Introduction
Renal cell carcinoma (RCC) is the most common form
of kidney cancer and its incidence has risen markedly over the past
decade (1). With recent advances in
imaging technology, stage I RCC is currently acknowledged to
account for ~60% of cases of RCC (2). In the early stage, tumors (size, <7
cm) are confined to the kidneys with no lymph node involvement,
allowing for local treatment strategies.
According to recently published guidelines, partial
nephrectomy is considered to be the standard treatment for clinical
T1a and selected T1b tumors (3).
Based on the importance of preserving the renal parenchyma and
avoiding chronic kidney disease (CKD), patients with a single
kidney, bilateral renal tumors or renal insufficiency are also
candidates for partial nephrectomy, where technically possible
(3,4). Percutaneous thermal ablation is an
alternative technique to radical nephrectomy, however, its
limitations with regard to exophytic tumors <4 cm in diameter
and a complication rate of 6.9–13.5%, may preclude certain patients
from this modality (5).
Furthermore, these two nephron-sparing treatments are not suitable
for certain patients with early-stage RCC, for example those with
poor performance status or major comorbidities. A less or
non-invasive modality is required for such patients as an
alternative treatment.
RCC is considered to be a radioresistant malignancy
and conventional radiotherapy has no curative role in the treatment
of primary tumors. In addition to advances in radiotherapy
techniques, stereotactic ablative radiotherapy (SABR), also termed
stereotactic body radiotherapy, has yielded a favorable local
control rate for primary and metastatic tumors in a variety of
tissues, including radioresistant tumors, such as melanoma and RCC
(6–16). The safety and efficiency of the
local control of SABR in RCC has also been demonstrated in previous
studies (6,11–15,17).
However, few studies have investigated the effect of SABR on renal
function and have been limited to patients with normal renal
function (12). Furthermore, to the
best of our knowledge, no study on SABR has focused on patients
with RCC and pre-existing CKD, which represents a serious
complication in the preservation of renal function. The current
study investigated three patients with RCC and pre-existing CKD and
presents the preliminary results of SABR using the
CyberKnife® image-guided radiosurgery system. The
present study aimed to analyze the safety and feasibility of SABR
using CyberKnife® as well as its impact on renal
function in patients with CKD.
Patients and methods
Patients
Three patients with CKD and stage I RCC were treated
at the Stereotactic Radiosurgery System Center, Tri-Service General
Hospital (Taipei, Taiwan) between August 2009 and February 2012.
The patients included one male and two females, aged 68, 83 and 85
years, all with a Karnofsky index of ≥60 (18). All patients had moderate to severe
CKD, with an estimated glomerular filtration rate (eGFR) <60
ml/min/1.73 m2, according to the Kidney/Dialysis
Outcomes Quality Initiative (K/DOQI) classification (19). Patient 3 had undergone right radical
nephrectomy for right renal pelvis urothelial carcinoma 22 years
previously. All patients had been histologically diagnosed with
clear cell RCC (CCRCC) using computed tomography (CT)-guided
biopsy. An abdominal CT was performed prior to treatment in order
to determine tumor size and staging. These patients had been
refused radical surgery due to major comorbidities and pre-existing
CKD. The demographic and staging data are presented in Table I. Patients provided written informed
consent.
 | Table IDemographics and cancer staging in
patients with renal cell carcinoma. |
Table I
Demographics and cancer staging in
patients with renal cell carcinoma.
| Case | Gender | Age | Tumor location | Tumor size
(cm)a | Tumor stage | Comorbiditiesb | Pre-SABR eGFR
(ml/min/1.73 m2) |
|---|
| 1 | Female | 68 | R’t lower pole | 3.6 | cT1aN0M0 | Type 2 DM, HTN | 17.51 |
| 2 | Male | 83 | R’t lower pole | 5.0 | cT1bN0M0 | Type 2 DM, HTN with
CHF, L’t RAS after stent placement | 33.88 |
| 3 | Female | 85 | L’t lower pole | 5.7 | cT1bN0M0 | R’t renal pelvis UCC
after nephrectomy, Type 2 DM, HTN with CHF | 34.79 |
Positioning and target delineation
Patients were placed in the supine position,
immobilized using customized whole-body vacuum pillows (CIVCO
Medical solutions, Kalona, IA, USA) and underwent planning CT with
a 1-mm slice thickness. The gross tumor volume (GTV) and organs at
risk, including the liver, bilateral kidneys (compartment involved
excluded), stomach, small intestine, large intestine and spinal
cord, were contoured using simulation CT. The GTV is defined as the
radiographically visible tumor based on CT images and the clinical
target volume (CTV) is the equivalent to the GTV. The planning
target volume (PTV) was obtained by adding 1–3 mm to the
corresponding CTV, with modification when dose-limiting organs
overlapped (with the exception of the normal kidney).
