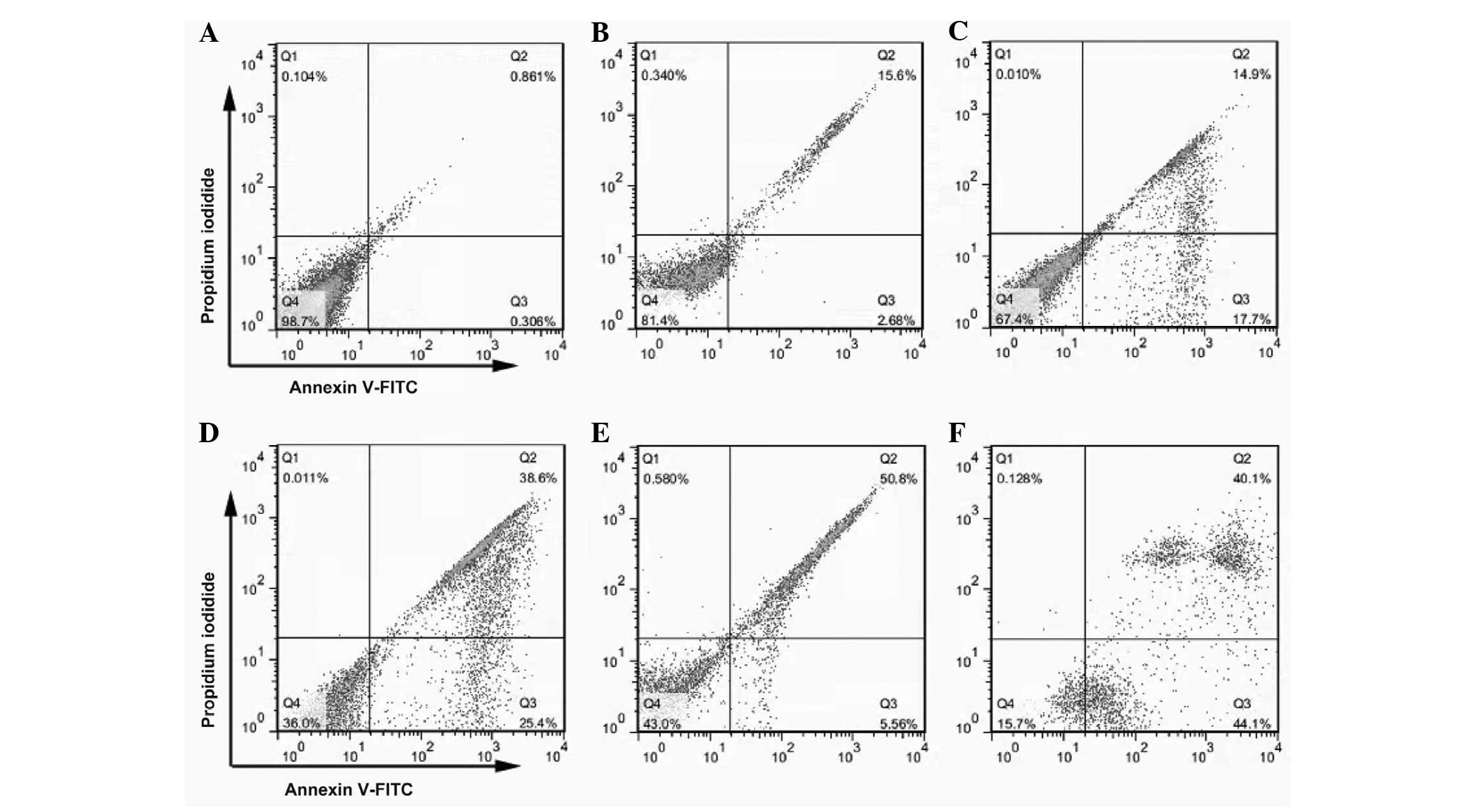 | Figure 4TMPyP4-PDT induces cell
apoptosis in A2780 cells. The cells were cultured with 3, 6, 15, 30
or 60 μM TMPyP4 for 4 h prior to exposure to radiation
and incubation at 37°C for 24 h. The apoptotic cells were
identified using the fluorescent marker, Annexin V-fluorescein
isothiocyanate (magnification, ×200). (A) Blank control group and
groups treated with TMPyP4-PDT concentrations of (B) 3,
(C) 6, (D) 15, (E) 30 and (F) 60 μM were exposed to a single laser
energy density of 6 J/cm2. TMPyP4,
5,10,15,20-tetra-(N-methyl-4-pyridyl)porphine; PDT, photodynamic
therapy. |
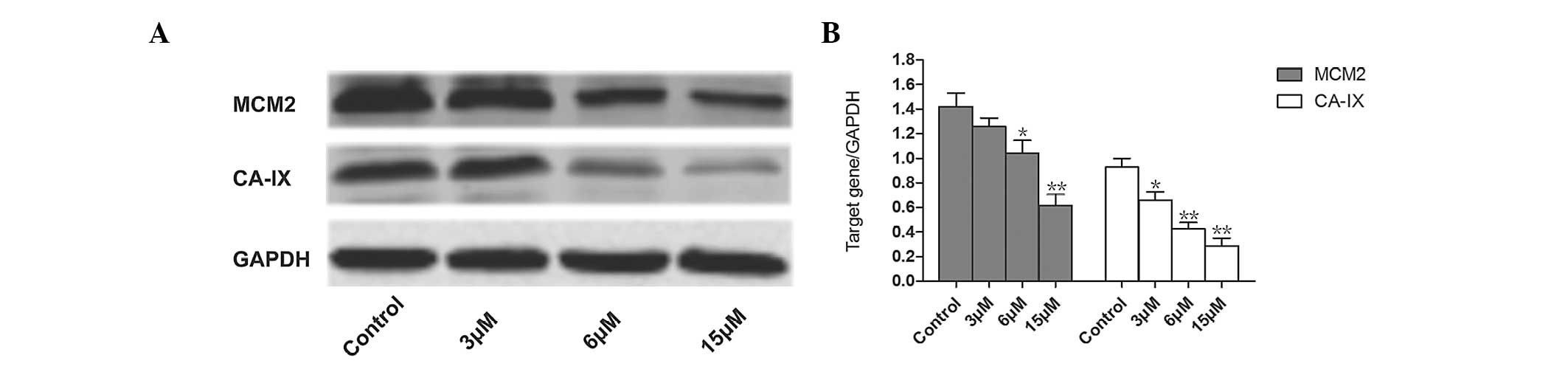
Effects of TMPyP4-PDT on the
expression levels of MCM2 and CA-IX proteins
Following irradiation with a laser energy density of
6 J/cm2 and treatment with TMPyP4-PDT at
doses of 3, 6 or 15 μM, the MCM2 and CA-IX expression levels were
analyzed. In contrast to the increased apoptosis observed in A2780
cells, the expression levels of MCM2 and CA-IX were significantly
downregulated in a dose-dependent manner (Fig. 5).
Discussion
PDT is minimally invasive, with good tolerance,
improved aesthetic outcomes, minimal functional disturbance, low
morbidity and the ability to be used more than once at the same
site in comparison to surgery, radiation and chemotherapy. The main
mechanism of PDT used for the diagnosis and treatment of cancer
involves the use of sensitized molecular components, which transfer
heat to generate singlet oxygen through a series of photochemical
and photobiological reactions. Furthermore, the generated singlet
oxygen and the release of prostaglandins, lymphokines, thromboxane
or other cytokines destroys the tumor microvessel and biofilm,
thereby killing the tumor cells (11–14).
TMPyP4 is a tetravalent cationic water-soluble porphyrin
that accumulates in tumors, with a good degree of selectivity
(15,16). TMPyP4 connects with
telomeres and promotes telomerase ends to form a stable
G-quadruplex structure, which reduces the telomerase activity and
its role in tumor cells, and leads to the arrest of tumor cell
growth, thus inducing the apoptosis of tumor cells (17,18).
In the current study, the growth inhibition of the human ovarian
carcinoma A2780 cells was not evident in the PDT group (P>0.05).
