Introduction
Lung cancer is one of the leading causes of
cancer-related mortality worldwide, due to its late diagnosis
(1), and non-small cell lung cancer
(NSCLC) accounts for >85% of all lung cancer cases (2). The majority of patients present with
the advanced stage, by which time treatment is not able to cure the
disease (3). Concurrent
chemotherapy plus thoracic radiotherapy have become the standard
therapeutic regimens for locally advanced NSCLC (4,5).
However, numerous patients demonstrate a poor, or occasionally, no
response to these therapies, with prompt progression of the
disease. Serum biomarkers are increasingly being evaluated for
their ability to facilitate early diagnosis and predict therapeutic
response, which may aid in the development of patient-tailored
treatment strategies for NSCLC.
Apoptosis serves as a natural barrier to cancer
development. Accumulated data (6)
demonstrate that alterations in the expression of death ligands and
their receptors are associated with carcinogenesis. FAS/FASL
(CD95/CD95 ligand) and tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL)/TRAIL receptor (TRAIL-R) are two
of the important death receptor-ligand systems that have been
demonstrated to be involved in processes of various human tumors
(7–10). The TRAIL/TRAIL-R system has been
shown to selectively induce apoptosis in various tumor cells but
not in normal cells. Due to this unique merit, there is a growing
interest in studying the significance of the TRAIL/TRAIL-R system
in various types of cancer (11).
TRAIL has five receptors which have been identified,
including two death receptors (DR4 and DR5), two decoy receptors
(DcR1and DcR2) and soluble receptor osteoprotegerin (12). The binding of TRAIL to its
transmembrane receptors DR4 and DR5 can activate the downstream
caspase cascade and finally induce the development of apoptosis
(13). DR5 has been demonstrated to
possess the highest affinity with TRAIL and play the most important
role in TRAIL-inducing apoptosis (14). Our previous data (15) showed that sDR5 levels played a vital
role in hepatitis B virus (HBV)-induced liver damage, and serum
sDR5 levels may be a useful prognostic indicator of HBV infection.
However, the significance of serum sDR5 levels in NSCLC patients
has not yet been elucidated. In the present study, we investigated
serum sDR5 concentrations in patients with locally advanced stage
III NSCLC, and analyzed the correlation with clinical parameters,
such as histopathological type, stage of disease, tumor burden and
progression-free survival (PFS). Therefore, the present study aimed
to evaluate its predictive and prognostic significance in patients
with locally advanced stage III NSCLC.
Materials and methods
Patients
In total, 122 patients with locally irresectable
stage III NSCLC, including 57 adenocarcinoma (ADC) patients and 65
squamous cell carcinoma (SCC) patients, who visited Shandong
Provincial Qianfoshan Hospital (Jinan, China) between January 2010
and July 2011, were selected as candidates. All patients were
histologically or cytologically confirmed. Any patient who received
surgery for lung cancer was not eligible to participate. Case
samples were collected at two time points: Before treatment and
after concurrent chemoradiotherapy (CRT). The control group
consisted of 50 healthy volunteers. All of the healthy controls
were age and gender-matched with the patients. All patients were
staged according to the seventh edition of the American Joint
Committee on Cancer (AJCC) system for lung cancer (16). TNM (tumor nodes metastasis) staging
method was used (17). Tumor
response was measured using the Response Evaluation Criteria In
Solid Tumors (RECIST) criteria (18).
Patients were treated with 60-Gy radiotherapy
administered as 2 Gy/day for 5 days a week over ~6 weeks with
platinum-doublet chemotherapy. The therapeutic dose was adjusted
according to individual conditions. Follow-up was performed from
the start of CRT to last confirmation of regression, including
physical examination, blood chemistry, ultrasound of the abdomen
and lymph node X-ray of the chest or CT scanning, scintigraphy of
the skeleton and brain CT scanning if necessary.
This study was approved by the ethics committee of
Shandong Provincial Qianfoshan Hospital. Written informed consent
was provided by all patients and controls before sample collection.
All serum samples were stored at −80°C until batch analysis by
enzyme-linked immunosorbent assay (ELISA).
Method
The concentration of sDR5 was detected by using a
solid phase sandwich ELISA kit (cat. no. IB-17792; Human DR5 ELISA
kit; Shanghai Jianglai Biotech, Shanghai, China) according to the
manufacturer’s instructions, with the detection range from 2 to 70
pg/ml. The value of absorbance at 450 nm was utilized to draw the
standard curve and the levels of sDR5 were obtained from the curve.