Treatment equipment and method
In all three cases, stage I CCRCC was treated using
only the CyberKnife® SABR system (Accuray Inc.,
Sunnyvale, CA, USA) with different tumor-tracking devices. Patient
1 underwent SABR with the aid of an abdominal compression device
and vertebral tracking (X-sight; Accuray, Inc.) in order to
minimize setup errors and diaphragmatic motion; therefore, limiting
tumor movement during radiotherapy. Patients 2 and 3 were treated
using the real-time respiration tracking technique (Synchrony;
Accuray, Inc.). This technique involved the implantation of five
fiducial markers in or near the tumor under CT guidance using a
19-G needle and local anesthesia, which acted as radiographic
markers for the Synchrony tracking system. One week after
implantation, planning CT scans were performed for these two
patients. During the procedure, appropriate symptomatic treatments
were administered to manage any complications, including nausea,
fatigue or dizziness.
Dose fractionation and dosimetric
analysis
Treatments were administered in five fractions, with
8 Gy per fraction prescribed to the periphery of the PTV. Treatment
planning was performed using the MultiPlan CyberKnife®
planning system version 2.1.0 (Accuray, Inc.). The dose constraint
for the ipsilateral uninvolved kidney was less than a third of the
volume of the unilateral normal kidney that received >15 Gy.
Other organs, with dose limitations and their constraints, have
previously been reported and are shown in Table II (9). With these dose-volume limitations, the
PTVs were encompassed by the 72.0, 83.3 and 83.9% isodose curves.
Dose-volume histograms provided the required data on dose
distribution, and a conformity index (CI) and dose heterogeneity
index (HI) were used for planning evaluation. The CI is defined as
the ratio of the tissue volume that receives equal to or more than
the prescription dose, to the tumor volume, which receives equal to
or more than the prescription dose. The HI is defined as the ratio
of the maximum dose to the prescription dose. The planning data are
shown in Table III.
 | Table IIDose-volume constraints for critical
organs. |
Table II
Dose-volume constraints for critical
organs.
| Constraint |
|---|
|
|
|---|
| Organ | Absorbed ratiation,
Gy | Volume of organ
receiving radiation | Maximum dose, Gy |
|---|
| Kidney | 15 | <1/3 | - |
| Liver | <15 | >700
cm3 | - |
| Stomach | 27 | <5
cm3 | <31 |
| Small intestine | 25 | <5
cm3 | <29 |
| Large intestine | 25 | <5
cm3 | <29 |
| Spinal cord | - | - | <25 |
 | Table IIIDose-volume parameters for
stereotactic ablative radiotherapy. |
Table III
Dose-volume parameters for
stereotactic ablative radiotherapy.
| Case | CTV (cc) | PTV (cc) | Margin (mm) | Coverage (%) | V15 (%) | CI | HI | Total delivery time
(h) |
|---|
| 1 | 40.0 | 46.3 | 1 | 90.26 | 28.16 | 1.54 | 1.39 | 10.5 |
| 2 | 46.6 | 68.2 | 3 | 83.27 | 18.37 | 1.43 | 1.43 | 9.5 |
| 3 | 67.0 | 97.1 | 2 | 83.93 | 15.73 | 1.24 | 1.43 | 8.0 |
Post-treatment follow-up
All patients were examined daily during treatment to
assess acute toxicity effects. Subsequent to treatment, patients
were followed up every 1–2 months for the initial 6 months and
every 3–4 months thereafter. History taking, clinical examination
and serum biochemistry analysis were performed at each follow-up.
Toxicity was recorded based on the worst toxicity experienced and
was graded according to the Radiation Therapy Oncology Group (RTOG)
radiation injury grading criteria (19). Acute toxicity was defined as an
adverse event occurring within three months of radiotherapy and
late toxicity was defined as an adverse event occurring after three
months (20). Surveillance CT scans
were performed at 3–4-month intervals subsequent to SABR and the
Response Evaluation Criteria in Solid Tumors (21) was used to assess the response.
