Introduction
The most common types of submucosal tumor (SMT)
include mesenchymal tumors, such as gastrointestinal (GI) stromal
tumors (STs), myogenic and neurogenic tumors, which collectively
account for 54% of all SMT cases, followed in frequency by aberrant
pancreases, cysts, lipomas, cartinoid tumors, lymphangiomas and
hemangiomas (1). Among these SMTs,
cases of GISTs are the most common. In 2004, the European Society
for Medical Oncology Consensus GIST meeting declared that GISTs
exhibit malignant potential and always require treatment by
surgical resection (2). Therefore,
it is clinically important to differentiate between GISTs and other
types of SMT. The typical endoscopic characteristics of all SMTs
include a lesion of hemispheric appearance with gently sloping
edges that is covered by normal mucosa, however, these features do
not aid with distinguishing between the histological types of SMT.
Endoscopic ultrasound (EUS) is a key procedure in the evaluation of
SMTs of the GI tract, as it enables determination of the layer of
origin of the GI wall and allows for diagnostic sampling (3). However, differentiating between GISTs,
leiomyomas and neurinomas using EUS is often complex as all of
these tumors are visualized as a hypoechoic mass arising from the
muscularis propria (MP), which is the typical EUS finding when
observing mesenchymal tumors (4).
Tissue sampling is therefore essential for obtaining an accurate
diagnosis of SMTs.
Recently, EUS-guided fine needle aspiration has
emerged as an important method for the diagnosis of SMTs. However,
as this technique provides limited diagnostic accuracy due to the
limited quantity of tissue sample that can be collected, an optimal
method for tissue sampling is required (5,6).
Endoscopic submucosal dissection (ESD), which involves the
insertion of an endoscope into the submucosa (SM) to facilitate the
dissection of the SM from the underlying muscle layer, enables an
en bloc resection of early epithelial neoplasm and has become the
standard approach for the resection of early GI cancer (7–10). The
submucosal endoscopy with mucosal flap (SEMF) method (11) incorporating the ESD technique has
been developed to permit a safer offset entry into the peritoneal
cavity during natural orifice translumenal endoscopic surgery
(12).
In our previous study, the value of core biopsy
using the SEMF method was developed and demonstrated as a novel
method for collecting tumor tissue under direct vision to assist in
the diagnosis of SMTs (13,14). One technical advantage of core
biopsy using the SEMF method is that once the ESD technique is
complete, and upon creating a tunnel into the SM toward the tumor,
the tumor can be visually identified, which enables the reliable
collection of tumor tissue. Using this method, which provides
direct visualization of the tumor, endoscopic images of the tumor