Each serum sample was tested in duplicate.
Statistical analysis
Serum sDR5 concentration was expressed as the mean ±
standard deviation. Differences between the two groups were
analyzed by Student’s t-test. Differences between multiple groups
were determined by analysis of variance or the Kruskal-Wallis test.
Survival analysis and curves were established according to the
Kaplan-Meier method and were compared using the log-rank test. PFS
was calculated as the time between the start date of the primary
treatment and the date of disease progression or the last follow-up
appointment. The cutoff point was chosen according to the receiver
operating characteristic (ROC) analysis. Differences were
considered to be statistically significant with P<0.05. All data
were analyzed using SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
Patient characteristics
The basic characteristics of the patients are shown
in Table I. The median age of
healthy controls was 48 years (range, 35–70 years) and that of
NSCLC patients was 51 years (range, 36–68 years). No statistical
difference was observed in gender or age between the controls and
patients. The objective response rate, referring to complete
responses (CRs) and partial responses (PRs), was 74%. Similarly,
the objective response rate in the ADC subgroup was 61.4% (35 out
of 57 patients) and in SCC subgroup was 60.0% (39 out of 65
patients), respectively.
 | Table IClinical characteristics of
patients. |
Table I
Clinical characteristics of
patients.
| Characteristics | n (%) | sDR5 (pg/ml) |
|---|
| Controls | 50 | 10.89±6.72 |
| Age, years | 48 (35–70)a | |
| Gender |
| Male | 25 (50.0) | 10.83±6.98 |
| Female | 25 (50.0) | 10.96±6.58 |
| Patients | 122 | 13.72±3.61 |
| Age, years | 51 (36–68)a | |
| Gender |
| Male | 63 (51.4) | 13.70±4.62 |
| Female | 59 (48.6) | 13.73±4.46 |
| Histopathological
type |
| Adenocarcinoma | 57 (40.1) | 13.67±3.89 |
| Squamousl
carcinoma | 65 (45.8) | 13.77±3.32 |
| Stage |
| IIIA | 75 (61.5) | 12.94±2.95 |
| IIIB | 47 (38.5) | 14.62±4.03 |
| T level |
| T1 | 22 (15.5) | - |
| T2 | 37 (26.1) | - |
| T3 | 42 (29.6) | - |
| T4 | 21 (14.8) | - |
| N level |
| N0 | 7 (5.70) | - |
| N1 | 37 (30.3) | - |
| N2 | 43 (35.2) | - |
| N3 | 35 (28.8) | - |
| Tumor burden,
cm |
| ≤3 | 57 (47.8) | 12.43±0.48 |
| >3 | 65 (52.2) | 13.95±0.47 |
| Evaluation |
| Total response
(rate) | 74 (60.6) | - |
| Adenocarcinoma |
| CR + PR | 35 (61.4) | 12.67±3.58 |
| PD + SD | 22 (38.6) | 15.24±3.93 |
| Squamous
carcinoma |
| CR + PR | 39 (60.0) | 12.95±3.12 |
| SD + PD | 26 (40.0) | 15.00±3.28 |
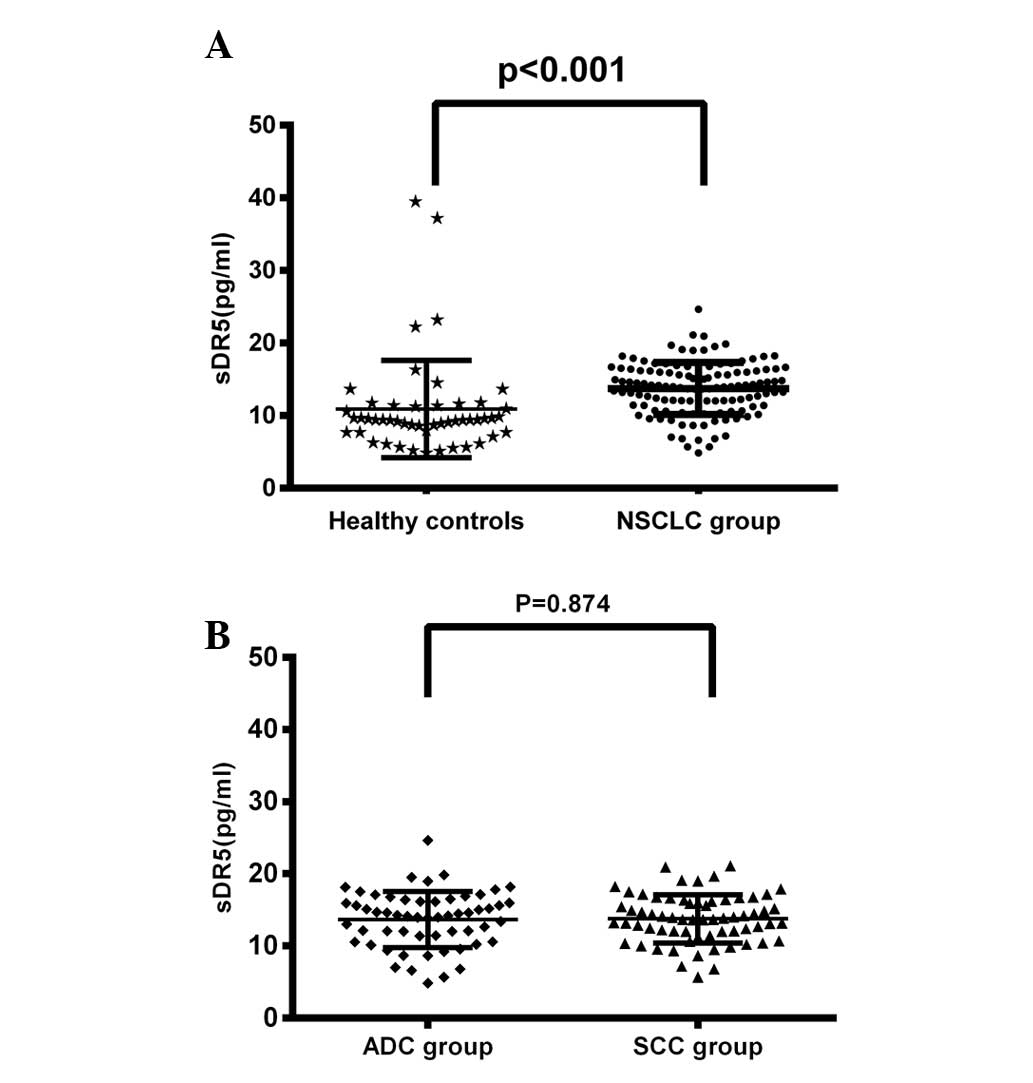
Detection of serum soluble DR5 levels in
NSCLC patients and healthy controls
The pretreatment serum sDR5 levels in the healthy
control group and the NSCLC group were 10.89±6.72 and 13.72±3.61
pg/ml, respectively. As presented in Fig. 1A, the pretreatment serum sDR5 levels
in all patients were significantly increased compared with the sDR5