Renal function assessment
Renal function was assessed using the eGFR, which
was calculated using the Modification of Diet in Renal Disease
Study formula as follows: eGFR (ml/min/1.73 m2) = 186 ×
serum creatinine (Scr) - 1.154 × age - 0.203 × (0.742 if
female) × (1.233 if Chinese) (22).
The severity of CKD was graded according to the K/DOQI
classification: Stage 1 (>90 ml/min/1.73 m2; kidney
damage/normal GFR); stage 2 (60–89 ml/min/1.73 m2;
kidney damage/mild decrease in GFR); stage 3 (30–59 ml/min/1.73
m2; kidney damage/moderate decrease in GFR); stage 4
(15–29 ml/min/1.73 m2; kidney damage/severe decrease in
GFR); and stage 5 (<15 ml/min/1.73 m2; kidney
failure) (20).
Results
Tumor response and survival
At the censor date, all patients were alive and
their total follow-up times were 40, 13 and 12 months post-SABR.
Non-enhanced abdominal CT scans were performed every three months
to assess the tumor response, with results indicating that all
patients had a stable condition in the area that was irradiated.
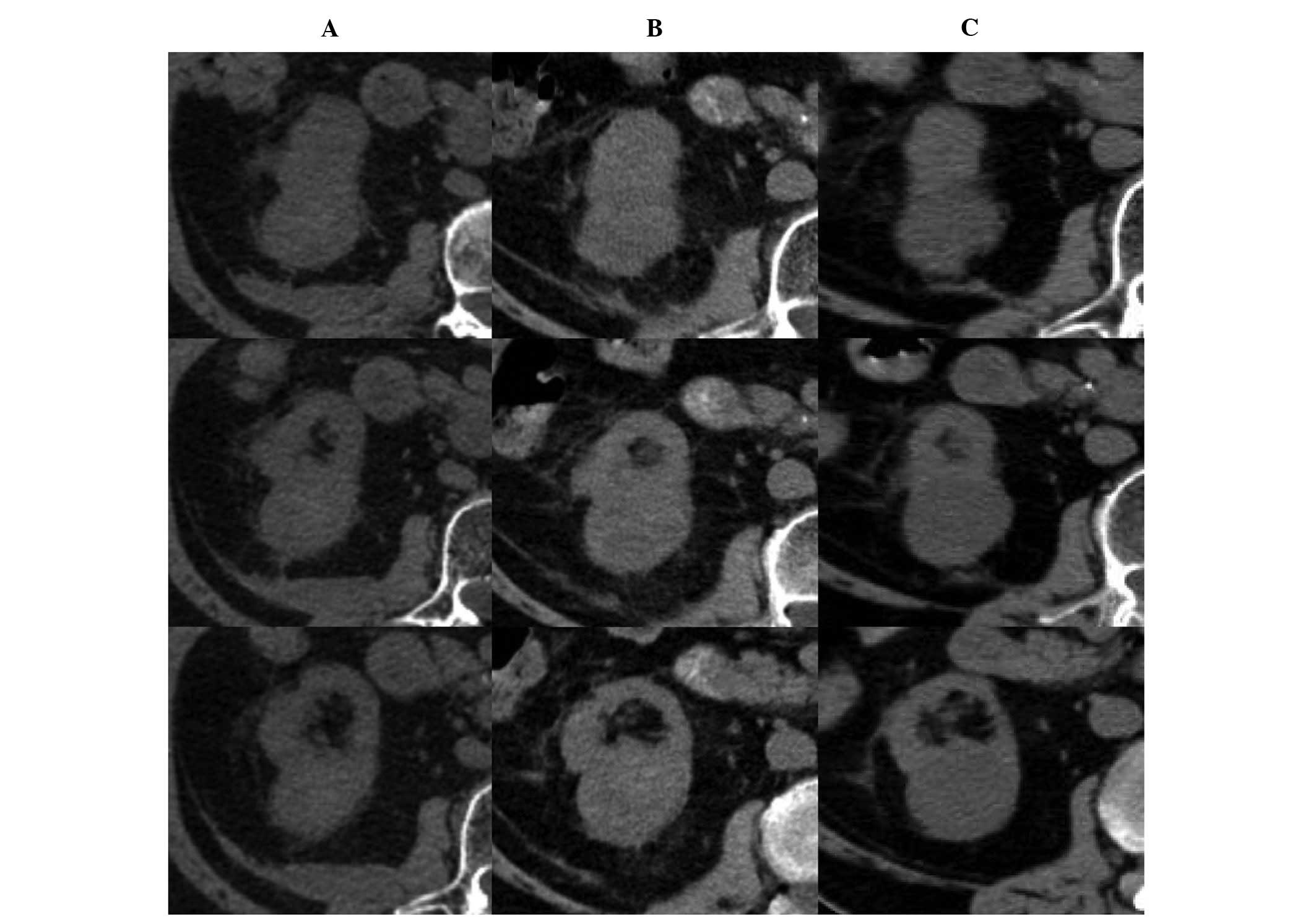
Fig. 1 shows representative images
of the patients pre- and post-SABR. Patient 3 developed
asymptomatic multiple metastases nine months after SABR, for which
sorafenib was administered for disease control.
