Introduction
Cathepsin D (Cat D) is a ubiquitous aspartyl-family
endoproteinase synthesized as a 52-kDa glycosylated preprotein,
which is subsequently converted into an active two-chained (34 and
14 kDa) enzyme (1). It is
distributed in lysosomes where it is involved in protein
degradation and generation. Therefore, it is important for the
maintenance of normal cell metabolism (2).
Previous studies have demonstrated that Cat D is
involved in tumor progression. Cat D was studied in human primary
breast cancer, and enzyme overexpression was found to be associated
with an increased risk of metastasis and shorter survival (3,4). A
similar association was identified in thyroid (5) and skin (6) cancer.
Urinary bladder cancer (UBC) is the ninth most
common cancer worldwide. It is the seventh most common malignancy
in males and seventeenth in females and the global standardized
incidence rate is 9/100,000 in males and 2/100,000 in females
(7). Annually, ~110,500 new cases
in males and 70,000 new cases in females are diagnosed, and 38,200
patients in the European Union and 17,000 patients in the USA
succumb to UBC (8).
Transitional cell carcinoma (TCC) biology is not
completely understood. Surgical removal of the tumor mass remains
the most effective treatment method. Understanding the mechanisms
affecting tumor origin and progression may provide a novel
theoretical basis for therapeutic methods and contribute to
treatment that results in disease amelioration.
Approximately 75% of bladder cancer carcinomas are
diagnosed as superficial (confined to mucosa and submucosa) and
~25% exhibit muscle-invasive disease (8).
In the present study, Cat D concentration in the
serum and urine was investigated using the surface plasmon
resonance imaging (SPRI) biosensor. The SPRI technique in
combination with the development of sensitive biosensors is a
promising tool for the determination of biologically active
species. This method is label-free, easy to perform and does not
require the use of radioisotopes or special substrates. The SPRI
method uses an extremely specific interaction between enzymes and
inhibitors (9) or antibody-antigens
(10). Methods for the SPRI
determination of several diagnostically significant species,
including cathepsins B, D (11,12)
and G (13), proteasome S20
(14), podoplanin (15) and cystatin C (16) have been developed. The SPR signal
reacts to an increase in mass by changing wavelength and
polarization angle. This signal is then converted to an image.
Co-operation of a biosensor with the SPRI instrument ensures
selectivity of the analytical signal. The biosensor contains an
immobilized antibody (15) or
inhibitor (9), which specifically
reacts with the species to be determined. Therefore, only the
species which have specifically bonded contribute to the analytical
signal.
Few studies have investigated the role of Cat D in
TCC. The majority of studies have focused on the evaluation of Cat
D expression in TCC (17,18), and all of these studies have
identified high Cat D expression in TCC tissues. Few studies have
determined the concentration of various cathepsins in the serum and
urine (19); however, a single
study (20) reported Cat D activity
in serum. The aim of this study was to determine the Cat D
concentration in the blood serum and urine of patients with bladder
cancer. The effects of various parameters of the urothelial cancer
on the Cat D concentration were compared.
Materials and methods
Preparation of biological samples
Urine and serum samples of patients with bladder
cancer were obtained prior to surgery or admission to the J.
Sniadecki Provincial Hospital of Bialystok (Bialystok, Poland). The
urine and serum samples were frozen immediately and maintained at
−70°C until Cat D was analyzed. Individuals with additional
malignant or inflammatory disease were excluded. Blood samples were
obtained from the median cubical vein. Cancer diagnosis was
detected by histological examination of tumor specimens obtained
from transurethral resection or cystectomy.
Prepared serum samples were diluted two-fold with
phosphate-buffered saline and transferred onto the sensor surface
for 10 min. The volume of the sample applied on each measuring
field was 2 μl.
Urine was centrifuged at 1,850 × g for 15 min and
the supernatant was separated. Finally, the sample was filtered
once through a paper filter of medium density. The prepared urine
samples were then transferred onto the sensor surface for 10 min.
The volume of the sample applied on each measuring field was 2
μl.
The total protein concentration was determined using
Lowry’s method and creatinine (CREA) concentration was determined
using Jaffe’s method.