However, in the TMPyP4 and TMPyP4 PDT groups, the growth inhibition
of the human ovarian carcinoma A2780 cells gradually increased with
increasing drug concentration and laser energy density, although,
when the treatment reached a certain level, the inhibition of the
cell increase slowed down (P<0.05). Therefore, in clinical PDT,
an appropriate dose of the photosensitizer and laser energy density
must be selected, as blindly increasing the dose of the
photosensitizer and laser energy density is not conducive to
improving the efficacy and may lead to toxicity and side-effects as
a result of the high dose. The effect of 5-aminolevulinic
acid-mediated PDT on the induction of apoptosis has also been
extensively described (19,20). Consistent with previous studies, a
similar phenomenon was observed in the current study, whereby the
TMPyP4-PDT-induced cell apoptosis and the effect on the
induction of apoptosis were in a laser energy- and dose-dependent
manner (21).
MCM2 is one of the conserved set of six related
proteins of the MCM complex (MCM2–7), which is essential in the
regulation of DNA replication (22). MCM2 has been studied in a wide range
of human organs, and its overexpression has been identified in
various types of tumors and tumor-like lesions of the oral mucosa,
larynx, stomach, colon, esophagus, breasts, lungs, ovaries,
kidneys, prostate, bladder, brain and soft tissues (23,24).
CA-IX is a member of the CA family, which are a group of
zinc-containing metalloenzymes that catalyze the reversible
hydration of carbon dioxide to carbonic acid and are involved in
respiration and the acid-base balance (25). CA-IX is important in the regulation
of cell proliferation and transformation, and is conducive to tumor
growth and metastasis (26). The
present study demonstrated that the antitumor effects of
TMPyP4-PDT are accompanied by the downregulation of MCM2
and CA-IX. According to this result, we hypothesized that
TMPyP4-PDT may inhibit the DNA replication of tumor
cells and change pH homeostasis, thereby inhibiting tumor cell
proliferation and metastasis. However, the specific mechanism of
TMPyP4-PDT requires further study.
In conclusion, the present study demonstrated that
TMPyP4-PDT potently suppressed the cell growth of the
A2780 cells in a laser energy- and dose-dependent manner. In
addition, the study indicated that TMPyP4-PDT may
exhibit its antitumor activity by downregulating the expression of
MCM2 and CA-IX in human ovarian carcinoma cells. Furthermore,
TMPyP4 enhances laser sensitivity. These results
indicated that TMPyP4-PDT may be supplementary to
conventional therapy in the treatment of ovarian carcinoma. Further
study to clarify the molecular mechanism of the
TMPyP4-PDT-induced antitumor activity may provide a
rationale for the development of antitumor drug targeted therapies
for ovarian carcinoma.
Acknowledgements
The authors would like to thank the professors of
the Key Laboratory of Cardiovascular Remodeling and Function
Research, Qilu Hospital of Shandong University (Jinan, Shandong,
China). This study was supported by the National Natural Science
Foundation (grant no. 81072122).
References
|
1
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010.
|
|
2
|
Jackson KS and Naik R: Pelvic floor
dysfunction and radical hysterectomy. Int J Gynecol Cancer.
16:354–363. 2006.
|
|
3
|
Bosgraaf RP, Mast PP, Struik-van der
Zanden PH, et al: Overtreatment in a see-and-treat approach to
cervical intraepithelial lesions. Obstet Gynecol. 121:1209–1216.
2013.
|
|
4
|
Chen FP and Li XJ: A profile of
radiotherapy side effects and complications in gynecological
malignant tumors. Int Med Health Guidance N. 15:117–118. 2009.
|
|
5
|
Hashimoto K: Radical operation of cervical
cancer of the uterus (Okabayashi’s method). Sanfujinka No Jissai.
19:348–351. 1970.(In Japanese).
|
|
6
|
Koning CC, Wouterse SJ, Daams JG, et al:
Toxicity of concurrent radiochemotherapy for locally advanced
non-small-cell lung cancer: a systematic review of the literature.
Clin Lung Cancer. 14:481–487. 2013.
|
|
7
|
Buytaert E, Dewaele M and Agostinis P:
Molecular effectors of multiple cell death pathways initiated by
photodynamic therapy. Biochim Biophys Acta. 1776:86–107. 2007.
|
|
8
|
Lemarteleur T, Gomez D, Paterski R, et al:
Stabilization of the c-myc gene promoter quadruplex by specific
ligands’ inhibitors of telomerase. Biochem Biophys Res Commun.