Toxicities
All acute toxicities were grade 1. Patient 3 had
grade 1 nausea and dizziness following the administration of the
first two fractions of SABR, however, these symptoms were
self-limiting and rapidly improved following the completion of the
course of radiotherapy. Patients 1 and 2 tolerated the treatment
well and exhibited no adverse acute effects. After three months,
toxicity analysis revealed no adverse late reactions. At the time
of analysis, no grade 3 or 4 toxicity was observed.
Renal function following SABR
No patients received dialysis up to the censor date.
The eGFR pre- and post-SABR is shown in Fig. 1. In patient 1, at the 26-month
follow-up the eGFR was found to have reduced from 17.51 to 12.28
ml/min/1.73 m2 [Scr, 3.4–4.6 mg/dl, (35%)]
with the K/DOQI stage increasing from 4 to 5 (kidney failure) and
the eGFR was predicted to reduce further. The other two patients
showed little change in Scr levels; however, the K/DOQI
stage increased from 3 to 4.
Discussion
Renal cancer is commonly associated with CKD as
tumors impair renal function (23).
Thus, patients with RCC are at a high risk of developing CKD
complications, including cardiovascular diseases and renal failure,
and mortality (24). For these
reasons, it is of particular importance to preserve renal function
while treating patients who have primary renal cancer with
pre-existing CKD, without compromising their treatment response.
The present study aimed to investigate a novel application for SABR
using CyberKnife® as a non-invasive, nephron-sparing
treatment for stage I RCC in patients diagnosed with significant
kidney dysfunction.
In the present study, the efficacy of SABR was
retrospectively assessed in three patients diagnosed with stage I
CCRCC (tumor size, 3.6–5.7 cm) and moderate to severe CKD. Local
control was achieved in all three patients following the delivery
of 40-Gy radiotherapy over five consecutive days. Distant
metastasis was detected in one patient after nine months of
follow-up. To the best of our knowledge, this is the first study to
analyze the efficiency of SABR in patients with CKD and RCC, which
is most commonly used for treating patients with RCC who have
normal kidney function (17). The
present study indicates that the same dosimetry may be used for
patients with CKD through patient-specific optimization of the
targeted area using the CyberKnife® Multiplan system,
which is a high precision radiation delivery system that spares the
surrounding normal tissue. In patients with RCC with normal kidney
function, the crude local control rate and estimated two-year local
control rate following SABR CyberKnife® treatment have
been reported to be between 84 and 100%, and 86 and 100%,
respectively (17). Overall,
results from these studies are consistent with the treatment
success reported in patients with RCC with normal kidney function
(6,11–15,17).
The safety of SABR was assessed according to the
RTOG radiation injury grading criteria for adverse effects and with
regard to the preservation of renal function. The treatment was
well tolerated in terms of general adverse effects, with only one
patient reporting transient nausea and dizziness. The most
important limiting factor of this treatment appears to be the
initial level of renal function. While patient 1 had stage 4 CKD,
the other two patients had stage 3. The patient with the most
advanced CKD experienced a gradual loss of kidney function
following treatment, which culminated in kidney failure after 26
months. The two patients with stage 3 CKD also exhibited altered
Scr levels following SABR, with the stage of CKD
severity increasing from 3 to 4, but with no renal failure. The
poor pre-SABR renal function in patient 1 may have contributed to
this result and patients with impending renal failure may benefit
from this type of treatment, which may delay the requirement for
dialysis.
One important factor that should be considered in
radiation therapy of renal tumors, regardless of the technique
used, is the quantity of renal volume that should be spared to
prevent the occurrence of renal failure. The QUANTEC group proposed
that almost complete sparing of a substantial proportion of the
kidney volume is associated with the preservation of renal
function, even with the focal delivery of high-dose radiation, for
example SABR, and a no dose constraint is recommended for kidney
sparing during SABR (20).