The urine and serum concentrations of Cat D were
measured in 68 patients (48 males and 20 females; mean age, 66
years) with TCC of the bladder and 54 healthy patients. Approval
for this study was obtained from the Bioethics Committee of the
Medical University of Bialystok (Bialystok, Poland) and written
informed consent was obtained from all the patients and donors.
Procedure of Cathepsin D
determination
Cat D obtained from human liver was purchased from
Sigma-Aldrich (Steinheim, Germany) and the concentration was
determined using the SPRI biosensor. The SPRI technique allows
sensitive determination of proteins using highly specific
enzyme-inhibitor interactions. An immobilized pepstatin A
(inhibitor) obtained from human liver was purchased from
Sigma-Aldrich and used for the Cat D entrapment on the biosensor
surface. The biosensor construction and optimization of measurement
conditions used were previously described (12).
Briefly, plasma or urine samples were placed
directly on the prepared biosensor for ~10 min to allow interaction
with the inhibitor (pepstatin A). The biosensor was washed with
water and HBS-ES buffer solution pH=7.4 (0.01 M
4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, 0.15 M sodium
chloride, 0.005% Tween 20, 3 mM EDTA) (all Biomed-Lublin, Lublin,
Poland) to remove unbound molecules from the surface. The SPRI
signal was measured twice on the basis of registered images,
following the immobilization of pepstatin A and then following
interaction with Cat D from the samples. The signal, which is
proportional to coupled biomolecules, was obtained by calculating
the difference between the signal prior to and following the
interaction with biomolecules. The concentration was determined
using the calibration curves of the SPRI signal depending on the
concentration of Cat D.
Statistical analysis
The results are presented as the median ± standard
deviation. Statistical analyses were performed using Student’s
t-test, and P<0.05 and P<0.01 were considered to indicate a
statistically significant difference.
Results
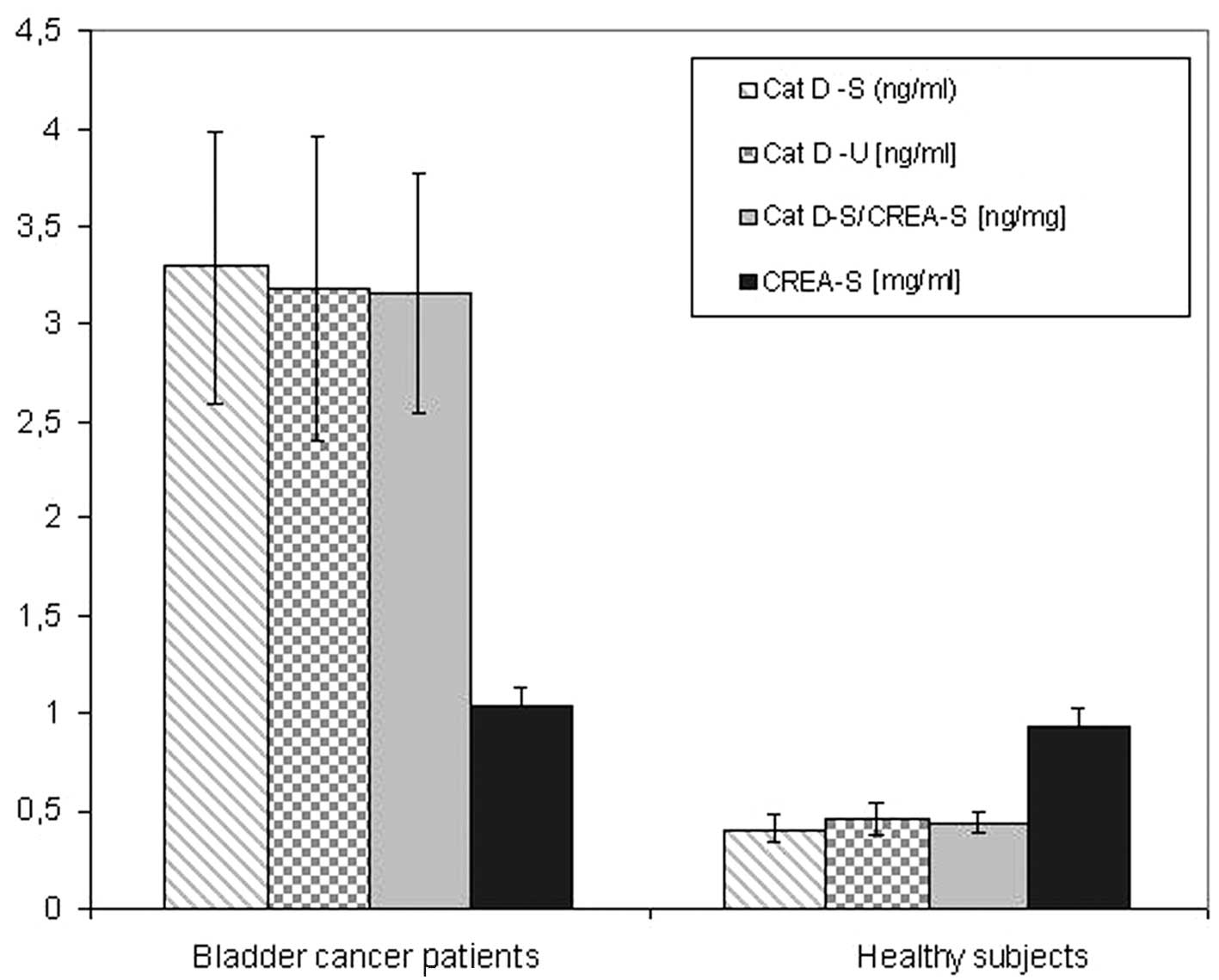
Changes in Cat D concentration
The Cat D concentration in serum (Table I) and urine (Table II) samples was investigated with