can be obtained, which can be quantified for the macroscopic
characteristics of SMTs, including the color, clarity and shape of
the tumor surface, the presence or absence of a tumor capsule, and
the solidity of the tumor (as assessed by pressure that is applied
using forceps). Using closed forceps the mass can be probed to
determine whether it is rigid, soft, or indents when depressed. The
consistency of the mass can be symptomatic and aid with diagnosis.
A mobile mass that is soft and indents when depressed using biopsy
forceps is highly indicative of a benign tumor, such as a lipoma or
a vascular or cystic tumor. By contrast, if a mass does not indent,
it may indicate a firm lesion, such as a GIST or a leiomyoma.
However, the specificity of these endoscopic characteristics has
not been rigorously evaluated (1).
A typical macroscopic GIST image is characterized as a
multi-nodular, gray/white, hard tumor. Improved characterization of
the endoscopic appearance of the surface of SMTs may further
improve the diagnostic accuracy of core biopsies using the SEMF
method combined with an endoscopic examination of the tumor
surface.
Patients and methods
Patients
In total, 26 patients were enrolled in the present
study (males, n=10 and females, n=16; mean age, 64.07 years; age
range, 41–82 years) who were histologically diagnosed with gastric
SMTs (GISTs, n=13; leiomyomas, n=5; granular cell tumors, n=2;
heterotopic pancreases, n=2; cysts, n=2; schwannoma, n=1; and
lipoma, n=1) by core biopsy using the SEMF method between November
2011 and October 2013 (Table I).
All tumors were evaluated by routine EUS (20-MHz high-frequency
miniprobe, UM-3R; Olympus Medical Systems, Tokyo, Japan) and
computed tomography. SMTs originating from the SM or the MP were
included and tumors presenting primarily with extra luminal growth
were excluded, as such cases were considered to be high risk for
perforation. The Clinical Ethics Committee of Kagawa University
Hospital (Kagawa, Japan) approved the use of this procedure for
gastric SMTs, and written informed consent was obtained from
patients prior to the procedure.
 | Table IClinicopathological data of patients
with submucosal tumors. |
Table I
Clinicopathological data of patients
with submucosal tumors.
| Case | Age/Gender | Tumor size, mm | Layer | Echoic | Pathology |
|---|
| 1 | 74/M | 20 | MP | Hypo | GIST, low risk |
| 2 | 63/M | 20 | MP | Hypo | GIST, low risk |
| 3 | 77/M | 45 | MP | Hypo | GIST, low risk |
| 4 | 53/F | 12 | MP | Hypo | Leiomyoma |
| 5 | 71/F | 15 | MP | Hypo | GIST, low risk |
| 6 | 66/F | 15 | MP | Hyper | Heterotopic
pancreas |
| 7 | 76/F | 15 | MP | Hypo | GIST, low risk |
| 8 | 55/F | 20 | MP | Hypo | GIST, low risk |
| 9 | 82/F | 15 | MP | Hypo | GIST, low risk |
| 10 | 51/F | 15 | MP | Hypo | Leiomyoma |