Preservation of function may be due to the compensatory increase in
renal function of the spared kidney volume. Compensatory capacity
is reduced with increases in the irradiated kidney volume (25). Although, to the best of our
knowledge, no reports of clinically relevant symptomatic renal
dysfunction following SABR have been reported to date (17), few studies have investigated kidney
tolerance and the effects of SABR on renal function in patients
with CKD.
The dose-volume constraints on the normal kidney
during SABR for RCC are critical, however, have yet to be
established. Cassady (26) proposed
a threshold dose of 15 Gy for renal injury based on data on
bilateral whole kidney irradiation. Although the safety of the
administration of higher doses in partial kidney irradiation has
been demonstrated, the majority of the data on partial kidney
radiation tolerance is based on small fraction sizes (0.4–2.0 Gy
per fraction) (20). Svedman et
al (12) investigated kidney
injury following SABR in seven patients, each with only one
functioning kidney. With a maximum V15 of 37.3%, five patients were
found to have stable renal function following SABR, whereas the
other two exhibited modest changes in Scr after two and
six years of follow-up, without the requirement for dialysis or
other medical intervention. The V15 in the unilateral normal kidney
may be an appropriate dose-volume constraint in SABR treatment
planning. Pre-existing renal insufficiency may further reduce
kidney radiation tolerance to a variable degree, thus a more
stringent dose-volume constraint may be required for patients with
CKD compared with those used in previous studies (20). In the present study, in accordance
with previous findings with SABR on HCC with certain modifications,
the V15 at less than a third of the unilateral normal kidney was
set as our dose constraint in SABR treatment planning, aiming to
spare as much normal kidney volume as possible (9).
The limitations of the present study are its
retrospective nature, low patient numbers and relatively short
follow-up for renal function. It would be valuable to assess the
impact of SABR on renal function in this patient population over a
longer period of time and in a prospective manner.
In conclusion, the present study has demonstrated
the preliminary findings for the local control, side-effects and
renal function status following SABR using CyberKnife®
in patients with RCC and pre-existing CKD. Therefore, SABR may be
an acceptable alternative treatment option in patients for whom
nephron-sparing surgery is contraindicated.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012.
|
|
2
|
Sun M, Thuret R, Abdollah F, Lughezzani G,
Schmitges J, Tian Z, Shariat SF, Montorsi F, Patard JJ, Perrotte P
and Karakiewicz PI: Age-adjusted incidence, mortality, and survival
rates of stage-specific renal cell carcinoma in North America: a
trend analysis. Eur Urol. 59:135–141. 2011.
|
|
3
|
Campbell SC, Novick AC, Belldegrun A,
Blute ML, Chow GK, Derweesh IH, Faraday MM, Kaouk JH, Leveillee RJ,
Matin SF, Russo P and Uzzo RG: Guideline for management of the
clinical T1 renal mass. J Urol. 182:1271–1279. 2009.
|
|
4
|
Huang WC, Levey AS, Serio AM, Snyder M,
Vickers AJ, Raj GV, Scardino PT and Russo P: Chronic kidney disease
after nephrectomy in patients with renal cortical tumours: a
retrospective cohort study. Lancet Oncol. 7:735–740. 2006.
|
|
5
|
del Laguna MP, Zondervan PJ and de la
Rosette JJ: Focal therapy in the management of small renal masses.
Curr Opin Urol. 22:372–378. 2012.
|
|
6
|
Beitler JJ, Makara D, Silverman P and
Lederman G: Definitive, high-dose-per-fraction, conformal,
stereotactic external radiation for renal cell carcinoma. Am J Clin
Oncol. 27:646–648. 2004.
|
|
7
|
Blomgren H, Lax I, Näslund I and Svanström
R: Stereotactic high dose fraction radiation therapy of
extracranial tumors using an accelerator. Clinical experience of
the first thirty-one patients. Acta Oncol. 34:861–870. 1995.
|
|
8
|
Gunvén P, Blomgren H and Lax I:
Radiosurgery for recurring liver metastases after hepatectomy.