regard to the bladder cancer parameters. Cat D/total protein and
Cat D/CREA ratios are also shown in Tables I and II. A summary of the results are presented
in Fig. 1.
 | Table IDiagnostic characteristics of serum
Cat D/protein and Cat D/CREA concentration ratios compared with
various parameters of urothelial cancer. |
Table I
Diagnostic characteristics of serum
Cat D/protein and Cat D/CREA concentration ratios compared with
various parameters of urothelial cancer.
| | Cat D/protein
(ng/nl) | | Cat D/S-CREA
(ng/ml) | |
|---|
| |
| |
| |
|---|
| Parameter | n | Range | Mean ± SD | P-value | Range | Mean±SD | P-value |
|---|
|
Primary/recurrent | | | | NS | | | NS |
| Primary | 33 | 0.037–0.072 | 0.059±0.030 | | 1.65–3.85 | 2.79±1.33 | |
| Recurrent | 35 | 0.028–0.089 | 0.062±0.024 | | 2.45–4.29 | 3.63±1.06 | |
| Multiplicity | | | | NS | | | NS |
| Single | 22 | 0.041–0.086 | 0.056±0.023 | | 1.98–4.13 | 3.04±1.61 | |
| Multiply | 46 | 0.038–0.089 | 0.065±0.022 | | 2.04–4.62 | 3.39±1.23 | |
| Stage | | | | NS | | | <0.05 |
| Superficial (Ta +
T1) | 47 | 0.027–0.089 | 0.060±0.025 | | 2.76–5.11 | 3.86±1.56 | |
| Invasive (T2 +
T3=T4) | 21 | 0.048–0.079 | 0.066±0.020 | | 1.64–3.69 | 2.27±1.12 | |
| Grade | | | | NS | | | NS |
| Low-grade | 22 | 0.027–0.072 | 0.057±0.018 | | 1.06–4.30 | 3.17±1.43 | |
| High-grade | 46 | 0.041–0.089 | 0.065±0.028 | | 1.14–4.89 | 3.23±1.79 | |
| Size (mm) | | | | NS | | | NS |
| <30 | 39 | 0.038–0.098 | 0.062±0.020 | | 2.59–5.04 | 3.47±1.28 | |
| >30 | 29 | 0.038–0.087 | 0.059±0.028 | | 1.78–4.98 | 2.87±1.74 | |
| Gender | | | | NS | | | NS |
| Female | 20 | 0.038–0.098 | 0.056±0.017 | | 1.67–4.85 | 3.35±1.20 | |
| Male | 48 | 0.038–0.073 | 0.059±0.028 | | 1.85–5.13 | 3.12±1.50 | |
| Age (years) | | | | NS | | | NS |
| <65 | 35 | 0.027–0.089 | 0.056±0.022 | | 2.60–5.05 | 3.24±1.06 | |
| ≥65 | 33 | 0.038–0.085 | 0.065±0.019 | | 2.87–5.98 | 3.26±1.94 | |
 | Table IIDiagnostic characteristics of urine
protein, Cat D and Cat D/protein ratio compared with various
parameters of urothelial cancer. |
Table II
Diagnostic characteristics of urine
protein, Cat D and Cat D/protein ratio compared with various
parameters of urothelial cancer.
