Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies worldwide, accounting for >740,000 new cases
and 690,000 mortalities per year (1). Half of these new cases and mortalities
were estimated to occur in China. The high rates of HCC in China
are largely due to the prevalence of chronic hepatitis B virus
(HBV) infection (2). The RAS/RAF
and PI3K/PTEN signaling pathways play central roles in
hepatocarcinogenesis (3). The
aberrant activation of the RAS/RAF and PI3K/PTEN signaling pathways
is associated with poor prognosis in cancer patients (4,5). HBV
also utilizes the pathways for the control of hepatocyte survival
and viral replication (6,7). Mutations of key components (such as
RAS, RAF, PIK3CA, PIK3R1 and
PTEN) in the RAS/RAF and PI3K/PTEN pathways lead to the
dysregulation of the two cascades (8). The RAS family comprises three
members: KRAS, NRAS and HRAS. Somatic
mutations in the RAS family are common in numerous human
cancer types, including pancreatic, thyroid, colorectal, liver,
kidney and lung (9). BRAF is
the most frequently mutated gene in the RAF family, and the
BRAF mutation has been reported in 61% of melanoma, 53% of
papillary thyroid cancer and 11.5% of colorectal cancer patients
(10–12). The PI3K gene comprises
PIK3CA, which encodes the catalytically active p110α
subunit, and PIK3R1, encoding the p85α regulatory subunit
(13). PIK3CA is mutated in
numerous tumor types, with the frequency ranging from 4 to 32% in
breast, colorectal, endometrial, brain, gastric and lung cancer
(14–17). PIK3R1 mutations were
identified in 43% of endometrial cancer, 4% of ovarian cancer and
2% of colon cancer (18–19). PTEN acts as a negative regulator of
the PI3K pathway and PTEN mutations lead to a reduction of
its phosphatase activity (20).
Mutations of the PTEN gene are associated with a wide
variety of human tumors (21).
Inhibitors targeting the RAS/RAF and PI3K/PTEN
pathways have been developed and the clinical responses of patients
were observed to differ according to the genetic alterations of the
critical components of the two cascades (22). However, few data are available
regarding the prevalence of KRAS, NRAS, HRAS,
BRAF, PIK3CA, PIK3R1 and PTEN mutations
in Chinese patients with HCC. In the present study, we conducted
mutational analysis of 57 somatic hotspot mutations in KRAS,
NRAS, HRAS, BRAF, PIK3CA, PIK3R1
and PTEN in 36 Chinese patients with HCC.
Materials and methods
Patients and tissue samples
Thirty-six patients with HCC undergoing surgery at
Nantong Tumor Hospital (Nantong, China) between 2009 and 2011 were
enrolled in this study. Tumor samples and adjacent normal liver
tissues from the corresponding patients were fixed with 10%
formalin, embedded in paraffin and stained with hematoxylin and
eosin (H&E). Tumor staging was performed according to the
Barcelona Clinic Liver Cancer (BCLC) staging classification
(23). This study was approved by
the Ethics Committee of Nantong Tumor Hospital. Written informed
consent was obtained from each patient prior to sample
collection.
Genomic DNA extraction
Tumor areas and non-tumorous tissue areas were
identified on H&E-stained slides. Genomic DNA was extracted
from formalin-fixed paraffin-embedded tissues of HCC with the
QIAamp DNA FFPE Tissue kit (Qiagen GmbH, Hilden, Germany) according
to the manufacturer’s instructions. Briefly, samples were placed
into Eppendorf tubes and the paraffin was removed. Next, the tubes
were incubated with proteinase K (Qiagen GmbH) at 56°C for 1 h.
Following proteinase K digestion, the samples were incubated at
90°C for 1 h and DNA was extracted using QIAamp MinElute columns
(Qiagen GmbH).
Mutation analysis
Polymerase chain reaction (PCR) was performed to
amplify the gene fragments including the hotspot mutations shown in
Table I. The selection of the
hotspots was based on the prevalence of mutations in cancers
identified in the COSMIC database (24). A 50 μl volume of PCR was prepared
using the Taq PCR Master Mix kit (Qiagen GmbH), according to the
manufacturer’s instructions. The thermocycling was performed at
94°C for 3 min; 35 cycles of 94°C for 30 sec, 56°C for 30 sec and
72°C for 60 sec; followed by a final 10 min at 72°C. PCR products
were run on 1.5% agarose gel electrophoresis and visualized with
ultraviolet light to confirm sizes. DNA purification was performed
with the QIAprep Gel Extraction kit (Qiagen GmbH) according to the
manufacturer’s instructions. Briefly, the DNA fragments were
excised from the agarose gel with a scalpel and placed in a
colorless tube. DNA cleanup was conducted using QIAquick spin
columns (Qiagen GmbH). Direct DNA sequencing was performed using a
Big Dye Terminator (v3.1) kit (Applied Biosystems, Foster City, CA,
USA). The sequencing products were run on an Applied Biosystems
3130XL Genetic Analyzer (Applied Biosystems). DNA sequencing
results were analyzed using Chromas software (Technelysium,
Brisbane, Queensland, Australia). The primers used for the PCR are
listed in Table I.
