1. Introduction
Human papillomavirus (HPV), the most extensively
studied virus of the past decade, is composed of a particularly
heterogeneous family of DNA viruses, which has the ability to
infect keratinocytes of the human skin and mucosa (1). HPV, which appears to invade the basal
layer of epithelial cells, is a common pathogen associated with a
wide range of cutaneous and mucosal infections (2). HPV can be transmitted through physical
contact via autoinoculation or fomites, sexual contact, as well as
vertically from the HPV-positive mother to her newborn and can
cause subclinical or clinical infections (1,2).
HPV-associated clinical infections include skin warts, genital
warts, recurrent respiratory papillomatosis (RRP), low-grade and
high-grade squamous intraepithelial lesions (SILs) and cervical
cancer, which globally represents the second most frequent cancer
in females (3).
In infancy and childhood, HPV infection involving
skin warts, genital warts and juvenile RRP among both male and
female neonates and children, as well as cervical SILs among
adolescent girls (Fig. 1), has been
excessively investigated [see reviews by Mammas et al
(2) and Syrjänen (4)]. This scientific effort began in 1978,
almost 35 years ago, when the first report involving children in
HPV research was published by Pfister and zur Hausen (5). To date, several researchers,
worldwide, have studied HPV from the paediatric point of view,
expanding the usage of molecular techniques, such as the polymerase
chain reaction (PCR) in samples obtained from children. During the
past years, a great expansion has taken place in the field due to
the introduction of the vaccination programmes against HPV into
clinical practice. In this review, we briefly summarize some of the
historical aspects of peadiatric HPV research until the time point
of this great expansion.
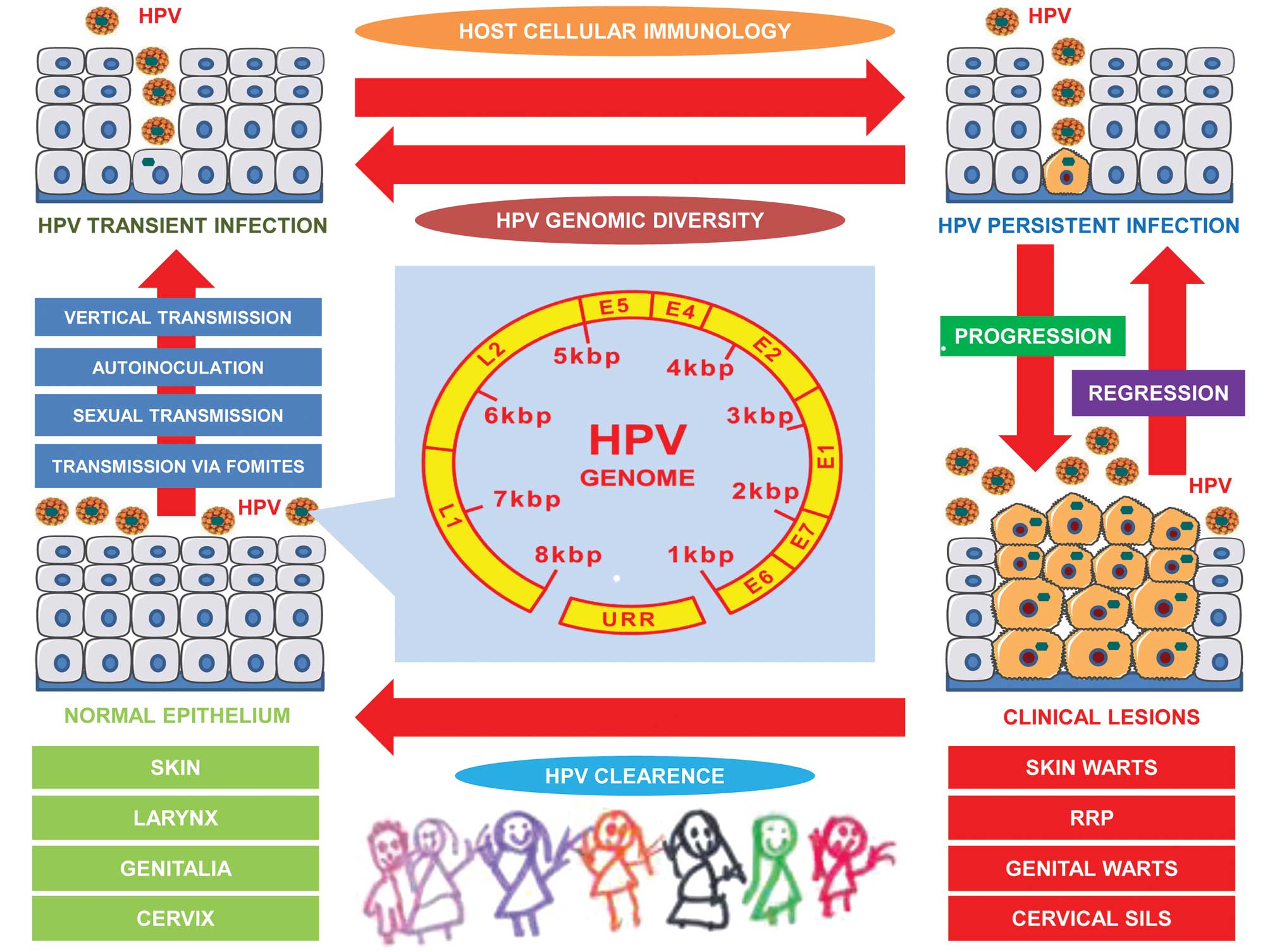 | Figure 1Association between HPV and clinical
lesions in neonates and children. HPV can be transmitted via
autoinoculation or via fomites, sexual contact or vertically from
the HPV-positive mother to her newborn, initially causing HPV
transient infection, which can consequently progress to HPV
persistent infection. HPV persistence can either regress, or can
become symptomatic, establishing clinical lesions in different
anatomical sites. In infancy and childhood, HPV clearance can occur
automatically. HPV, human papilloma virus; RRV, recurrent