| | Cat D (ng/ml) | | Cat D/protein
(ng/mg) | |
|---|
| |
| |
| |
|---|
| Parameter | n | Range | Mean±SD | P-value | Range | Mean±SD | P-value |
|---|
|
Primary/recurrent | | | | NS | | | <0.01 |
| Primary | 33 | 2.51–4.35 | 3.41±0.95 | | 5.25–12.5 | 9.48 ±2.28 | |
| Recurrent | 35 | 2.25–5.31 | 3.09±0.93 | | 2.68–7.28 | 4.23±1.19 | |
| Multiplicity | | | | NS | | | <0.01 |
| Single | 22 | 2.42–5.19 | 3.46±1.01 | | 5.60–9.29 | 8.65±1.35 | |
| Multiply | 46 | 2.15–5.31 | 2.90±0.94 | | 4.13–8.36 | 6.59±1.68 | |
| Stage | | | | NS | | | NS |
| Superficial
(Ta+T1) | 47 | 2.90–5.85 | 3.21±0.76 | | 3.90–11.60 | 7.64±2.72 | |
| Invasive
(T2+T3=T4) | 21 | 1.35–7.14 | 3.57±1.80 | | 4.85–9.10 | 6.15±1.69 | |
| Grade | | | | NS | | | <0.01 |
| Low-grade | 22 | 2.42–4.35 | 3.41±0.70 | | 8.96–16.10 | 13.37±2.72 | |
| High-grade | 46 | 1.79–6.32 | 3.45±1.26 | | 7.16–10.70 | 8.02±1.42 | |
| Size (mm) | | | | NS | | | <0.01 |
| <30 | 39 | 1.85–7.14 | 3.69±1.49 | | 9.20–14.20 | 11.8±1.75 | |
| >30 | 29 | 2.55–6.32 | 3.52±1.14 | | 6.43–12.15 | 9.78±2.14 | |
| Gender | | | | NS | | | NS |
| Female | 20 | 1.89–6.32 | 3.77±1.59 | | 7.37–11.15 | 8.77±1.29 | |
| Male | 48 | 2.42–7.14 | 3.51±1.20 | | 6.09–10.80 | 8.36±1.69 | |
| Age (years) | | | | NS | | | <0.05 |
| <65 | 35 | 1.89–7.14 | 3.39±1.43 | | 6.91–14.50 | 9.68±2.89 | |
| ≥65 | 33 | 2.55–6.32 | 3.84±1.14 | | 5.90–9.10 | 7.38±1.69 | |
A significant difference in serum and urine Cat D
concentration levels was observed between bladder cancer patients
and healthy subjects (Fig. 1). This
indicates the potential of Cat D as a cancer marker. No significant
differences in CREA concentration were identified between bladder
cancer patients and healthy subjects. To further investigate the
results of the present study, Cat D concentration was corrected by
serum CREA concentration to eliminate the impact of renal
impairment on the observed results. Furthermore, the Cat D/protein
ratio was introduced as a novel parameter. In this way, one of the
causes of inflammatory proteinuria was eliminated.
Blood serum analysis
In terms of different cancer parameters, few
parameters in the serum were statistically significant. When
comparing invasive and superficial tumors, values were almost
identical; however, the Cat D/CREA ratio was found to be
significantly higher in superficial tumors when compared with
invasive tumors (P<0.05; Table
I). This was due to the significantly higher CREA
concentrations (data not shown) identified in invasive tumors when
compared with superficial tumors (P<0.05).
In recurrent, multifocal, high-grade and smaller
(<30 mm) tumors, serum Cat D levels were elevated; however, no
significant differences were identified. This pattern was confirmed
by the Cat D/CREA ratio in all the aforementioned groups. Males and
older individuals were characterized by higher levels of Cat D;
however, this difference was not statistically significant and did
not confirm this correlation in relation to the Cat D/CREA ratio
(Table I).