levels of healthy controls (P<0.001). However, the sDR5 levels
showed no significant difference between the ADC and SCC patient
groups (13.67±3.89 vs. 13.77±3.32 pg/ml; P=0.874; Fig. 1B).
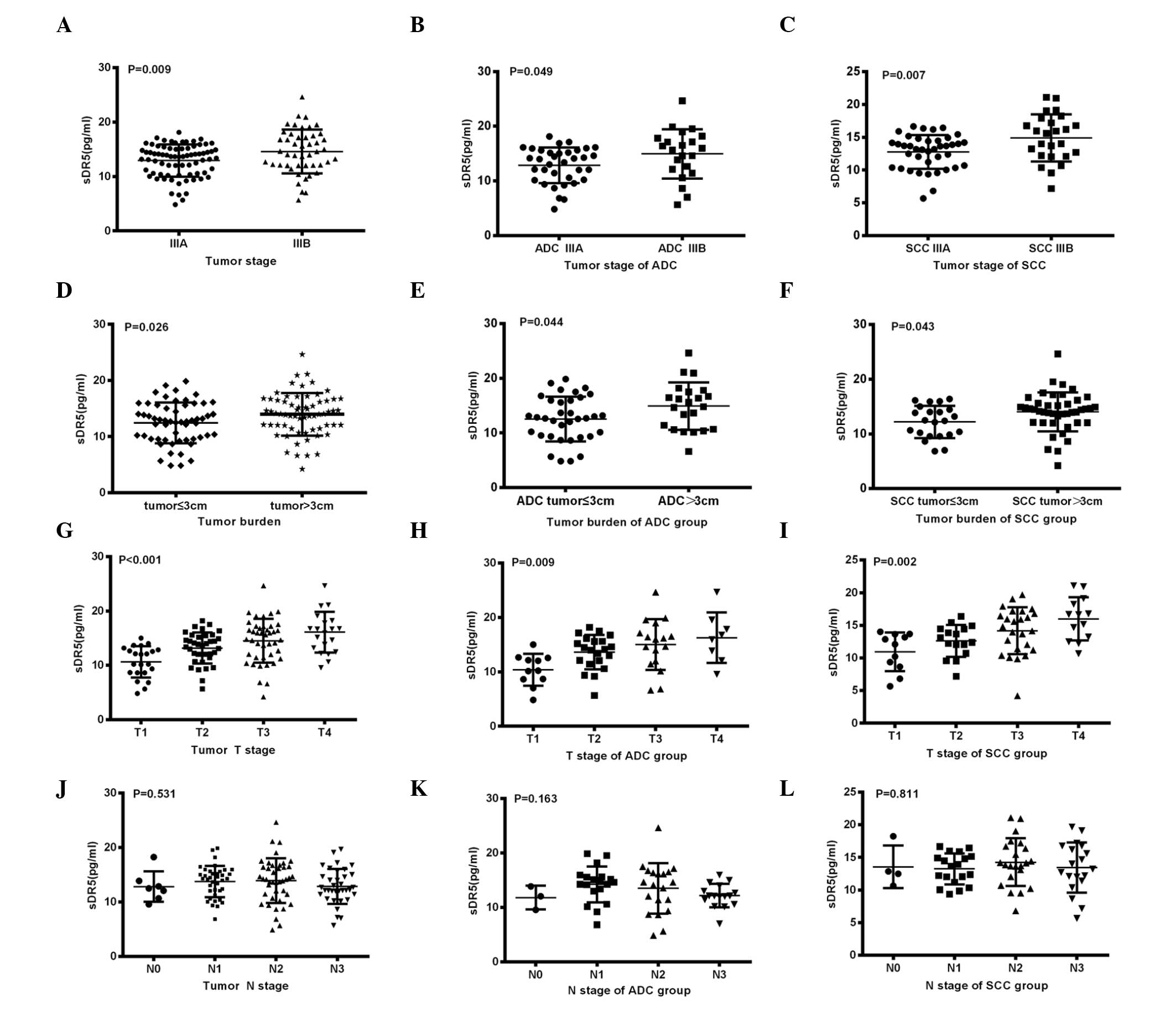
Expression of sDR5 in association with
the clinical characteristics of NSCLC patients
When the clinical classifications of the NSCLC
patients were considered, a significant increase in pretreatment
serum sDR5 levels could be observed in IIIB stage patients compared
with IIIA stage patients (P=0.009; Fig.
2A). Similar results were observed between the IIIA and IIIB
stage patients when patients were separated into ADC (P=0.049;
Fig. 2B) and SCC (P=0.007; Fig. 2C) subgroups. Regarding the tumor
burden, analysis revealed a marked increase in pretreatment sDR5
concentration in patients with a tumor load of ≤3 cm compared with
patients with a load of >3 cm (12.43±0.48 vs. 13.95±0.47 pg/ml;
P=0.026; Fig. 2D). Similar results
were identified between the patients with different tumor burdens
in the ADC subgroup (P=0.044; Fig.
2E) and SCC (P=0.043; Fig. 2F)
subgroups. Pretreatment serum sDR5 levels in patients with T4 stage
tumors were significantly higher than those in patients with T1
stage tumors (P<0.001; Fig. 2G).
Similar results were observed between patients with T1 and T4 stage
tumors in the ADC (P=0.009; Fig.
2H) an SCC (P=0.002; Fig. 2I)
subgroups. However, no such correlation was found with N stage
(Fig. 2J–L).
