Introduction
Colorectal cancer (CRC) is an important contributor
to cancer-related mortality and morbidity. Accumulated data has
uncovered several critical genes and pathways important in the
initiation and progression of CRC (1–3).
Large-scale sequencing analyses have identified numerous
recurrently mutated genes and chromosomal translocations (4–6). In
addition, a number of microRNAs (miRNAs) have been previously
reported to be associated with CRC (7,8).
However, how miRNA changes contribute to colorectal tumorigenesis
has not yet been defined. Further insight into these changes may
identify potential biomarkers or therapeutic targets.
miRNAs are small non-protein coding RNA molecules
that regulate gene expression (9,10).
miRNAs are important in crucial cellular processes, including
development, differentiation, proliferation, apoptosis and
metabolism (11,12). miRNAs have been proven to interact
with potential oncogenes or tumor suppressors, and a number of
miRNAs are differentially expressed in normal and neoplastic
tissues and in tumors. The differential expression of miRNA has
been previously evaluated as a predictive signature of cancer
(13–17).
The present study investigated the expression
profile of miRNAs in the serum of CRC patients. In total, 19 miRNAs
were identified by high-throughput sequencing. A network analysis
was performed based on a computational approach to identify
associations between CRC and miRNAs. The network analysis of
miRNA-target genes may aid in identifying altered miRNA regulatory
networks that are involved in tumor pathogenesis.
Materials and methods
Samples and RNA extraction
Primary tumor and neighboring non-tumorous tissues
were obtained from five CRC patients. All samples were collected
according to procedures approved by the Institutional Review Board
of the Guangzhou Medical University (Guangzhou, China) and
individuals can not be identified from data or images included in
the present study.
Tissue samples were flash frozen in liquid nitrogen
and stored at −80°C until nucleic acid extraction. In total, 200 mg
of fresh frozen tissues were used to isolate total RNA by phenol
extraction (TRIzol reagent; Invitrogen, Life Technologies,
Carlsbad, CA, USA). RNA concentration and purity were controlled by
A NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham,
MA, USA). The Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.,
Santa Clara, CA, USA) was used to measure the quantity, integrity
and purity of the small RNA. Patients provided written informed
consent and the study was approved by the ethics committee of the
Third Hospital of the Guangzhou Medical University.
miRNA sequencing and sequence
analysis
The miRNA expression profile was determined using
the Ion Torrent PGM™ sequencer (Life Technologies). Briefly, the
Ion Total RNA-Seq kit v2 (Life Technologies) was used to make small
RNA libraries that preserve strand information. The templates were
prepared using the Ion OneTouch™ system (Life Technologies), and
small RNA analysis was performed using the Ion PGM™ sequencer. The
miRNA sequences were analyzed by the Torrent Suite software (Life
Technologies), and by miRWalk (http://mirwalk.uni-hd.de) and miRBase (http://www.mirbase.org). In addition, gene ontology
(GO) and pathway analyses were performed using several tools that
identify pathways and GO based on data sets from sequencing with
the intent to identify miRNA-related genes and pathways. The tools
include Ingenuity Systems Bioinformatics Software (Ingenuity
Systems, Inc., Redwood City, CA, USA), FatiGO (http://fatigo.bioinfo.cnio.es) and Gene Expression
Omnibus (http://www.ncbi.nlm.nih.gov/gds).
Results
Altered miRNA expression in CRC
patients
The miRNA profiles of five pairs of solid tumor and
adjacent tissues were compared. In total, 16 miRNAs exhibited
higher expression in the solid tumor tissues, while three miRNAs
exhibited higher expression profiles in the normal tissues; these
are listed in Table I.
 | Table IIdentification of miRNAs with higher
expression profiles in normal or solid tumor tissues. |
Table I
Identification of miRNAs with higher
expression profiles in normal or solid tumor tissues.