Urine analysis
In urine, a significantly higher Cat D/protein ratio
was demonstrated in primary, single, smaller and low-grade groups
of cancer. Notably, in the case of low-grade tumors, Cat D/protein
ratio was significantly higher than that of high-grade tumors,
while Cat D concentration alone was marginally elevated in
high-grade tumors. This may be explained by the significant
difference in protein concentration (data not shown) identified
between low- and high-grade tumors (P<0.05).
Discussion
The majority of UBCs are TCCs. The effect of various
parameters of TCCs on Cat D concentrations were analysed in this
study. The most significant result of the present study is that all
bladder tumor cases exhibited significantly higher serum
(eight-fold) and urine (seven-fold) Cat D concentrations when
compared with healthy control subjects. This shows the efficacy of
Cat D concentration as a tumor marker. In the serum, the lowest Cat
D concentration for TCC was 1.3 ng/ml, whereas the highest Cat D
concentration for healthy donors was 0.52 ng/ml. In the case of
urine, the lowest Cat D concentration for TCC was 1.35 ng/ml,
whereas the highest Cat D concentration for healthy donors was 0.68
ng/ml. This comparison shows that the concentration of Cat D may
have prognostic value for excluding TCC, and Cat D may be used as a
tumor marker to reduce the number of cystoscopies.
TCC patients were found to exhibit extremely high,
but relatively stable, levels of serum and urine Cat D, which were
independent of tumor parameters. Serum Cat D concentrations were
found to range between 1.30 and 5.59 ng/ml with the majority of the
results at ~3.3 ng/ml and, in the case of urine, the concentration
was found to range between 1.35 and 7.14 ng/ml with the majority of
the results at ~3.2 ng/ml.
A high recurrence rate is characteristic of TCC of
the bladder. In superficial stages, Ta and T1, as well as in
particular cases of T2a, it may be effectively cured by
bladder-sparing treatment (21).
Effectively controlling bladder TCC prolongs survival; however,
this requires strict follow-up procedures to guarantee early
detection. The European Association of urology (22) and American Association of Urology
(23) consistently recommend
performing cystoscopy with established procedures. Previous studies
have attempted to identify a tumor marker in the blood or urine to
facilitate diagnosis and eliminate invasive procedures (24,25).
Urine cytology, which is recognized as a traditional test, has low
sensitivity. Therefore, a negative result does not exclude the
patient from obligatory cystoscopy (26). Novel substances are verified as
potential highly sensitive markers to reduce the number of
cystoscopies (27).
Further studies using larger numbers of patients are
required, which investigate the association between Cat D and the
individual parameters that characterize bladder cancer, in
particular the recurrence and prediction of progression.
References
|
1
|
Faust PL, Kornfeld S and Chirgwin JM:
Cloning and sequence analysis of cDNA for human cathepsin D. Proc
Natl Acad Sci USA. 82:4910–4914. 1985.
|
|
2
|
Barrett AJ and Cathepsin D: Purification
of isoenzymes from human and chicken liver. Biochem J. 117:601–607.
1970.
|
|
3
|
Têtu B, Brisson J, Côté C, et al:
Prognostic significance of cathepsin-D expression in node-positive
breast carcinoma: an immunohistochemical study. Int J Cancer.
55:429–435. 1993.
|
|
4
|
Veneroni S, Daidone MG, Di Fronzo C, et
al: Quantitative immunohistochemical determination of cathepsin-D
and its relation with other variables. Breast Cancer Res Treat.
26:7–13. 1993.
|
|
5
|
Métayé T, Millet C, Kraims JL, et al:
Estrogen receptors and cathepsin D in human thyroid tissue. Cancer.
72:1991–1996. 1993.
|
|
6
|
Warwas M and Taurowska E: Cathepsin D in
diagnosis of neoplastic diseases. Postepy Hig Med Dosw. 47:277–288.
1993.(In Polish).
|
|
7
|
Ferlay J, Shin HR, Bray F, et al: Cancer
Incidence and Mortality Worldwide: IARC CancerBase No. 10.
International Agency for Research on Cancer. GLOBOCAN; Lyon,
France: 2008, http://globocan.iarc.fr.