| 11 | 75/F | 25 | SM | Hyper | Lipoma |
| 12 | 67/F | 12 | MP | Hypo | GIST, low risk |
| 13 | 56/M | 25 | SM | Anechoic | Gastric cyst |
| 14 | 73/F | 22 | MP | Hypo | Schwannoma |
| 15 | 62/M | 15 | MP | Hypo | Leiomyoma |
| 16 | 49/F | 15 | SM | Hypo | Heterotopic
pancreas |
| 17 | 41/M | 14 | MP | Hypo | GIST, low risk |
| 18 | 63/M | 14 | MP | Hypo | Granular cell
tumor |
| 19 | 63/M | 8 | MP | Hypo | Granular cell
tumor |
| 20 | 62/F | 26 | SM | Anechoic | Gastric cyst |
| 21 | 63/M | 22 | MP | Hypo | GIST, low risk |
| 22 | 63/F | 32 | MP | Hypo | GIST, low risk |
| 23 | 72/F | 14 | MP | Hypo | GIST, low risk |
| 24 | 82/F | 13 | MP | Hypo | GIST, low risk |
| 25 | 53/M | 15 | MP | Hypo | Leiomyoma |
| 26 | 54/F | 22 | MP | Hypo | Leiomyoma |
The SEMF method
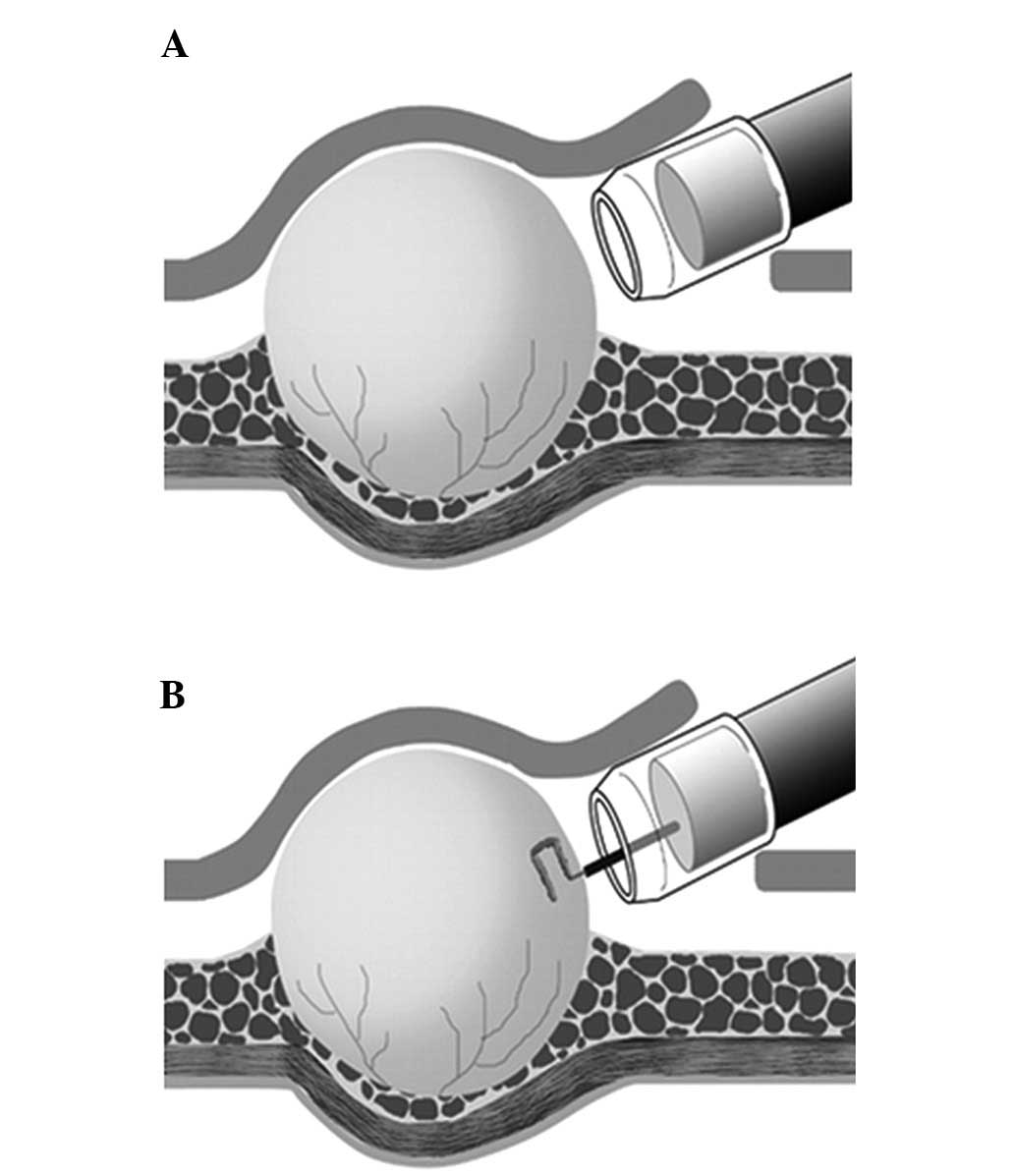
The SEMF method consists of five major procedures as
previously described (13,14): i) After demarcating the tumor
borders with a margin of ~5 mm, a small incision is made to create
a 10-mm opening flap (i.e. the ESD procedure). ii) A short tunnel,
which is used to access the tumor, is made through the opening flap
via an additional submucosal dissection (i.e. a short SEMF method).
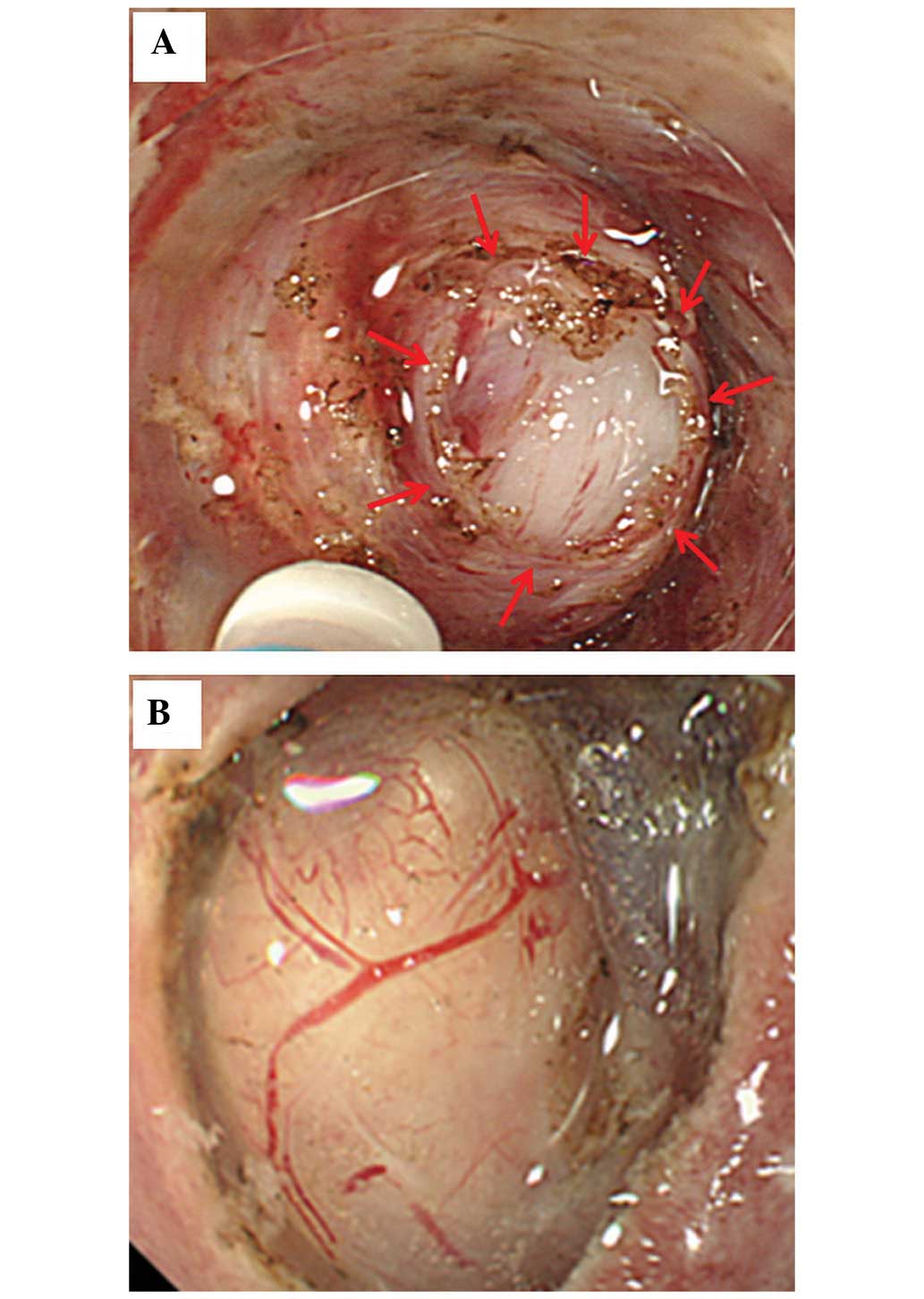
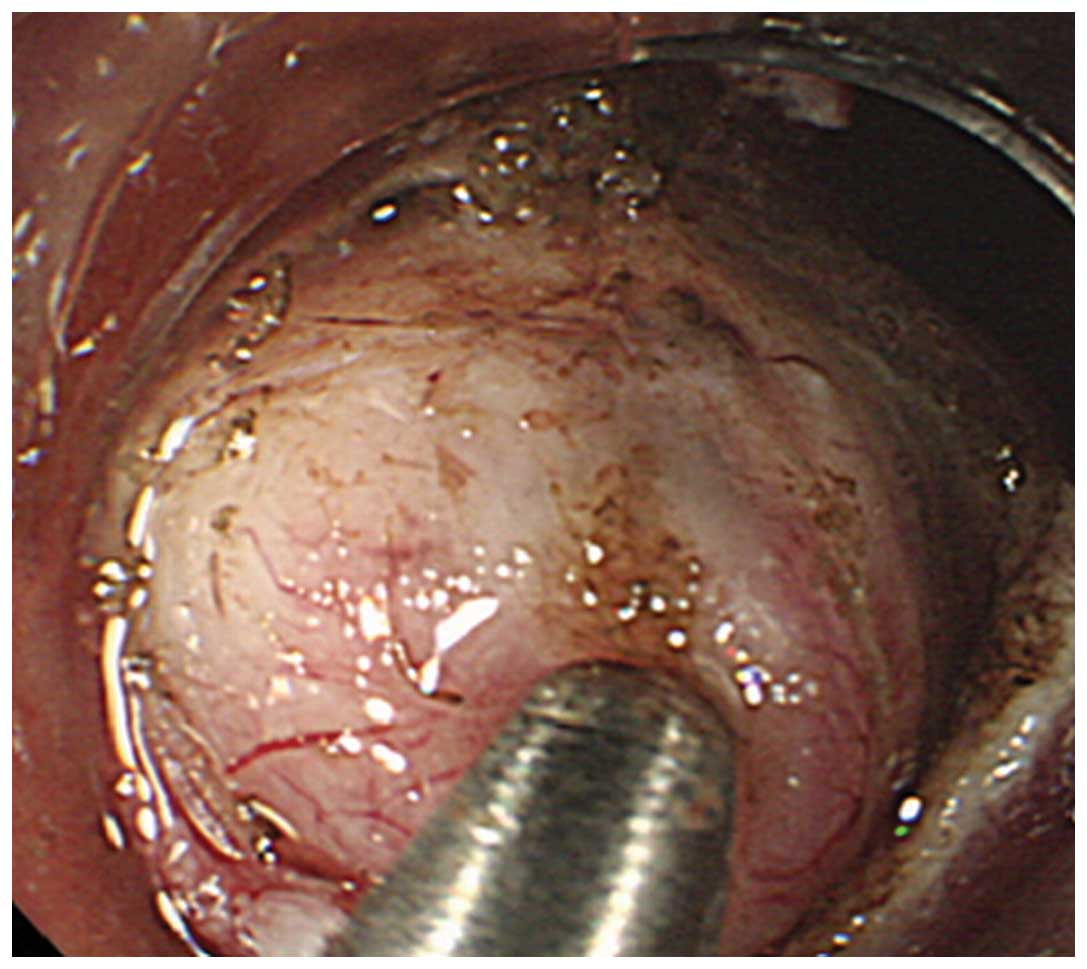
The tumor is visually identified and exposed (Fig. 1A). iii) A core specimen (5×5×2 mm)
is obtained using a needle-knife (Olympus KD-441Q; Olympus Medical
Systems) in the cutting mode provided by the electrosurgical unit
(VIO 300D, EndoCut® mode effect 2, duration 3; ERBE
Elektromedizin GmbH, Tübingen, Germany) while minimizing
compression of the tissue (i.e. a core biopsy; Fig. 1B). iv) The specimen is collected
into a transparent cap that is designed to be longer at the tip
(Elastic Touch F-01; TOP Corporation, Tokyo, Japan; i.e. the long
attachment method for tissue collection), with care taken to
prevent the tissue from coming into contact with the inner wall of
the tunnel. v) The entire detached surface is sutured away from the
periphery of the tumor with clips to prevent tumor fragments from
flowing back into the tunnel (i.e. clip closure from the tumor
side) and finally, a specimen that is sufficient for
immunohistochemical analysis (~5-mm diameter), is obtained.
Endoscopic images, still and moving, obtained for the 26 SMT
patients during the second (Fig.
1A) and third (Fig. 1B)
procedures were retrospectively reviewed. The short SEMF method
(the second step) provides endoscopic visualization of the tumor
under direct vision (endoscopically visualized features; EVF);
these images may be quantified for the macroscopic characteristics
of SMTs, including color, clarity, shape and presence or absence of
a tumor capsule. The core biopsy (the third step) demonstrates the
solidity of the tumor as assessed by pressure applied using closed
forceps.