respiratory papillomatosis; SILs, squamous intraepithelial lesions;
URR, upstream regulatory region. |
2. Historical background
HPV-associated lesions, including skin and genital
warts, have been reported since ancient times (6). In the 4th century B.C. Hippokrates the
Asclepiad, first described skin warts, genital warts and cervical
neoplasia (6–8). Although Hippokrates was certainly not
the first to discover cervical neoplasia, he referred to a cervical
lesion, which in Greek is termed ‘ἔλκoς’ (6), meaning ‘ulcer’ that can potentially
progress to cervical cancer, indicating that HPV-associated SILs
can progress to invasive cervical cancer. This knowledge referring
to the physical history of HPV infection in the cervix is apparent
in the impressive description by Hippokrates: ‘ɛἰ δὲ μὴ ἐμɛλɛδάνθη,
μηδὲ oἱἡ κάθαϱσις ἐϱϱάγη αὐτόματη, τὸἓλκoς μέζoν ἐπoίησεν καὶ μὴ
ἀνει】σα ἑκινδὸνɛυσɛν ɛἰς τὸ καϱϰινωθη̑ναι τὰ ἓλκɛα’ (7), meaning that ‘if it (the infection) is
not taken care of, catharsis will not take place automatically, and
thus the ulcer will increase in size and if it does not regress,
there is a risk of the ulcers becoming cancerous’.
Despite the fact that skin and genital warts have
been considered infectious since this early period, the development
of cervical cancer due to infection was only suspected in the 19th
century A.C. by an Italian scientist from Asiago, Italy, the
surgeon Antonio Domenico Rigoni-Stern (9). In 1928, a Greek scientist originating
from the island of Euboea, Dr George N. Papanicolaou [a brief
referral to his life is presented in the article by Mammas and
Spandidos (10)] observed
precancerous HPV-associated lesions in vaginal smears collected
from females, an observation which led to the development of the
Pap smear test (11,12). The first description of HPV was
provided in 1949 by Strauss et al (13), who used electron microscopy
to examine aqueous extracts of wart tissues, while in 1963 the
physical properties of HPV DNA were described in the study by
Crawford and Crawford (14). It was
not until the 1970s, that a role of HPV in cervical cancer was
postulated for the first time by Professor Harald zur Hausen, the
‘Father of HPV Virology’ (3). It is
currently well established that HPV is involved in human
carcinogenesis, causing not only the vast majority of cervical, but
also a substantial proportion of other non-genital cancers
(15).