Comparison of sDR5 levels before and
after CRT
Analysis of the sDR5 concentrations before and after
CRT demonstrated that there were no significant differences between
pre- and post-treatment sDR5 concentrations among all NSCLC
patients (P=0.462), the ADC subgroup (P=0.066) or the SCC subgroup
(P=0.052), as shown in Table
II.
 | Table IIComparasion of sDR5 level (pg/ml)
before and after chemoradiotherapy. |
Table II
Comparasion of sDR5 level (pg/ml)
before and after chemoradiotherapy.
| NSCLC | ADC group | SCC group |
|---|
| Pre-CRT | 13.72±3.61 | 13.73±3.88 | 13.82±3.33 |
| Post-CRT | 13.39±3.39 | 13.32±3.73 | 13.46±3.08 |
| P-value | 0.462 | 0.066 | 0.052 |
Change in serum DR5 levels according to
clinical response after CRT
The treatment response is one of vital indices of
the effectiveness of CRT in NSCLC patients. We defined patients
with CRs or PRs as responders, while those with stable or
progressive disease were considered non-responders, according to
RECIST criteria (18). When the
patients were grouped according to response to CRT, pretreatment
sDR5 levels in the responder group were significantly lower than
those in the non-responder group (P<0.0001; Table III). Further analysis in the ADC
and SCC subgroups demonstrated the same trend (Table III). However, there was no
correlation between the post-treatment sDR5 levels and clinical
response (Table IV).
 | Table IIIPretreatment sDR5 level (pg/ml)
according to treatment response. |
Table III
Pretreatment sDR5 level (pg/ml)
according to treatment response.
| NSCLC | ADC group | SCC group |
|---|
| Responders | 12.57±0.37 | 12.67±3.58 | 12.95±3.12 |
| Non-responders | 15.16±0.49 | 15.24±3.93 | 15.00±3.28 |
| P-value | <0.0001 | 0.014 | 0.011 |
 | Table IVPost-treatment sDR5 levels (pg/ml)
according to treatment response. |
Table IV
Post-treatment sDR5 levels (pg/ml)
according to treatment response.
| NSCLC | ADC group | SCC group |
|---|
| Responders | 12.97±0.32 | 12.93±0.48 | 13.01±0.45 |
| Non-responders | 14.02±0.43 | 13.88±0.75 | 14.13±0.51 |
| P-value | 0.054 | 0.269 | 0.108 |
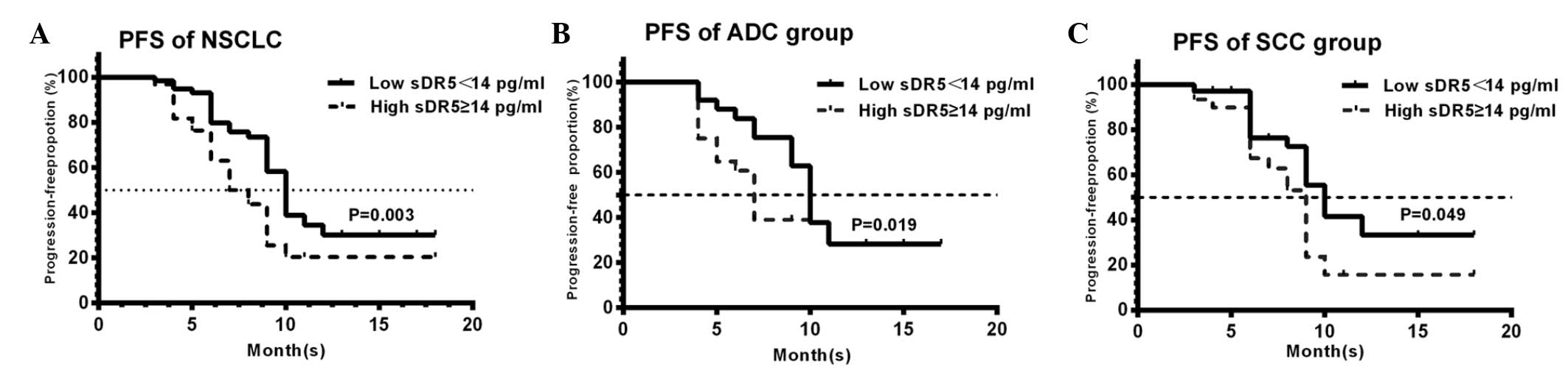
Correlation between sDR5 levels and PFS
time
To evaluate the correlation between sDR5 levels and
the outcome of patients following CRT, we calculated the PFS time
of patients. At the median follow-up of 18 months (range, 3–24
months), the median PFS time was 8.9 months. Patients were then
subdivided into two groups according to the sDR5 cutoff value (14
pg/ml), which was calculated by ROC analysis. In the NSCLC group,
the median PFS time in patients with pretreatment sDR5 levels of