Assessment I
The five EVF for each type of SMT were evaluated and
each type of SMT was classified based on these five EVF as follows:
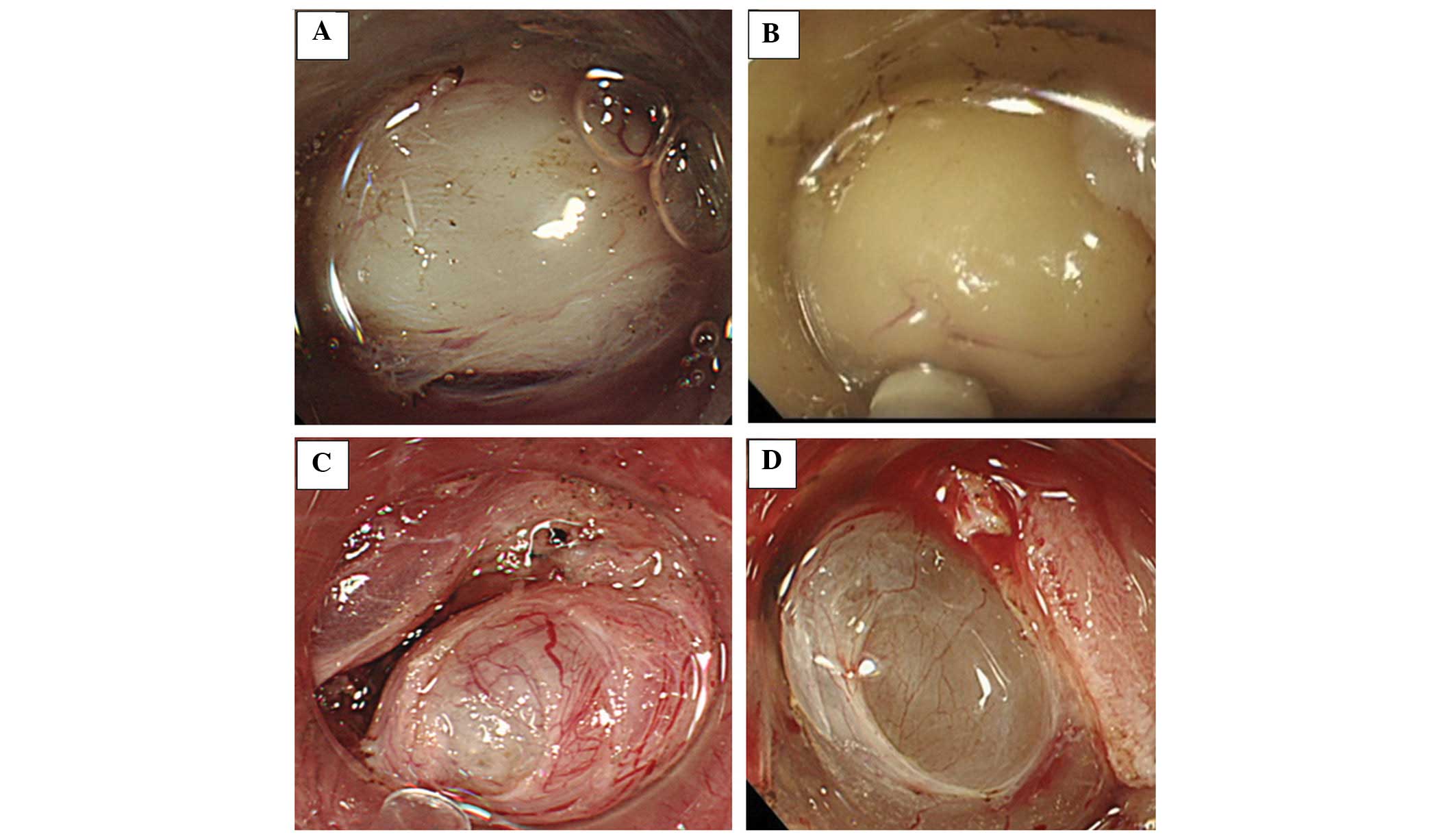
Color, clarity, shape, tumor coating and solidity. Colors were
classified into four typical EVF colors: White, yellow, blue and
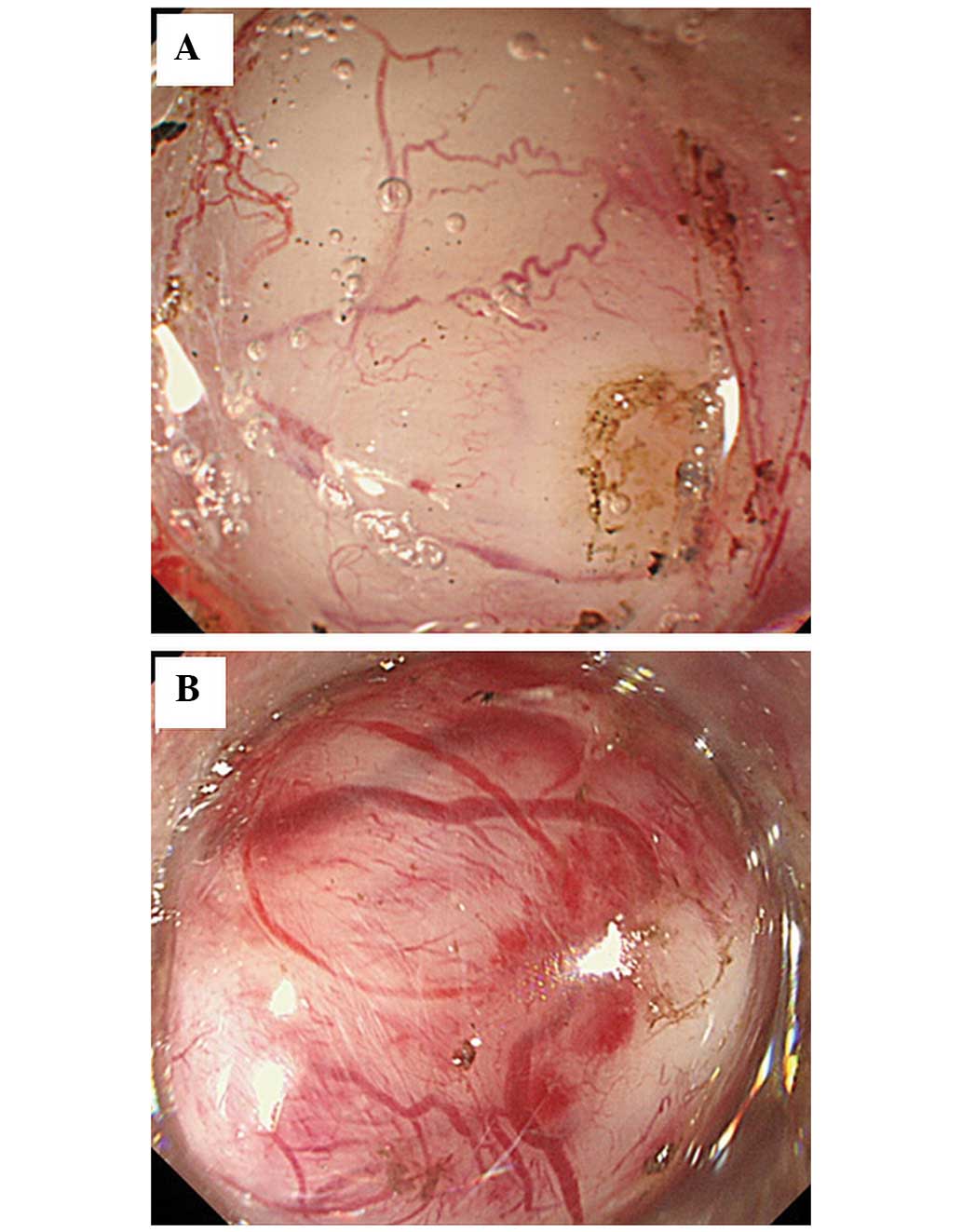
colorless (Fig. 2A–D). The clarity
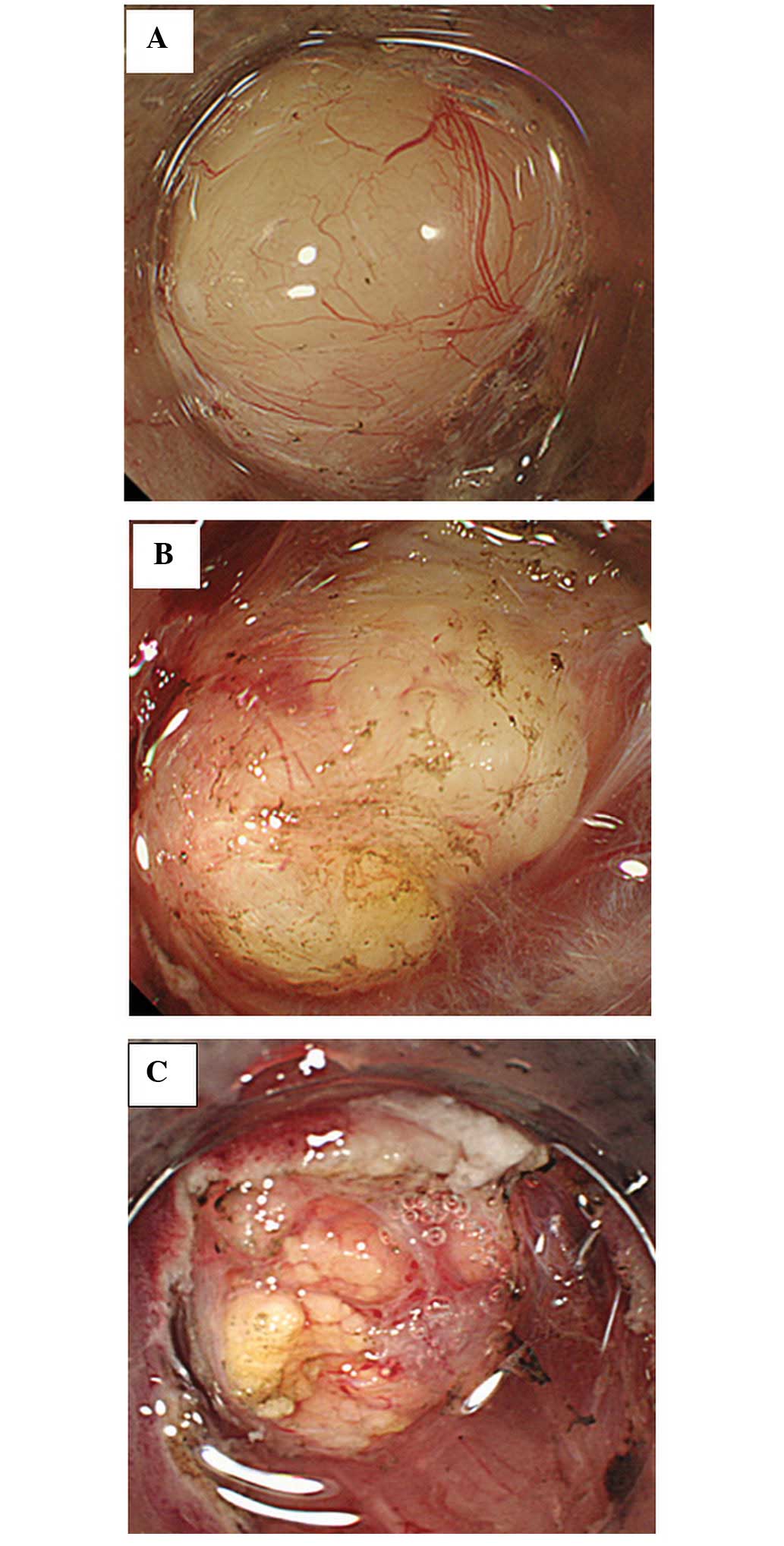
was classified as either clear or cloudy (Fig. 3A and B). The shape was classified as
either round or nodular (Fig.
4A–C). Additionally, the nodules were subdivided by size as
small or large (Fig. 4B and C). The
tumor coating was classified as either visible or not visible
(Fig 5A and B). In addition, the
solidity was classified as rigid (Fig.
6) or soft (Fig. 2B). Rigid
tumors were defined as elastic and non-elastic hard tumors.
Assessment II