3. HPV in children: a brief overview
Although the infectious cause of warts in children
was known by the end of the 19th century (16), initial studies on children using
molecular hybridization techniques were performed in the end of the
1970s. In an early study by Pfister and zur Hausen (5) in 1978, it was well documented that HPV
1, HPV 2 and HPV 3 predominate in skin warts in children between
the ages of 5 and 15 years, while HPV 4 is most often isolated in
children of older ages. As is presented in Table I, this article was the first in the
literature (5,17–50)
involving samples obtained from children in HPV research. Evidence
of the presence of HPV in juvenile RRP also dates back to the
beginning of the 1980s (17–19).
These studies have provided strong evidence of the etiology of
tumors caused by HPV that was verified by subsequent studies on
RRP.
 | Table IHPV pre-vaccination research and
children: a brief overview. |
Table I
HPV pre-vaccination research and
children: a brief overview.
| Year of
publication | Authors/(Refs.) | Contribution |
|---|
| 1978 | Pfister and zur
Hausen (5) | HPV types in skin
warts in children |
| 1981 | Costa et al
(17) | HPV types in juvenile
RRP |
| 1982 | Braun et al
(18) | HPV types in juvenile
RRP |
| Mounts et al
(19) | HPV types in juvenile
RRP |
| 1986 | Roman and Fife
(20) | HPV types in foreskin
in neonates |
| Rock et al
(21) | HPV types in genital
warts in children |
| 1987 | Vallejos et al
(22) | HPV types in genital
warts in children |
| 1988 | Steinberg (23) | HPV types in genital
warts in children |
| 1989 | Hanson et al
(24) | HPV mother-to-infant
transmission |
| Sedlacek et al
(25) | HPV mother-to-infant
transmission |
| 1990 | Gibson et al
(26) | HPV types in genital
warts in children |
| Padel et al
(27) | HPV types in genital
warts in children |
| Jenison et al
(28) | HPV types in oral
samples in asymptomatic children |
| 1991 | Smith et al
(29) | HPV mother-to-infant
transmission |
| 1993 | Fredericks et
al (30) | HPV mother-to-infant
transmission |
| 1994 | Pakarian et al
(31) | HPV mother-to-infant
transmission |
| Kaye et al
(32) | HPV viral load as a
determinant for mother-to-infant transmission |
| 1995 | Cason et al
(33) | HPV
mother-to-infant transmission |
| 1996 | Alberico et
al (34) | HPV
mother-to-infant transmission |
| 1998 | Tseng et al
(35) | Evaluation of HPV
infection and mode of delivery |
| 2000 | Rice et al
(36) | HPV types in oral
samples in asymptomatic children |
| 2005 | Rintala et
al (37) | HPV
mother-to-infant transmission |
| Chen et al
(38) | HPV types in
tonsils in asymptomatic children |
| 2006 | Sisk et al
(39) | HPV types in
tonsils in asymptomatic children |
| Mammas et al
(40) | HPV types in
tonsils in asymptomatic children |
| 2008 | Sarkola et
al (41) | Evaluation of HPV
types in breast milk |
| Mammas et al
(42) | HPV types in skin
warts in children |
| 2009 | Cazzaniga et
al (43) | Evaluation of HPV
types in breast milk |
| 2010 | Mammas et al
(44) | HPV
mother-to-infant transmission |
| Mammas et al
(45) | Novel HPV types in
juvenile RRP |
| Mammas et al
(46) | Evaluation of HPV
infection and neonatal prematurity |
| 2011 | Mammas et al
(47) | HPV types in lower
respiratory tract in children |
| Yoshida et
al (48) | Evaluation of HPV
types in breast milk |
| Mammas et al
(49) | Evaluation of HPV
types in breast milk |
| 2012 | Mammas et al
(50) | Evaluation of HPV
persistence and mode of delivery |
During the second half of the 1980s, a clear picture
of the presence of specific types of HPV in genital warts in
children was obtained (21,22,24).
These initial reports enthusiastically supported HPV typing as an
important prognostic tool for HPV-infected children, particularly
in those infected with HPV 16 and HPV 18, due to the highly
oncogenic potential of these two HPV types (24). For this reason, at that time,
several paediatric departments were requesting HPV typing in cases
with genital warts in order to identify children who were at a risk
of developing cancer. At the same time, researchers evaluated the
impact of the presence of HPV in the diagnosis of sexual abuse.
However, early enough, it was made clear that HPV typing using
molecular techniques is not sufficient to determine the source of
HPV infection and pursue the possibility of sexual abuse (24,26,27).
In the study by Padel et al (27), it was well established that HPV
typing does not provide substantial evidence of the presence or
absence of sexual transmission.