>14 pg/ml was 8 months, while that of patients whose
pretreatment sDR5 levels were ≤14 pg/ml was 10 months. There was a
statistically significant difference in PFS time between the two
groups (P=0.003; Fig. 3A). Further
analysis of the ADC and SCC subgroups demonstrated the same trend
(P=0.019; Fig. 3B; and P=0.049;
Fig. 3C, respectively). That is,
high serum sDR5 levels were associated with a lower PFS compared
with low sDR5 levels, both in the ADC and SCC subgroups. However,
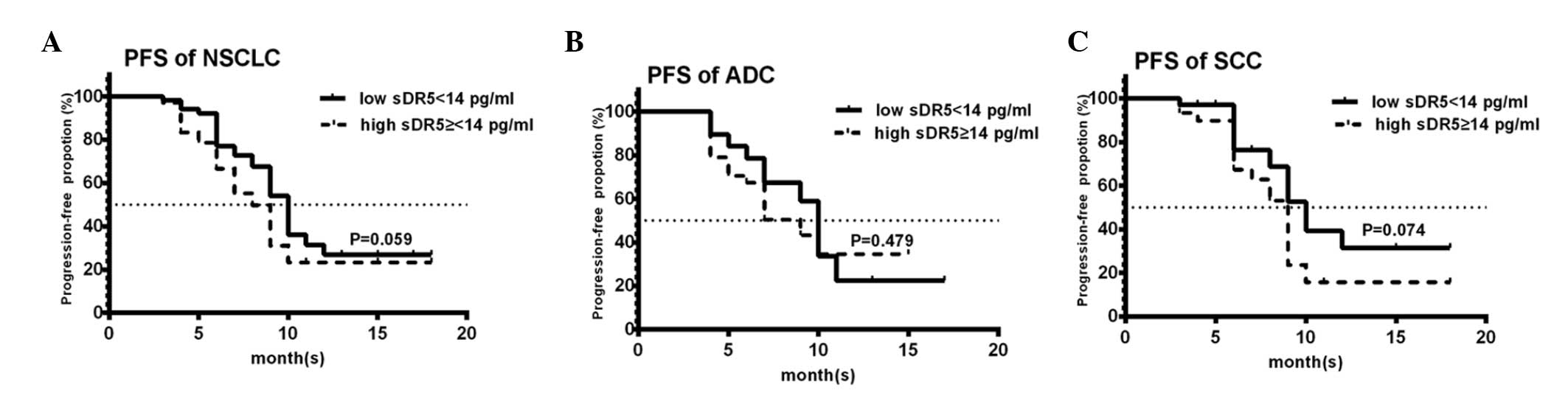
there was no correlation between the post-treatment sDR5 levels and
PFS (Fig. 4).
Discussion
Apoptosis plays a significant role in maintaining
body homeostasis. TRAIL/TRAIL-R induced apoptosis is an important
regulatory pathway, which serves its potential role as a mediator
of tumor immune surveillance (19).
DR5 is a prominent death domain-containing receptor for TRAIL
(20). Our previous study (21) showed that downregulation of DR5 was
involved in the apoptosis of the HBV-related hepatoma cell line. To
the best of our knowledge, the present study is the first to
demonstrate that the serum levels of sDR5 may be a useful biomarker
for the diagnosis and prognosis of patients with locally advanced
stage III NSCLC.
In several studies, the clinical significance of DR5
expression in human tumors has been determined. Ganten et al
(22) showed that DR5 expression
was negatively associated with poor clinical outcome in breast
cancer patients. Leithner et al (23) demonstrated that nuclear and
cytoplasmic DR5 were prognostic factors in patients with NSCLC
treated with chemotherapy. Zhuang et al (24) found that decreased DR5 expression
was associated with the progression of melanoma. However, all of
the above results were obtained by the immunhistochemical analysis
of tumor tissues, which is an invasive immunodiagnostic method. In
the present study, we used the non-invasive method, ELISA assay, to
detect serum soluble DR5 levels and evaluate their diagnostic and
prognostic significance in locally advanced NSCLC patients.
The current study found that pretreatment sDR5 serum
levels in locally advanced stage III NSCLC patients were higher
than the serum sDR5 levels of healthy controls (P<0.001).
According to multiple clinical classification analysis, a
significant increase in pretreatment sDR5 serum levels could be
observed between IIIB and IIIA stage patients (P=0.009), and
patients with T4 stage tumors had significantly higher pretreatment
sDR5 levels compared with those with T1 stage tumors (P<0.001).