In the retrospective comparative study, the EVF were
compared between the 13 patients with gastric GISTs and the 13
patients with benign submucosal tumors (BSTs) with respect to color
(white or not white), clarity, shape of the tumor surface, the
presence or absence of a visible capsule and the rigidity (whether
the mass indents when depressed) as evaluated by two endoscopists.
Additionally, a combination of three EVF was compared between the
two groups.
Statistical analysis
The two-sided Fisher’s exact test was used for the
comparison of the five tumor characteristics between the two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Assessment I
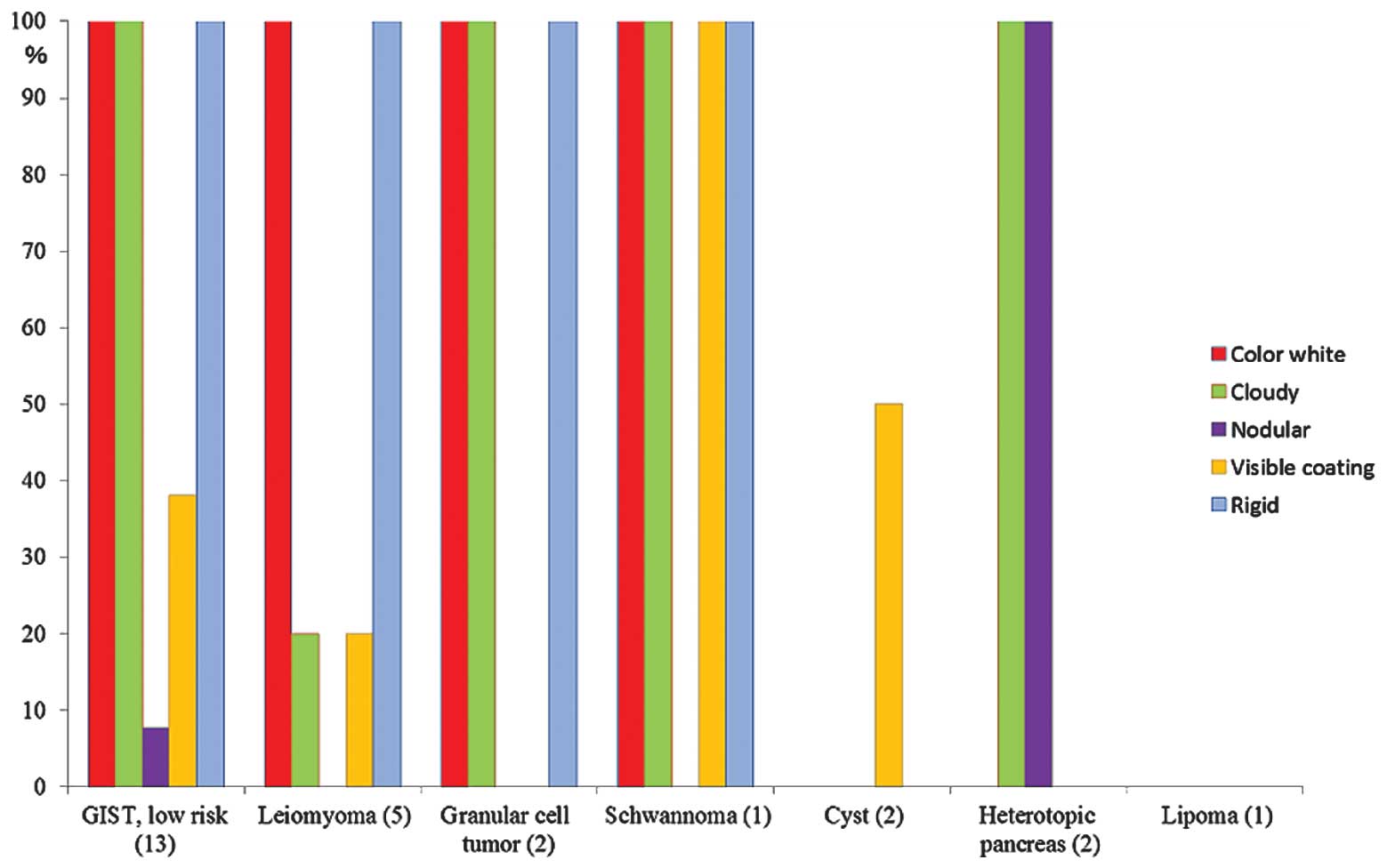
The EVF of SMTs in the individual cases are
summarized in Table II. A
histogram of the results was constructed to clearly demonstrate the
differences between each SMT (Fig.