Accessed 4 July, 2010
|
|
8
|
Burger M, Catto JW, Dalbagni G, et al:
Epidemiology and risk factors of urothelial bladder cancer. Eur
Urol. 63:234–241. 2013.
|
|
9
|
Fernández-González A, Rychłowska J, Badía
R and Salzer R: SPR imaging as a tool for detecting mucin -
anti-mucin interaction. Outline of the development of a sensor for
near-patient testing for mucin. Microchim Acta. 158:219–225.
2007.
|
|
10
|
Lee HJ, Nedelkov D and Corn RM: Surface
plasmon resonance imaging measurements of antibody arrays for the
multiplexed detection of low molecular weight protein biomarkers.
Anal Chem. 78:6504–6510. 2006.
|
|
11
|
Gorodkiewicz E, Regulska E and
Roszkowska-Jakimiec W: Determination of the active form
concentration of cathepsins D and B by SPRI biosensors. J Lab
Diagn. 46:107–109. 2010.
|
|
12
|
Gorodkiewicz E and Regulska E: SPR imaging
biosensor for aspartyl cathepsins: sensor development and
application for biological material. Protein Pept Lett.
17:1148–1154. 2010.
|
|
13
|
Gorodkiewicz E, Regulska E and Wojtulewski
K: Development of an SPR imaging biosensor for determination of
cathepsin G in saliva and white blood cells. Mikrochim Acta.
173:407–413. 2011.
|
|
14
|
Gorodkiewicz E, Ostrowska H and Sankiewicz
A: SPR imaging biosensor for the 20S proteasome: sensor development
and application to measurement of proteasomes in human blood
plasma. Microchim Acta. 175:177–184. 2011.
|
|
15
|
Gorodkiewicz E, Charkiewicz R, Rakowska A,
et al: SPR imaging biosensor for podoplanin: sensor development and
application to biological materials. Microchim Acta. 176:337–343.
2012.
|
|
16
|
Gorodkiewicz E: Surface Plasmon Resonance
Imaging sensor for cathepsin determination based on immobilized
cystatin. Protein Pept Lett. 16:1379–1385. 2009.
|
|
17
|
Tokyol C, Köken T, Demirbas M, et al:
Expression of cathepsin D in bladder carcinoma: correlation with
pathological features and serum cystatin C levels. Tumori.
92:230–235. 2006.
|
|
18
|
Salman T, el-Ahmady O, el-Shafee M, et al:
Cathepsin-D and TNF-alpha in bladder cancer. Dis Markers.
12:253–259. 1996.
|
|
19
|
Kotaska K, Dusek P, Prusa R, et al: Urine
and serum cathepsin B concentration in the transitional cell
carcinoma of the bladder. J Clin Lab Anal. 26:61–65. 2012.
|
|
20
|
Szajda SD, Darewicz B, Kudelski J, et al:
Cancer procoagulant and cathepsin D activity in blood serum in
patients with bladder cancer. Pol Merkur Lekarski. 18(108):
651–653. 2005.
|
|
21
|
Herr HW: Transuretheral resection of
muscle-invasive bladder cancer: 10-year outcome. J Clin Oncol.
19:89–93. 2001.
|
|
22
|
European Association of Urology.
Non-muscle-invasive (Ta, T1 and CIS) bladder cancer. http://www.uroweb.org/guidelines/online-guidelines/.
Accessed March 13, 2012
|
|
23
|
American Urological Association. Guideline
for the management of nonmuscle invasive bladder cancer: (stages
Ta, T1 and Tis): Update 2007. http://www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines.cfm.
Accessed November 15, 2007
|
|
24
|
Elissa S, Swellam M, Sadek M, et al:
Comparative evaluation of the nuclear matrix protein, fibronectin,
urinary bladder cancer antigen and voided urine cytology in the
detection of the bladder tumors. J Urol. 168:465–469. 2002.
|
|
25
|
Menéndez V, Fernández-Suárez A, Galán JA,
et al: Diagnosis of bladder cancer by analysis of urinary
fibronectin. Urology. 65:284–289. 2005.
|
|
26
|
Lotan Y, Shariat SF, Schmitz-Dräger BJ, et
al: Considerations on implementing diagnostic markers into clinical
decision making in bladder cancer. Urol Oncol. 28:441–448.
2010.
|
|
27
|
Catto JWF: Old and new urinary markers:
Which one is the PSA for bladder cancer? Eur Urol Suppl. 7:422–425.
2008.
|