Furthermore, patients with a tumor burden of >3 cm had higher
pretreatment sDR5 concentrations compared with those with tumor
burdens of ≤3 cm. The results showed that pretreatment sDR5 serum
concentrations may be a usefully adjunctive factor in the diagnosis
of locally advanced stage III NSCLC patients.
Further analysis found that when patients were
divided according to therapeutic response (responders versus
non-responders), the pretreatment sDR5 levels were significantly
lower in responders compared with non-responders (P=0.007).
Therefore, CRT was more effective in patients with lower
pretreatment sDR5 levels than in those with higher pretreatment
sDR5 levels. The results indicated that pretreatment serum sDR5
levels may aid in the development of more powerful strategies to
improve the treatment efficacy for locally advanced stage III NSCLC
patients.
To investigate the correlation between the sDR5
levels and the outcome of the NSCLC patients, PFS survival analysis
was performed. It was found that high sDR5 serum levels were
associated with a shorter PFS time compared with low sDR5 levels in
NSCLC patients; patients whose pretreatment sDR5 levels were ≤14
pg/ml (cutoff value, 14 pg/ml) had an improved disease outcome
compared with patients whose pretreatment sDR5 levels were >14
pg/ml. These results indicated that serum sDR5 levels may be a
useful prognostic biomarker for patients with locally advanced
stage III NSCLC.
At present, the cellular origin of the increased
serum sDR5 levels observed in the present study is unknown.
Although Yildiz et al detected the expression of serum sDR5
levels in metastatic colorectal cancer, the authors did not
investigate the generation of serum sDR5 (9). We propose that another important death
receptor, serum sFas, may originate from the tumor tissues
themselves, as a correlation between sFas/CD95 serum concentration
and the patient’s stage of disease has been observed (25). In the present study, it was also
found that serum sDR5 levels correlated with the patient’s stage of
disease and disease progression. Therefore, according to the above
evidence, we hypothesize that sDR5 may be generated by the lung
cancer tissue itself.
However, no correlation was identified between the
post-sDR5 level and the treatment response or the PFS time in the
present study. These results may be due to the fact that
post-treatment sDR5 levels were affected by six weeks of CRT.
Post-treatment sDR5 levels had no prognostic significance in
locally advanced stage III NSCLC patients
In conclusion, pretreatment sDR5 serum concentration
s may be a usefully adjunctive diagnostic index for locally
advanced stage III NSCLC patients. Notably, pretreatment sDR5
levels in the patient’s serum may be a predictive and prognostic
biomarker for the effectiveness of CRT in locally advanced stage
III NSCLC patients.
Acknowledgements
The authors thank Dr Jan Clay and Dr Suzanne Bither
for manuscript assistance. This study was supported by grants from
the Natural Science Foundation of Shandong Province (grant no.
ZR2011HQ010), the National Natural Science Foundation of China
(grant nos. 81272501, 30901712 and 81000731) and the Fund for
Excellent Young and Middle-Aged Scientists of Shandong Province
(grant no. BS2010YY045).
References
|
1
|
Spierings DC, de Vries EG, Timens W, Groen
HJ, Boezen HM and de Jong S: Expression of TRAIL and TRAIL death
receptors in stage III non-small cell lung cancer tumors. Clin
Cancer Res. 9:3397–3405. 2003.
|
|
2
|
Ettinger DS, Akerley W, Borghaei H, et al:
Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw.
11:645–653. 2013.
|
|
3
|
Chute JP, Chen T, Feigal E, Simon R and
Johnson BE: Twenty years of phase III trails for patients with
extensive-stage small-cell lung cancer: perceptible progress. J
Clin Oncol. 17:1794–1801. 1999.
|
|
4
|
Machtay M, Bae K, Movsas B, et al: Higher
biologically effective dose of radiotherapy is associated with
improved outcomes for locally advanced non-small cell lung
carcinoma treated with chemoradiation: an analysis of the Radiation
Therapy Oncology Group. Int J Radiat Onclo Biol Phys. 82:425–434.
2012.
|
|
5
|
Aupérin A, Le Péchoux C, Rolland E, et al:
Meta-analysis of concomitant versus sequential radiochemotherapy in
locally advanced non-small-cell lung cancer. J Clin Oncol.
28:2181–2191. 2010.
|
|
6
|
Igney FH and Krammer PH: Death and
anti-death: tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002.
|
|
7
|
Malhi H and Gores GJ: TRAIL resistance
results in cancer progression: a TRAIL to perdition? Oncogene.