7). The mesenchymal tumors, including the 13 GISTs, five
leiomyomas, two granular cell tumors and one schwannoma tended to
exhibit similar characteristics. Among the SMTs, heterotopic
pancreas revealed small nodules with an appearance similar to that
of the pancreatic tissue itself or showing pancreatic-like tissue
characteristics (Fig. 4C). A
classification system of gastric SMTs using EVF is proposed on the
basis of these results (Table
III). The typical endoscopic findings of GISTs were tumors that
were white, cloudy, round and rigid (Figs. 2A, 3B, 4B and
6). In the five cases of
leiomyomas, the tumors were characterized as white, clear (n=4)
> cloudy (n=1), round and elastic hard tumors (Figs. 3A and 4A). Although the sample size was small,
two granular cell tumors (Fig. 5B)
and one schwannoma were white, cloudy, round and rigid tumors,
which is similar to GISTs. Conversely, in the two cases of gastric
cysts, the tumor was colorless or blue, clear, round, soft and the
surface was wet (Fig. 2C and D). In
the two cases of heterotopic pancreas, the tumors were yellow,
cloudy, soft and the tumor surfaces exhibited small nodules with an
appearance similar to that of the pancreatic tissue itself or
showing pancreatic-like tissue characteristics. (Fig. 4C). In the single case of lipoma, the
tumor was yellow, clear, round, soft and the tumor surface appeared
to be fatty and adipose tissue-like (Fig. 2B).
 | Table IIFive selective characteristic EVF
findings of SMTs. |
Table II
Five selective characteristic EVF
findings of SMTs.
| | Color | Clarity | Shape | Tumor coating | Solidity |
|---|
| |
|
|
|
|
|
|---|
| SMT | n | White | Blue | Colorless | Yellow | Clear | Cloudy | Round | Nodular | Visible | Not visible | Rigid | Soft |
|---|
| GIST, low risk | 13 | 13 | - | - | - | - | 13 | 12 | 1 (Large) | 5 | 8 | 13 (7 E, 6 NE) | - |
| Leiomyoma | 5 | 5 | - | - | - | 4 | 1 | 5 | - | 1 | 4 | 5 (5 E) | - |
| Granular cell
tumor | 2 | 2 | - | - | - | - | 2 | 2 | - | - | 2 | 2 (2 E) - | |
| Schwannoma | 1 | 1 | - | - | - | - | 1 | 1 | - | 1 | - | 1 (1 NE) | - |
| Cyst | 2 | - | 1 | 1 | - | 2 | - | 2 | - | 1 | 1 | - | 2 |
| Heterotopic
pancreas | 2 | - | - | - | 2 | - | 2 | - | 2 (Small) | - | 2 | - | 2 |
| Lipoma | 1 | - | - | - | 1 | 1 | - | 1 | - | - | 1 | - | 1 |
 | Table IIIClassification by EVF findings of
gastric SMTs (proposed as a result of the present study). |
Table III
Classification by EVF findings of
gastric SMTs (proposed as a result of the present study).
| SMT | EVF findings of
gastric SMTs |
|---|
| GIST, low risk | White, cloudy,
round or nodular, rigid |
| Granular cell
tumor | White, cloudy,
round, rigid |
| Schwannoma | White, cloudy,
round, rigid |
| Leiomyoma | White, clear >
cloudy, round, rigid (elastic hard) |
| Cyst | Blue or colorless,
clear, round, soft |
| Heterotopic
pancreas | Yellow, cloudy,
small nodular, soft |
| Lipoma | Yellow, clear,
round, soft |
Assessment II
The results of the statistical analysis of the
comparison between the GIST and BST groups with regards to the five
EVF are summarized in Table IV.
Significant differences were identified between the GIST and BST
groups in terms of the frequency of white (100% [13/13] vs. 61.5%
[8/13]), cloudy (100% [13/13] vs. 53.8% [7/13]) and rigid tumors
(100% [13/13] vs. 61.5% [8/13]; P<0.05 for all three),
respectively. No significant differences were identified between
the GIST and BST groups in terms of the frequency of nodular tumors
(7.7% [1/13] vs. 15.4% [2/13]) and tumors with visible coatings
(38.5% [5/13] vs. 23.1% [3/13]; P>0.05 for the two).
Additionally, significant differences were observed between the two
groups regarding the frequency of tumors with the combination of
three EVF (white, cloudy and rigid), which was demonstrated in all
13 GISTs (100% [13/13] vs. 30.8% [4/13]; P<0.05 for the
two).
 | Table IVStatistical analysis between the GIST
and BST groups with regard to the five selective characteristic EVF
findings and the combination of the three EVF findings (white,
cloudy and rigid). |
Table IV
Statistical analysis between the GIST
and BST groups with regard to the five selective characteristic EVF
findings and the combination of the three EVF findings (white,
cloudy and rigid).
| Characteristic | GIST, n=13 (%) | BST, n=13 (%) | P-valuea |
|---|
| White color | 13 (100) | 8 (61.5) | 0.039 |
| Cloudy | 13 (100) | 7 (53.8) | 0.014 |
| Nodule | 1 (7.7) | 2 (15.4) | 1.000 |
| Visible
coating | 5 (38.5) | 3 (23.1) | 0.673 |
| Rigid | 13 (100) | 8 (61.5) | 0.014 |
| Three EVFs (White,
cloudy, rigid) | 13 (100) | 4 (30.8) | 0.0005 |
Discussion
SMTs are non-epithelial tumors that are covered by a
normal mucosa. Unlike epithelial tumors, the majority of SMTs are
endoscopically visualized as masses that protrude into the GI
lumen. Thus, it is difficult to morphologically distinguish between
the different types of SMT. Our novel tissue sampling method, i.e.
a core biopsy using the SEMF method, enables a reliable
histological diagnosis and the visualization of the tumor surface
under endoscopic direct vision. This provides EVF of SMTs in the SM
via a dissected submucosal tunnel, which can be assessed to
differentiate between SMTs. To the best of our knowledge, this is
the first report to characterize EVF of each type of SMT,
particularly of GISTs, a type of SMT that is considered to possess
malignant features. Therefore, the characteristic EVF may have
potential diagnostic value for the differentiation of GISTs from
other BSTs.