The presence of HPV DNA in asymptomatic neonates was
initially documented in foreskins by Roman and Fife (20). Soon after the report in 1988 by
Steinberg (23) addressing the
transmission of HPV to the fetus, a number of studies investigated
the perinatal modes of HPV transmission in childhood (25,29).
These studies supported a vertical transmission mechanism of HPV in
children based on the presence of HPV DNA in asymptomatic neonates
in oral and genital samples at or shortly after birth (25). The detection of HPV DNA in the
amniotic fluid also suggested an in utero mechanism of HPV
transmission (25). Smith et
al provided evidence indicating the prevalence of HPV among
pregnant women increases with the gestational age from 8.0% in the
first trimester to 23.1% in the third trimester (29), while, in 1994, Kaye et al
(32) suggested that viral load is
a determinant for HPV transmission to the neonate. In the study by
Fredericks et al (30) in
1993, it was well established that the contamination of neonates
occurs commonly at birth and persists for at least 6 weeks. In a
subsequent report in 1995 by Cason et al (33), the authors supported a bimodal
distribution of IgM seropositivity, which peaked between 2 and 5,
and 13 and 16 years of age, suggesting that two distinct modes of
transmission may occur. Perinatal HPV in infants has also been
shown to be related to the mode of delivery and it was suggested
that neonates are at a higher risk of exposure to HPV after vaginal
delivery than after caesarean delivery (35).
In 1998, a research team from the University of
Turku School of Medicine in Finland, initiated the Finnish Family
HPV Study, which was the first prospective attempt to assess HPV
dynamics at multiple anatomical sites in parents and infants
(37). The large number of
mother-infant pairs analyzed made it possible to explore the
consequences of the presence of HPV in the placenta, umbilical cord
blood and breast milk. Studies supporting perinatal HPV
transmission have been reviewed by two separate research teams, one
at the Department of Virology at Kings College in London, UK
(51) and the other at the
University of Turku, Finland (52).
However, these reports (51,52)
have been met with skepticism as regards definitive interpretation.
Nevertheless, the potential impact of early acquired HPV neonatal
infection on the efficacy of current vaccines for HPV-positive
children remains undetermined.
The early findings by Jenison et al (28) in the 1990s that HPV types exist in
the oral cavity of asymptomatic children were re-evaluated a decade
later. In 2000, Rice et al (36) reported the presence of HPV in oral
samples obtained from healthy children, while other researchers
documented tonsillar tissue as a reservoir of HPV DNA (38–40).
These findings attracted the attention of Reuters Health, raising
questions concerning the modes of HPV transmission in childhood
(53). Moreover, the presence of
HPV in the lower tract in children may be involved in the recent
increasing scientific interest of the role of HPV in lung
carcinogenesis (47). Despite the
detection of HPV DNA in human breast samples (41), it was clarified that this event is
rare and there is no contraindication of HPV-positive mothers to
breast feed their children (43,44,48,49).
In the following years, our research led out to the
detection of novel HPV types, including HPV 13, HPV 39, HPV 40 HPV
56, in juvenile RRP (45). Two more
studies (46,50) evaluating HPV infection in relation
to neonatal prematurity and the mode of delivery remain unique in
the field of pediatric HPV research. The first of these studies
(46) did not find any significant
evidence that maternal HPV infection is related to neonatal
prematurity, while the other study (50) suggested that a caesarean section
does not decrease the risk for oral HPV persistence in children. In
a recent study, we used for the first time the term ‘Trojan horse
oncogenic strategy’ to describe the physical history of HPV in
childhood (54). This hypothesis
that children act as a reservoir of silent high risk HPV types,
analogous to the Trojan horse in Greek mythology, requires further
investigation.
4. Future perspectives
Following the approval of the two current vaccines
against HPV (55,56), a great expansion of studies
involving HPV research and children was observed. These studies
aimed to clarify several unresolved issues involving the efficacy
and safety of the vaccination programmes against HPV (57–60).
Moreover, they attempted to provide evidence to resolve the issues
of whether or not the current target ages should be changed, and to
determine the necessity of including boys into the vaccination
programmes against HPV (59,60).
Epidemiological studies aim to determine the factors that influence
the participation of adolescents into the vaccination programmes
against HPV and propose scheduled strategies to increase this
participation. As the vaccination period has already begun, a
re-evaluation of the potential modes of HPV transmission in infancy
and the physical history of HPV-associated infections in childhood
is expected. In the future, further research is required to fully
investigate and clarify all these issues, highlighting the fact
that the paediatric story of HPV remains a challenging research
target for the next generation of researchers. Indeed, the war
against HPV continues.