25:7333–7335. 2006.
|
|
8
|
Baader E, Toloczko A, Fuchs U, et al:
Tumornecrosis factor-related apoptosis-inducing ligand-mediated
proliferation of tumor cells with receptor-proximal apoptosis
defects. Cancer Res. 65:7888–7895. 2005.
|
|
9
|
Yildiz R, Benekli M, Buyukberber S, et al:
The effect of bevacizumab on serum soluble FAS/FASL and TRAIL and
its receptors (DR4 and DR5) in metastatic colorectal cancer. J
Cancer Res Clin Oncol. 136:1471–1476. 2010.
|
|
10
|
Yang H, Li H, Wang Z, Gao J and Guo Y: Is
urinary soluble fas an independent predictor of non-muscle-invasive
bladder cancer? A prospective chart study. Urol Int. 91:456–461.
2013.
|
|
11
|
van Dijk M, Halpin-McCormick A, Sessler T,
Samali A and Szegezdi E: Resistance to TRAIL in non-transformed
cells is due to multiple redundant pathways. Cell Death Dis.
4:e7022013.
|
|
12
|
Wiley SR, Schooley K, Smolak PJ, et al:
Identification and characterization of a new member of the TNF
family that induces apoptosis. Immunity. 3:673–682. 1995.
|
|
13
|
LeBlanc HN and Ashkenazi A: Apo2L/TRAIL
and its death and decoy receptors. Cell Death Differ. 10:66–75.
2003.
|
|
14
|
Truneh A, Sharma S, Silverman C, et al:
Temperature-sensitive differential affinity of TRAIL for its
receptors. DR5 is the highest affinity receptor. J Biol Chem.
275:2319–2325. 2000.
|
|
15
|
Du J, Wang L, Han J, Gao L, Ma C and Liang
X: Serum soluble death receptor 5 concentration in patients with
chronic hepatitis B is associated with liver damage and viral
antigen level. Clin Biochem. 45:845–847. 2012.
|
|
16
|
Edge SB, Byrd DR, Compton CC, et al:
Thorax. AJCC Cancer Staging Manual. 7th edition. Springer; New
York, NY: pp. 299–323. 2010
|
|
17
|
Goldstraw P, Crowley J, Chansky K, et al:
The IASLC Lung Cancer Staging Project: proposals for the revision
of the TNM stage groupings in the forthcoming (seventh) edition of
the TNM classification of malignant tumors. J Thorac Oncol.
2:706–714. 2007.
|
|
18
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
|
|
19
|
Takeda K, Hayakawa Y, Smyth MJ, et al:
Involvement of tumor necrosis factor-related apoptosis-inducing
ligand in surveillance of tumor metastasis by liver natural killer
cells. Nat Med. 7:94–100. 2001.
|
|
20
|
Kelley RF, Totpal K, Lindstrom SH, et al:
Receptor-selective mutants of apoptosis-inducing ligand 2/tumor
necrosis factor-related apoptosis-inducing ligand reveal a greater
contribution of death receptor (DR) 5 than DR4 to apoptosis
signaling. J Biol Chem. 280:2205–2212. 2005.
|
|
21
|
Du J, Liang X, Liu Y, et al: Hepatitis B
virus core protein inhibits TRAIL-induced apoptosis of hepatocytes
by blocking DR5 expression. Cell Death Differ. 16:219–229.
2009.
|
|
22
|
Ganten TM, Sykora J, Koschny R, et al:
Prognostic significance of tumour necrosis factor-related
apoptosis-inducing ligand(TRAIL) receptor expression in patients
with breast cancer. J Mol Med (Berl). 87:995–1007. 2009.
|
|
23
|
Leithner K, Stacher E, Wurm R, et al:
Nuclear and cytoplasmic death receptor 5 as prognostic factors in
patients with non-small cell lung cancer treated with chemotherapy.
Lung Cancer. 65:98–104. 2009.
|
|
24
|
Zhuang L, Lee CS, Scolyer RA, et al:
Progression in melanoma is associated with decreased expression of
death receptors for tumor necrosis factor-related
apoptosis-inducing ligand. Hum Pathol. 37:1286–1294. 2006.
|
|
25
|
Ugurel S, Rappl G, Tilgen W and Reinhold
U: Increased soluble CD95 (sFas/CD95) serum level correlates with
poor prognosis in melanoma patients. Clin Cancer Res. 7:1282–1286.
2001.
|