323:802–808. 2004.
|
|
9
|
Gomez D, Paterski R, Lemarteleur T, et al:
Interaction of telomestatin with the telomeric single-strand
overhang. J Biol Chem. 279:41487–41494. 2004.
|
|
10
|
Zhuang XY and Yao YG: Mitochondrial
dysfunction and nuclear-mitochondrial shuttling of TERT are
involved in cell proliferation arrest induced by G-quadruplex
ligands. FEBS Lett. 587:1656–1662. 2013.
|
|
11
|
Hong Sang: The present situation in the
study on application of photodynamic therapy. Clin Dermatol.
31:3322002.
|
|
12
|
Juarranz A, Jaén P, Sanz-Rodríguez F, et
al: Photodynamic therapy of cancer. Basic principles and
applications. Clin Transl Oncol. 10:148–154. 2008.
|
|
13
|
Meng B, Wen BG, Li GY and Shen ZY:
Research progress in the study of mechanism of apoptosis induced by
photodynamic therapy. Sheng Li Ke Xue Jin Zhan. 33:269–272.
2002.(In Chinese).
|
|
14
|
Saczko J, Chwilkowska A, Kulbacka J, et
al: Photooxidative action in cancer and normal cells induced by the
use of photofrin in photodynamic therapy. Folia Biol (Praha).
54:24–29. 2008.
|
|
15
|
Villanueva A, Caggiari L, Jori G and
Milanesi C: Morphological aspects of an experimental tumour
photosensitized with a meso-substituted cationic porphyrin. J
Photochem Photobiol B. 23:49–56. 1994.
|
|
16
|
Yamakawa N, Ishikawa Y and Uno T: Solution
properties and photonuclease activity of cationic bis-porphyrins
linked with a series of aliphatic diamines. Chem Pharm Bull
(Tokyo). 49:1531–1540. 2001.
|
|
17
|
Liu W, Sun D and Hurley LH: Binding of
G-quadruplex-interactive agents to distinct G-quadruplexes induces
different biological effects in MiaPaCa cells. Nucleosides
Nucleotides Nucleic Acids. 24:1801–1815. 2005.
|
|
18
|
Guo K, Pourpak A, Beetz-Rogers K, et al:
Formation of pseudosymmetrical G-quadruplex and i-motif structures
in the proximal promoter region of the RET oncogene. J Am Chem Soc.
129:10220–10228. 2007.
|
|
19
|
Chen X, Zhao P, Chen F, et al: Effect and
mechanism of 5-aminolevulinic acid-mediated photodynamic therapy in
esophageal cancer. Lasers Med Sci. 26:69–78. 2011.
|
|
20
|
He GF, Bian ML, Zhao YW, et al: A study on
the mechanism of 5-aminolevulinic acid-mediated photodynamic
therapy in vitro and in vivo in cervical cancer. Oncol Rep.
21:861–868. 2009.
|
|
21
|
Zhao YL, Cao Z and Xu CS: Effects of
TMPyP4-PDT on human tongue carcinoma Tca8113 cells in vitro. Laser
Journal. 4:78–79. 2008.
|
|
22
|
Bell SP and Dutta A: DNA replication in
eukaryotic cells. Annu Rev Biochem. 71:333–374. 2002.
|
|
23
|
Giaginis C, Vgenopoulou S, Vielh P and
Theocharis S: MCM proteins as diagnostic and prognostic tumor
markers in the clinical setting. Histol Histopathol. 25:351–370.
2010.
|
|
24
|
Torres-Rendon A, Roy S, Craig GT and
Speight PM: Expression of Mcm2, geminin and Ki67 in normal oral
mucosa, oral epithelial dysplasias and their corresponding
squamous-cell carcinomas. Br J Cancer. 100:1128–1134. 2009.
|
|
25
|
Pugh CW and Ratcliffe PJ: Regulation of
angiogenesis by hypoxia: role of the HIF system. Nat Med.
9:677–684. 2003.
|
|
26
|
Airley R, Loncaster J, Davidson S, et al:
Glucose transporter glut-1 expression correlates with tumor hypoxia
and predicts metastasis-free survival in advanced carcinoma of the
cervix. Clin Cancer Res. 7:928–934. 2001.
|