Abbreviations:
|
HPV
|
human papillomavirus
|
|
PCR
|
polymerase chain reaction
|
|
RRP
|
recurrent respiratory
papillomatosis
|
|
SILs
|
squamous intraepithelial lesions
|
|
URR
|
upstream regulatory region
|
References
|
1
|
zur Hausen H: Papillomaviruses and cancer:
from basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002.
|
|
2
|
Mammas IN, Sourvinos G and Spandidos DA:
Human papilloma virus (HPV) infection in children and adolescents.
Eur J Pediatr. 168:267–273. 2009.
|
|
3
|
zur Hausen H: Papillomaviruses in the
causation of human cancers - a brief historical account. Virology.
384:260–265. 2009.
|
|
4
|
Syrjänen S: Current concepts on human
papillomavirus infections in children. APMIS. 118:494–509.
2010.
|
|
5
|
Pfister H and zur Hausen H:
Seroepidemiological studies of human papilloma virus (HPV-1)
infections. Int J Cancer. 21:161–165. 1978.
|
|
6
|
Lipourlis D: Hippokrates, Gynaikologia
Maieutiki. Zitros Publications; Thessaloniki: 2001
|
|
7
|
Karaberopoulos D, Aristotelis P and Kouzis
O: karkinos para tois arxaiois ellisin iatrois. 1902. Stamoulis
Publications; Athens: 2004
|
|
8
|
Gasparini R and Panatto D: Cervical
cancer: from Hippocrates through Rigoni-Stern to zur Hausen.
Vaccine. 27(Suppl 1): A4–A5. 2009.
|
|
9
|
Rigoni-Stern DA: Fatti statistici relative
alle malattie cancerose. Giorn Serv Progr Patol Terap. 2:507–517.
1842.
|
|
10
|
Mammas IN, Spandidos DA and George N:
Papanicolaou (1883–1962): Fifty years after the death of a great
doctor, scientist and humanitarian. J BUON. 17:180–184. 2012.
|
|
11
|
Papanicolaou GN and Traut HF: The
diagnostic value of vaginal smears in carcinoma of the uterus. Am J
Obst Gynecol. 42:193–206. 1941.
|
|
12
|
Papanicolaou GN: A new procedure for
staining vaginal smears. Science. 95:438–439. 1942.
|
|
13
|
Strauss MJ, Shaw EW, Bunting H and Melnick
JL: Crystalline virus-like particles from skin papillomas
characterized by intranuclear inclusion bodies. Proc Soc Exp Biol
Med. 72:46–50. 1949.
|
|
14
|
Crawford LV and Crawford EM: A comparative
study of polyoma and papilloma viruses. Virology. 21:258–263.
1963.
|
|
15
|
Mammas IN, Sourvinos G, Zaravinos A and
Spandidos DA: Vaccination against human papilloma virus (HPV):
epidemiological evidence of HPV in non-genital cancers. Pathol
Oncol Res. 17:103–119. 2011.
|
|
16
|
Payne J: On the contagious rise of common
warts. Br J Dermatol. 3:1851891.
|
|
17
|
Costa J, Howley PM, Bowling MC, Howard R
and Bauer WC: Presence of human papilloma viral antigens in
juvenile multiple laryngeal papilloma. Am J Clin Pathol.
75:194–197. 1981.
|
|
18
|
Braun L, Kashima H, Eggleston J and Shah
K: Demonstration of papillomavirus antigen in paraffin sections of
laryngeal papillomas. Laryngoscope. 92:640–643. 1982.
|
|
19
|
Mounts P, Shah KV and Kashima H: Viral
etiology of juvenile- and adult-onset squamous papilloma of the
larynx. Proc Natl Acad Sci USA. 79:5425–5429. 1982.
|
|
20
|
Roman A and Fife K: Human papillomavirus
DNA associated with foreskins of normal newborns. J Infect Dis.