EUS is widely used for characterizing SMTs, and the
information obtained by EUS, such as the layer from which an SMT
arises, echogenicity and the internal structure of the tumor,
enables the differential diagnosis between different types of SMT
with a certain level of accuracy (15,16).
For example, GISTs typically appear as a hypoechoic mass arising
from the fourth hypoechoic GI wall layer (i.e. the MP) (17–20).
GISTs with malignant potential are significant for
the differentiation from leiomyomas during diagnosis. Leiomyomas
characteristically arise from the MP and are hypoechoic and
homogeneous in their internal structures (17,18).
Thus, it is difficult to distinguish GISTs from leiomyomas based
only on the homogeneity of their internal structures. Furthermore,
a substantial proportion of SMT cases exhibiting a hyperechoic
submucosal layer include lipomas or heterotopic pancreas (3,17,18),
which also cannot be reliably diagnosed by EUS. Thus, EUS should
only be used as a supplementary diagnostic tool for determining the
treatment strategy for SMTs and it is not intended to replace
direct tissue sampling for the definitive diagnosis of SMTs.
However, EUS-guided fine needle aspiration and tissue sampling
procedures using the ESD technique have been shown to provide
limited benefits (21–24). Furthermore, we have previously
reported on the suitability of core biopsy using the SEMF method as
a novel tissue sampling technique (13,14);
in the present study, this technique provided accurate diagnoses in
all cases.
According to Assessment I in the present study, and
based on EVF obtained by core biopsy using the SEMF method, the
endoscopic characteristics of the different types of SMTs may be
summarized, which produces a novel classification of SMTs (Table III). Typical EVF are as follows:
i) Low risk gastric GISTs, white, cloudy, round and rigid; ii)
leiomyomas, white, almost clear, elastic hard tumors; iii) granular
cell tumors and schwannomas, white, cloudy, round, rigid tumors
comparable with GISTs (it is considered to be difficult to
distinguish these tumors from GISTs using EVF); iv) gastric cysts,
colorless or blue, clear, round, soft tumors with wet surfaces; v)
heterotopic pancreas, yellow and small-nodular tumors. Among the
SMTs, only heterotopic pancreases revealed a specific tumor surface
with small nodules, which was characteristic of pancreatic tissues
(25); and vi) lipomas, yellow,
clear, soft tumors with adipose tissue-like characteristics.
According to Assessment II, each of the five EVF
between the GIST and BST groups were compared; white tumors were
observed in all of the 13 GIST cases, the five leiomyomas, the two
granular cell tumors and the schwannoma. The two cases of
heterotopic pancreas and the single case of lipoma presented yellow
tumors, and the two cases of cysts demonstrated colorless or blue
tumors. Although it is difficult to distinguish GISTs and benign
tumors, including leiomyomas, granular cell tumors and schwannoma
using color differences, it was possible to differentiate white
GISTs from non-white benign tumors, such as heterotopic pancreas,
lipoma and cysts. Furthermore, significant differences were
observed with regards to clarity between the two groups.
Specifically, GISTs and leiomyomas exhibited a difference with
regard to clarity (0% [0/13] vs. 80% [4/5]). Thus, the clarity of
the tumor surface between EVF may become an important index for
distinguishing between GISTs and leiomyomas. The clarity of the
tumor surface is considered to reflect the components and
heterogeneity of its internal structures. These may be
histological, and associated with the density of spindle cells and
hyaline degeneration. Notably, the cystic tumor contained a fluid
compartment, which may have contributed to its glossy and wet
appearance. The association between EVF of the tumor surface and
pathological characteristics will be investigated in our future
studies.
With regard to the shape of the tumor surface, a
large nodule was identified in one case (case 22: Tumor size, 32
mm; low risk GIST) of the 13 GIST cases, demonstrated that certain
GISTs >2 cm exhibit nodular features compared with the 10 small
GISTs (<2 cm in size), which had round surfaces (10/10 small
GISTs). Small nodules were observed in only two of the cases of
heterotopic pancreas among all of the SMTs. Therefore, the
evaluation of the presence or absence of nodules may facilitate the
distinction of specific tumors among SMTs. Visible tumor coatings
were observed in 38.5% of GISTs (5/13) and in 23.1% of BSTs (3/13);
the leiomyoma, schwannoma and the cyst. GISTs are generally
encapsulated tumors, however, eight of the 13 GIST cases were not
visually identified to have a thick capsule when observed under
direct endoscopic view, indicating the limited diagnostic potential
for the visual identification of a capsule.
Regarding solidity, there were significant
differences identified between the two groups, indicating GISTs
exhibit rigid tumors when compared with BSTs (100% [13/13] vs.
61.5% [8/13], respectively). Furthermore, concerning elastic or
non-elastic tumors, all five leiomyomas presented with the feature
of elastic hard tumors when compared with GISTs (100% [5/5] vs.
53.8% [7/13], respectively). Whether the mass indents, when
pressure is applied using biopsy forceps, is commonly used for
assessing the hardness of an SMT. GISTs and leiomyomas generally do
not indent, which complicates the endoscopic differentiation of the
tumors. Conversely, the elasticity of the mass, as obtained by core
biopsy using the SEMF method, enables the assessment of the
solidity of the tumor itself. This EVF provided the novel
information that gastric leiomyomas are characteristically an
elastic hard tumor.
There were statistically significant differences
identified between GISTs and BSTs with regard to three EVF: Color,
clarity and solidity. In addition, significant differences were
observed between the two groups regarding the frequency of tumors
that exhibited the combination of three specific EVF: White, cloudy
and rigid, which were observed in all 13 GISTs, indicating that
this combination of three EVF may be a useful parameter for
differentiating between GISTs and BSTs.
With regard to clinical implications, a combination
of TBB and visualizing the tumor surface, i.e. EVF, may be
beneficial. This combination may aid with the decision as to
whether the tumor requires resection. With an increasing number of
reports describing the curative endoscopic resection of SMTs by ESD
(26,27), further advances in diagnostics are
required. Therefore, if the application of EVF assists with the
diagnosis of SMTs, unnecessary and invasive resections may be
avoided. The continued efforts to evaluate the clinical advantages
of the current diagnostic techniques are anticipated to contribute
to the development of novel criteria for diagnosing SMTs based on
EVF. Finally, further studies are required to validate the
specificity of this novel differential diagnostic approach. A
prospective study to clarify the clinical application of EVF is
currently ongoing.