Hepatogastroenterology. 50:1201–1204. 2003.
|
|
9
|
Huang WY, Jen YM, Lee MS, Chang LP, Chen
CM, Ko KH, Lin KT, Lin JC, Chao HL, Lin CS, et al: Stereotactic
body radiation therapy in recurrent hepatocellular carcinoma. Int J
Radiat Oncol Biol Phys. 84:355–361. 2012.
|
|
10
|
Lax I, Blomgren H, Näslund I and Svanström
R: Stereotactic radiotherapy of malignancies in the abdomen.
Methodological aspects. Acta Oncol. 33:677–683. 1994.
|
|
11
|
Stinauer MA, Kavanagh BD, Schefter TE,
Gonzalez R, Flaig T, Lewis K, Robinson W, Chidel M, Glode M and
Raben D: Stereotactic body radiation therapy for melanoma and renal
cell carcinoma: impact of single fraction equivalent dose on local
control. Radiat Oncol. 6:342011.
|
|
12
|
Svedman C, Karlsson K, Rutkowska E,
Sandström P, Blomgren H, Lax I and Wersäll P: Stereotactic body
radiotherapy of primary and metastatic renal lesions for patients
with only one functioning kidney. Acta Oncol. 47:1578–1583.
2008.
|
|
13
|
Svedman C, Sandström P, Pisa P, Blomgren
H, Lax I, Kälkner KM, Nilsson S and Wersäll P: A prospective Phase
II trial of using extracranial stereotactic radiotherapy in primary
and metastatic renal cell carcinoma. Acta Oncol. 45:870–875.
2006.
|
|
14
|
Teh B, Bloch C, Galli-Guevara M, Doh L,
Richardson S, Chiang S, Yeh P, Gonzalez M, Lunn W, Marco R, et al:
The treatment of primary and metastatic renal cell carcinoma (RCC)
with image-guided stereotactic body radiation therapy (SBRT).
Biomed Imaging Interv J. 3:e62007.
|
|
15
|
Wersäll PJ, Blomgren H, Lax I, Kälkner KM,
Linder C, Lundell G, Nilsson B, Nilsson S, Näslund I, Pisa P and
Svedman C: Extracranial stereotactic radiotherapy for primary and
metastatic renal cell carcinoma. Radiother Oncol. 77:88–95.
2005.
|
|
16
|
Wulf J, Haedinger U, Oppitz U, Thiele W,
Mueller G and Flentje M: Stereotactic radiotherapy for primary lung
cancer and pulmonary metastases: a noninvasive treatment approach
in medically inoperable patients. Int J Radiat Oncol Biol Phys.
60:186–196. 2004.
|
|
17
|
Siva S, Pham D, Gill S, Corcoran NM and
Foroudi F: A systematic review of stereotactic radiotherapy
ablation for primary renal cell carcinoma. BJU Int. 110:E737–E743.
2012.
|
|
18
|
Karnofsky DA, Abelmann WH, Craver LF and
Burchenal JH: The use of the nitrogen mustards in the palliative
treatment of carcinoma. With particular reference to bronchogenic
carcinoma. Cancer. 1:634–656. 1948.
|
|
19
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the Radiation Therapy Oncology Group (RTOG) and the
European Organization for Research and Treatment of Cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995.
|
|
20
|
Dawson LA, Kavanagh BD, Paulino AC, Das
SK, Miften M, Li XA, Pan C, Ten Haken RK and Schultheiss TE:
Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys.
76(3 Suppl): S108–S115. 2010.
|
|
21
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
|
|
22
|
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y,
Xu JS, Huang SM, Wang LN, Huang W, et al: Modified glomerular
filtration rate estimating equation for Chinese patients with
chronic kidney disease. J Am Soc Nephrol. 17:2937–2944. 2006.
|
|
23
|
Russo P: End stage and chronic kidney
disease: associations with renal cancer. Front Oncol. 2:282012.
|
|
24
|
Go AS, Chertow GM, Fan D, McCulloch CE and
Hsu CY: Chronic kidney disease and the risks of death,
cardiovascular events, and hospitalization. N Engl J Med.
351:1296–1305. 2004.
|
|
25
|
Ritchey ML, Green DM, Thomas PR, Smith GR,
Haase G, Shochat S, Moksness J and Breslow NE: Renal failure in
Wilms’ tumor patients: a report from the National Wilms’ Tumor
Study Group. Med Pediatr Oncol. 26:75–80. 1996.
|
|
26
|
Cassady JR: Clinical radiation
nephropathy. Int J Radiat Oncol Biol Phys. 31:1249–1256. 1995.
|