153:855–861. 1986.
|
|
21
|
Rock B, Naghashfar Z, Barnett N, Buscema
J, Woodruff JD and Shah K: Genital tract papillomavirus infection
in children. Arch Dermatol. 122:1129–1132. 1986.
|
|
22
|
Vallejos H, Del Mistro A, Kleinhaus S,
Braunstein JD, Halwer M and Koss LG: Characterization of human
papilloma virus types in condylomata acuminata in children by in
situ hybridization. Lab Invest. 56:611–615. 1987.
|
|
23
|
Steinberg BM: Papillomavirus. Effects upon
mother and child. Ann N Y Acad Sci. 549:118–128. 1988.
|
|
24
|
Hanson RM, Glasson M, McCrossin I, Rogers
M, Rose B and Thompson C: Anogenital warts in childhood. Child
Abuse Negl. 13:225–233. 1989.
|
|
25
|
Sedlacek TV, Lindheim S, Eder C, Hasty L,
Woodland M, Ludomirsky A and Rando RF: Mechanism for human
papillomavirus transmission at birth. Am J Obstet Gynecol.
161:55–59. 1989.
|
|
26
|
Gibson PE, Gardner SD and Best SJ: Human
papillomavirus types in anogenital warts of children. J Med Virol.
30:142–145. 1990.
|
|
27
|
Padel AF, Venning VA, Evans MF, Quantrill
AM and Fleming KA: Human papillomaviruses in anogenital warts in
children: typing by in situ hybridisation. BMJ. 300:1491–1494.
1990.
|
|
28
|
Jenison SA, Yu XP, Valentine JM, Koutsky
LA, Christiansen AE, Beckmann AM and Galloway DA: Evidence of
prevalent genital-type human papillomavirus infections in adults
and children. J Infect Dis. 162:60–69. 1990.
|
|
29
|
Smith EM, Johnson SR, Jiang D, et al: The
association between pregnancy and human papilloma virus prevalence.
Cancer Detect Prev. 15:397–402. 1991.
|
|
30
|
Fredericks BD, Balkin A, Daniel HW,
Schonrock J, Ward B and Frazer IH: Transmission of human
papillomaviruses from mother to child. Aust N Z J Obstet Gynaecol.
33:30–32. 1993.
|
|
31
|
Pakarian F, Kaye JN, Cason J, et al:
Cancer associated human papillomaviruses: perinatal transmission
and persistence. Br J Obstet Gynaecol. 101:514–517. 1994.
|
|
32
|
Kaye JN, Cason J, Pakarian FB, et al:
Viral load as a determinant for transmission of human
papillomavirus type 16 from mother to child. J Med Virol.
44:415–421. 1994.
|
|
33
|
Cason J, Kaye JN, Jewers RJ, et al:
Perinatal infection and persistence of human papillomavirus types
16 and 18 in infants. J Med Virol. 47:209–218. 1995.
|
|
34
|
Alberico S, Pinzano R, Comar M, Toffoletti
F, Maso G, Ricci G and Guaschino S: Maternal-fetal transmission of
human papillomavirus. Minerva Ginecol. 48:199–204. 1996.
|
|
35
|
Tseng CJ, Liang CC, Soong YK and Pao CC:
Perinatal transmission of human papillomavirus in infants:
relationship between infection rate and mode of delivery. Obstet
Gynecol. 91:92–96. 1998.
|
|
36
|
Rice PS, Mant C, Cason J, Bible JM, Muir
P, Kell B and Best JM: High prevalence of human papillomavirus type
16 infection among children. J Med Virol. 61:70–75. 2000.
|
|
37
|
Rintala MA, Grénman SE, Puranen MH,
Isolauri E, Ekblad U, Kero PO and Syrjänen SM: Transmission of
high-risk human papillomavirus (HPV) between parents and infant: a
prospective study of HPV in families in Finland. J Clin Microbiol.
43:376–381. 2005.
|
|
38
|
Chen R, Sehr P, Waterboer T, Leivo I,
Pawlita M, Vaheri A and Aaltonen LM: Presence of DNA of human
papillomavirus 16 but no other types in tumor-free tonsillar
tissue. J Clin Microbiol. 43:1408–1410. 2005.
|
|
39
|
Sisk J, Schweinfurth JM, Wang XT and Chong
K: Presence of human papillomavirus DNA in tonsillectomy specimens.
Laryngoscope. 116:1372–1374. 2006.
|
|
40
|
Mammas IN, Sourvinos G, Michael C and
Spandidos DA: Human papilloma virus in hyperplastic tonsillar and
adenoid tissues in children. Pediatr Infect Dis J. 25:1158–1162.
2006.
|
|
41
|
Sarkola M, Rintala M, Grénman S and
Syrjänen S: Human papillomavirus DNA detected in breast milk.