| Solid tumor
tissues | Normal adjacent
tissues |
|---|
| hsa-miR-135b-5p | hsa-miR-100-5p |
| hsa-miR-146a-5p | hsa-miR-138-5p |
| hsa-miR-148b-3p | hsa-miR-191-5p |
| hsa-miR-17-5p | |
| hsa-miR-196a-5p | |
| hsa-miR-200a-3p | |
| hsa-miR-20a-5p | |
| hsa-miR-21-5p | |
| hsa-miR-223-3p | |
| hsa-miR-27a-5p | |
| hsa-miR-29b-3p | |
| hsa-miR-30e-5p | |
| hsa-miR-374b-5p | |
| hsa-miR-4787-5p | |
| hsa-miR-485-3p | |
| hsa-miR-660-5p | |
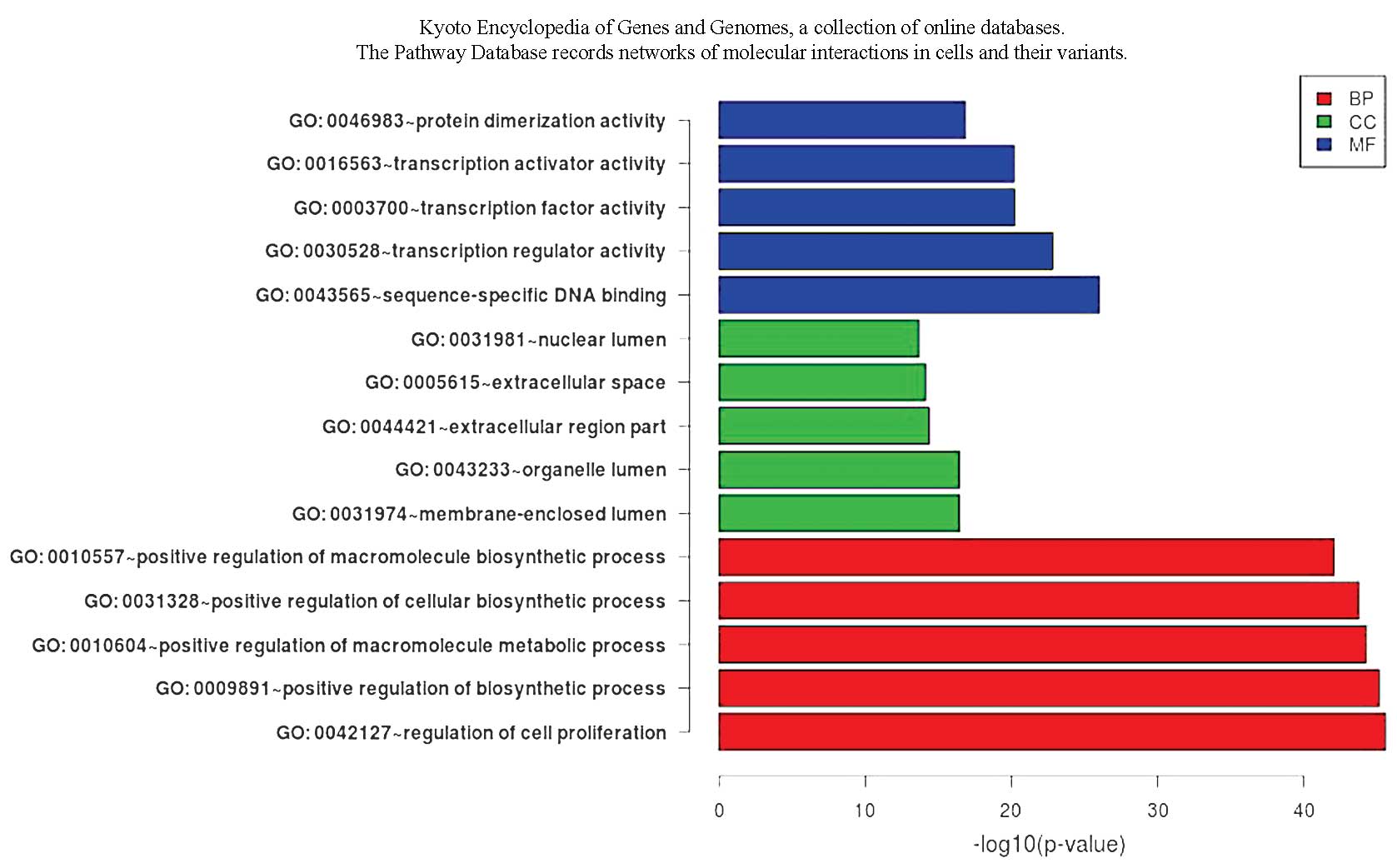
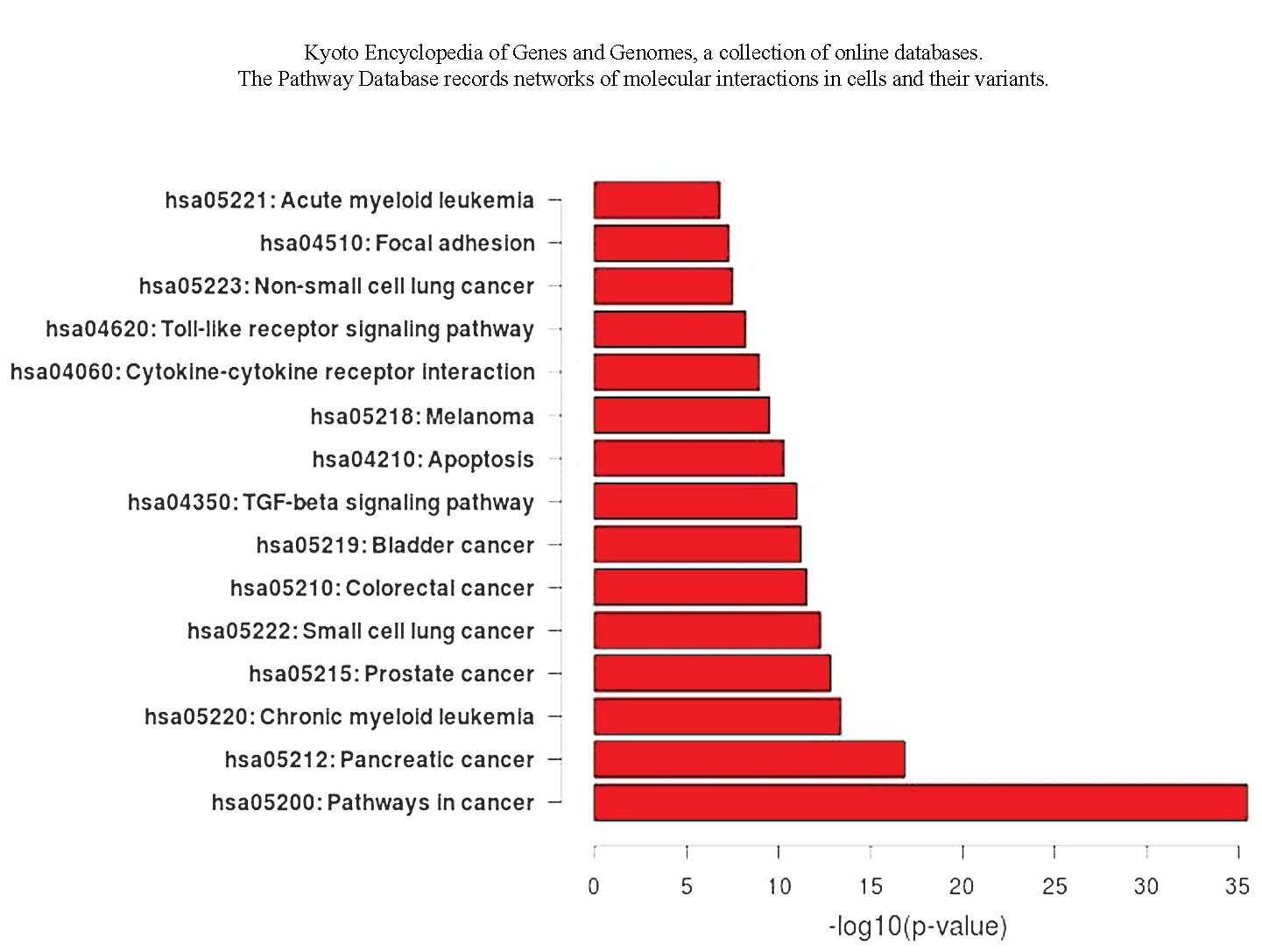
GO and pathway analyses of miRNA target
genes
The results of the GO analysis showed that a number
of target genes are involved in cell proliferation and apoptosis.
The pathway analysis showed that the transforming growth factor β
and Toll-like signal pathways are also involved (Fig. 1 and 2).