In conclusion, gastric SMTs may be classified based
on five EVF as follows: Color, clarity, shape, tumor coating and
solidity, which indicates that EVF may possess potential diagnostic
value for differentiating GISTs from BSTs.
Acknowledgements
The authors would like to thank Dr Makoto Oryu for
the technical and editorial assistance.
References
|
1
|
Hwang JH and Kimmey MB: The incidental
upper gastrointestinal subepithelial mass. Gastroenterology.
126:301–307. 2004.
|
|
2
|
Blay JY, Bonvalot S, Casali P, et al; GIST
consensus meeting panelists. Consensus meeting for the management
of gastrointestinal stromal tumors. Report of the GIST Consensus
Conference of 20–21 March 2004, under the auspices of ESMO. Ann
Oncol. 16:566–578. 2005.
|
|
3
|
Săftoiu A, Vilmann P and Ciurea T: Utility
of endoscopic ultrasound for the diagnosis and treatment of
submucosal tumors of the upper gastrointestinal tract. Rom J
Gastroenterol. 12:215–229. 2003.
|
|
4
|
Boyce GA, Sivak MV Jr, Rösch T, et al:
Evaluation of submucosal upper gastrointestinal tract lesions by
endoscopic ultrasound. Gastrointest Endosc. 37:449–454. 1991.
|
|
5
|
Hoda KM, Rodriguez SA and Faigel DO:
EUS-guided sampling of suspected GI stromal tumors. Gastrointest
Endosc. 69:1218–1223. 2009.
|
|
6
|
Philipper M, Hollerbach S, Gabbert HE, et
al: Prospective comparison of endoscopic ultrasound-guided
fine-needle aspiration and surgical histology in upper
gastrointestinal submucosal tumors. Endoscopy. 42:300–305.
2010.
|
|
7
|
Ono H: Endoscopic submucosal dissection
for early gastric cancer. Chin J Dig Dis. 6:119–121. 2005.
|
|
8
|
Jee YS, Hwang SH, Rao J, et al: Safety of
extended endoscopic mucosal resection and endoscopic submucosal
dissection following the Japanese Gastric Cancer Association
treatment guidelines. Br J Surg. 96:1157–1161. 2009.
|
|
9
|
Probst A, Pommer B, Golger D, Anthuber M,
Arnholdt H and Messmann H: Endoscopic submucosal dissection in
gastric neoplasia - experience from a European center. Endoscopy.
42:1037–1044. 2010.
|
|
10
|
Yamamoto Y, Fujisaki J, Ishiyama A,
Hirasawa T and Igarashi M: Current status of training for
endoscopic submucosal dissection for gastric epithelial neoplasm at
Cancer Institute Hospital, Japanese Foundation for Cancer Research,
a famous Japanese hospital. Dig Endosc. 24(Suppl 1): 148–153.
2012.
|
|
11
|
Sumiyama K, Gostout CJ, Rajan E, et al:
Submucosal endoscopy with mucosal flap safety valve. Gastrointest
Endosc. 65:688–694. 2007.
|
|
12
|
Kalloo AN, Singh VK, Jagannath SB, et al:
Flexible transgastric peritoneoscopy: a novel approach to
diagnostic and therapeutic interventionsin the peritoneal cavity.
Gastrointest Endosc. 60:114–117. 2004.
|
|
13
|
Kobara H, Mori H, Fujiwara S, Nishiyama N,
Kobayashi M and Masaki T: Bloc biopsy by tunneling method using the
endoscopic submucosal dissection for an upper gastrointestinal
submucosal tumor. Endoscopy. 44(Suppl 2): E197–E198. 2012.
|
|
14
|
Kobara H, Mori H, Fujihara S, et al: Bloc
biopsy by using submucosal endoscopy with a mucosal flap method for
gastric subepithelial tumor tissue sampling (with video).
Gastrointest Endosc. 77:141–145. 2013.
|
|
15
|
Bhatia V, Tajika M and Rastogi A: Upper
gastrointestinal submucosal lesions - clinical and endosonographic
evaluation and management. Trop Gastroenterol. 31:5–29. 2010.
|
|
16
|
Landi B and Palazzo L: The role of
endosonography in submucosal tumours. Best Pract Res Clin
Gastroenterol. 23:679–701. 2009.
|
|
17
|
Chak A, Canto MI, Rösch T, et al:
Endosonographic differentiation of benign and malignant stromal
cell tumors. Gastrointest Endosc. 45:468–473. 1997.
|
|
18
|
Palazzo L, Landi B, Cellier C, et al:
Endosonographic features predictive of benign and malignant
gastrointestinal stromal cell tumours. Gut. 46:88–92. 2000.
|
|
19
|
Fletcher CD, Berman JJ, Corless C, et al:
Diagnosis of gastrointestinal stromal tumors: A consensus approach.
Hum Pathol. 33:459–465. 2002.
|
|
20
|
Davila RE and Faigel DO: GI stromal
tumors. Gastrointest Endosc. 58:80–88. 2003.
|
|
21
|
Polkowski M, Gerke W, Jarosz D, et al:
Diagnostic yield and safety of endoscopic ultrasound-guided trucut
[corrected] biopsy in patients with gastric submucosal tumors: a
prospective study. Endoscopy. 41:329–334. 2009.
|
|
22
|
de la Serna-Higuera C, Pérez-Miranda M,
Díez-Redondo P, et al: EUS-guided single-incision needle-knife
biopsy: description and results of a new method for tissue sampling
of subepithelial GI tumors (with video). Gastrointest Endosc.
74:672–676. 2011.
|
|
23
|
Cantor MJ, Davila RE and Faigel DO: Yield
of tissue sampling for subepithelial lesions evaluated by EUS: a
comparison between forceps biopsies and endoscopic subepithelial
resection. Gastrointest Endosc. 64:29–34. 2006.
|
|
24
|
Lee CK, Chung IK, Lee SH, et al:
Endoscopic partial resection with the unroofing technique for
reliable tissue diagnosis of upper GI subepithelial tumors
originating from the muscularis propria on EUS (with video).
Gastrointest Endosc. 71:188–194. 2010.
|
|
25
|
Kobara H, Mori H, Fujihara S, Nishiyama N,
Tsutsui K and Masaki T: Gastric heterotopic pancreas can be
identified by endoscopic direct imaging with submucosal endoscopy.
J Gastrointestin Liver Dis. 22:345–348. 2013.
|
|
26
|
Kobara H, Mori H and Masaki T: Successful
en bloc resection of an esophageal hemangioma by endoscopic
submucosal dissection. Endoscopy. 44(Suppl 2): E134–E135. 2012.
|
|
27
|
Inoue H, Ikeda H, Hosoya T, et al:
Submucosal endoscopic tumor resection for subepithelial tumors in
the esophagus and cardia. Endoscopy. 44:225–230. 2012.
|