Pediatr Infect Dis J. 27:557–558. 2008.
|
|
42
|
Mammas I, Sourvinos G, Michael C and
Spandidos DA: High-risk human papilloma viruses (HPVs) were not
detected in the benign skin lesions of a small number of children.
Acta Paediatr. 97:1669–1671. 2008.
|
|
43
|
Cazzaniga M, Gheit T, Casadio C, et al:
Analysis of the presence of cutaneous and mucosal papillomavirus
types in ductal lavage fluid, milk and colostrum to evaluate its
role in breast carcinogenesis. Breast Cancer Res Treat.
114:599–605. 2009.
|
|
44
|
Mammas IN and Spandidos DA: No evidence of
mother-to-infant transmission of human papilloma virus via human
breast milk. Pediatr Infect Dis J. 29:932010.
|
|
45
|
Mammas IN, Sourvinos G, Vakonaki E,
Giamarelou P, Michael C and Spandidos DA: Novel human papilloma
virus (HPV) genotypes in children with recurrent respiratory
papillomatosis. Eur J Pediatr. 169:1017–1021. 2010.
|
|
46
|
Mammas IN, Sourvinos G and Spandidos DA:
Maternal human papillomavirus (HPV) infection and its possible
relationship with neonatal prematurity. Br J Biomed Sci.
67:222–224. 2010.
|
|
47
|
Mammas IN, Zaravinos A, Sourvinos G and
Spandidos DA: Detection of human papillomavirus in bronchoalveolar
lavage samples in immunocompetent children. Pediatr Infect Dis J.
30:384–386. 2011.
|
|
48
|
Yoshida K, Furumoto H, Abe A, et al: The
possibility of vertical transmission of human papillomavirus
through maternal milk. J Obstet Gynaecol. 31:503–506. 2011.
|
|
49
|
Mammas IN, Zaravinos A, Sourvinos G,
Myriokefalitakis N, Theodoridou M and Spandidos DA: Can ‘high-risk’
human papillomaviruses (HPVs) be detected in human breast milk?
Acta Paediatr. 100:705–707. 2011.
|
|
50
|
Mammas IN, Sourvinos G, Giamarelou P,
Michael C and Spandidos DA: Human papillomavirus in the oral cavity
of children and mode of delivery: a retrospective study. Int J STD
AIDS. 23:185–188. 2012.
|
|
51
|
Cason J, Rice P and Best JM: Transmission
of cervical cancer-associated human papilloma viruses from mother
to child. Intervirology. 41:213–218. 1998.
|
|
52
|
Syrjänen S and Puranen M: Human
papillomavirus infections in children: the potential role of
maternal transmission. Crit Rev Oral Biol Med. 11:259–274.
2000.
|
|
53
|
Boggs W: Human papilloma virus present in
hyperplastic tonsillar tissue in children. Reuters Health. December
28–2006, at www.reuters.comurisimplewww.reuters.com.
|
|
54
|
Mammas IN, Sourvinos G and Spandidos DA:
The ‘Trojan horse’ oncogenic strategy of HPVs in childhood. Fut
Virology. 8:801–808. 2013.
|
|
55
|
FUTURE II Study Group. Quadrivalent
vaccine against human papillomavirus to prevent high-grade cervical
lesions. N Engl J Med. 356:1915–1927. 2007.
|
|
56
|
Paavonen J, Naud P, Salmerón J, et al:
Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted
vaccine against cervical infection and precancer caused by
oncogenic HPV types (PATRICIA): final analysis of a double-blind,
randomised study in young women. Lancet. 374:301–314. 2009.
|
|
57
|
Rafle AE: Challenges of implementing human
papillomavirus (HPV) vaccination policy. BMJ. 335:375–377.
2007.
|
|
58
|
Maher F and Mammas I: HPV vaccination in
younger pre-adolescents? 13–September. 2007, at www.bmj.com/content/335/7616/375?tab=responsesurisimplewww.bmj.com/content/335/7616/375?tab=responses.
|
|
59
|
Mammas I, Maher F, Theodoridou M and
Spandidos DA: Human papilloma virus (HPV) vaccination in childhood:
challenges and perspectives. Hippokratia. 15:299–303. 2011.
|
|
60
|
Mammas IN and Spandidos DA: Vaccination
against human papillomavirus in childhood: the next rubella
analogue? J BUON. 17:389–390. 2012.
|