Discussion
miRNAs are small non-coding RNAs that enhance the
cleavage or translational repression of specific mRNAs with
recognition site(s) in the 3′-untranslated region. Since the
identification of the miRNAs, several large-scale studies have
compared the profiles of miRNA expression patterns between
non-tumor and tumor tissues (18,19). A
number of lines of evidence have shown that miRNA expression is
predictive of outcome in patients with solid tumors. In lung
cancer, low levels of let-7a have been associated with a short
survival time following surgery. In addition, previous miRNA
microarray expression profiling of tumors and paired non-tumorous
tissues has been performed in colon cancer patients to identify
miRNA expression patterns associated with outcome, and high levels
of miR-21 have been found to be associated with a short overall
survival time, independent of other factors (19,20).
In the current study, 19 miRNAs were identified by
high-throughput sequencing. A network analysis was performed based
on a computational approach to identify associations between CRC
and miRNAs. The current study may be useful to identify
cancer-specific signatures and potentially useful biomarkers for
the diagnosis of CRC. The network analysis of miRNA-target genes
may aid in identifying altered miRNA regulatory networks that are
involved in tumor pathogenesis.
Acknowledgements
The present study was supported by the Science and
Information Technology Foundation of Guangzhou (grant nos.
2010J-E141 and 2011J4100051), the Guangdong Provincial Science and
Technology Department (grant no. 2011B0904005260) and the Natural
Science Foundation of Guangdong Province (grant no.
S2012040006707).
References
|
1
|
Tjandra JJ, Kilkenny JW, Buie WD, et al:
Standards Practice Task Force; American Society of Colon and Rectal
Surgeons: Practice parameters for the management of rectal cancer
(revised). Dis Colon Rectum. 48:411–423. 2005.
|
|
2
|
Fearon ER: Molecular genetics of
colorectal cancer. Annu Rev Pathol. 6:479–507. 2011.
|
|
3
|
Aaltonen LA, Peltomäki P, Leach FS, et al:
Clues to the pathogenesis of familial colorectal cancer. Science.
260:812–816. 1993.
|
|
4
|
Bass AJ, Lawrence MS, Brace LE, et al:
Genomic sequencing of colorectal adenocarcinomas identifies a
recurrent VTI1A-TCF7L2 fusion. Nat Genet. 43:964–968. 2011.
|
|
5
|
Sjöblom T, Jones S, Wood LD, et al: The
consensus coding sequences of human breast and colorectal cancers.
Science. 314:268–274. 2006.
|
|
6
|
Wood LD, Parsons DW, Jones S, et al: The
genomic landscapes of human breast and colorectal cancers. Science.
318:1108–1113. 2007.
|
|
7
|
Schepeler T, Reinert JT, Ostenfeld MS, et
al: Diagnostic and prognostic microRNAs in stage II colon cancer.
Cancer Res. 68:6416–6424. 2008.
|
|
8
|
Schetter AJ, Leung SY, Sohn JJ, et al:
MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–436.
2008.
|
|
9
|
Lim LP, Glasner ME, Yekta S, Burge CB and
Bartel DP: Vertebrate microRNA genes. Science. 299:15402003.
|
|
10
|
Zeng Y and Cullen BR: Sequence
requirements for micro RNA processing and function in human cells.
RNA. 9:112–123. 2003.
|
|
11
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.
|
|
12
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006.
|
|
13
|
Perera RJ and Ray A: MicroRNAs in the
search for understanding human diseases. BioDrugs. 21:97–104.
2007.
|
|
14
|
Iorio MV, Visone R, Di Leva G, et al:
MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007.
|
|
15
|
Caldas C and Brenton JD: Sizing up miRNAs
as cancer genes. Nat Med. 11:712–714. 2005.
|
|
16
|
Jones KB, Salah Z, Del Mare S, et al:
miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012.
|
|
17
|
Volinia S, Galasso M, Costinean S, et al:
Reprogramming of miRNA networks in cancer and leukemia. Genome Res.
20:589–599. 2010.
|
|
18
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005.
|
|
19
|
Luo X, Burwinkel B, Tao S and Brenner H:
MicroRNA signatures: novel biomarker for colorectal cancer? Cancer
Epidemiol Biomarkers Prev. 20:1272–1286. 2011.
|
|
20
|
Jeong HC, Kim EK, Lee JH, Lee JM, Yoo HN
and Kim JK: Aberrant expression of let-7a miRNA in the blood of
non-small cell lung cancer patients. Mol Med Rep. 4:383–387.
2011.
|