 | Table IPrimers used for polymerase chain
reaction in this study. |
Table I
Primers used for polymerase chain
reaction in this study.
| Gene | Mutation | Forward primer
sequence (5′ to 3′) | Reverse primer
sequence (5′ to 3′) |
|---|
| KRAS | G12C, G12D, G13S,
G13D, L19F, Q22K |
CTTAAGCGTCGATGGAGGAG |
CCCTGACATACTCCCAAGGA |
| A59T, Q61E, Q61R,
Q61H |
GGTGCTTAGTGGCCATTTGT |
CCTAGGTTTCAATCCCAGCA |
| A146T |
TTGTGGACAGGTTTTGAAAGA |
AGAAACCAAAGCCAAAAGCA |
| NRAS | G12S, G12V, G13R,
G13V, A18T |
GCCCAAGGACTGTTGAAAAA |
CCGACAAGTGAGAGACAGGA |
| Q61K, Q61R,
Q61H |
GGCAGAAATGGGCTTGAATA |
AGGTTAATATCCGCAAATGAC |
| HRAS | G12S, G12V, G13R,
G13D |
GTGGGTTTGCCCTTCAGAT |
TGGTGGATGTCCTCAAAAGA |
| Q61K, Q61R,
Q61H |
TGGCTGTGTGAACTCCCC |
GTCAGTGAGTGCTGCTCCC |
| BRAF | G464V, G466V,
G469A, V471F |
CACTTGGTAGACGGGACTCG |
AGTTTATTGATGCGAACAGTGA |
| D594G, L597V,
V600E |
AACTCTTCATAATGCTTGCTCTGA |
AGCCTCAATTCTTACCATCCA |
| PIK3CA | R38G, Q75E,
R108H |
GCCTAATCAAGTCAAACTATGGAA |
AAGCTTTATGGTTATTTGCATTTT |
| G118D |
ATGTTTGCTGCCTTTGCTCT |
ATAAGCAGTCCCTGCCTTCA |
| C378R |
TAAGGGGATTGTGGGCCTAT |
AATGGGGTCTTGCTTTGTTG |
| C420R |
CTCATGCTTGCTTTGGTTCA |
TTGGCATGCTCTTCAATCAC |
| P539R, E542K,
E545K, E545G, Q546K |
GATTGGTTCTTTCCTGTCTCTG |
CCACAAATATCAATTTACAACCATTG |
| T1025A, M1043T,
M1043I, H1047Y, H1047R, H1047L |
CATTTGCTCCAAACTGACCA |
CACCCCAAGCATTTTTCTTC |
| PIK3R1 | G376R |
CAGACGGGACCTTTTTGGTA |
AACAAAATAGCTGACATGGAAACA |
| K459E, D464H |
GGCTTCTCTGACCCATTAACC |
CCCCACCTCATTCGTAAAAA |
| L570P |
GGAAGAGAAGCCACGCTTTA |
CCCAACCACTCGTTCAACTT |
| PTEN | R130G, R130Q |
CCGTATAGCGTAAATTCCCAGA |
TCTCAGATCCAGGAAGAGGAA |
| R233X |
TGCTTGAGATCAAGATTGCAG |
GCCATAAGGCCTTTTCCTTC | |
Results
Clinicopathological characteristics
Of 36 patients with HCC, the median age was 54 years
(range, 40–77 years), including 33 males and three females. The
majority of the cases had HCC associated with HBV infection (34/36;
94.4%). All patients were negative in hepatitis C virus infection.
The concentrations of serum AFP of 16 patients (16/36; 44.4%) were
higher than 400 ng/ml. The BCLC staging classification was used to
classify the cancer staging (23).
There were 2, 25, 8, 1 and 0 cases of stages 0 to D, respectively
(Table II).
 | Table IIClinical characteristics of
hepatocellular carcinoma patients. |
Table II
Clinical characteristics of
hepatocellular carcinoma patients.
| Characteristic | Value |
|---|
| Age, years |
| Median
(range) | 54 (40–77) |
| Gender, n (%) |
| Male | 33 (91.7) |
| Female | 3 (8.3) |
| Etiology, n
(%) |
| HBV(+) | 34 (94.4) |
| HBV(−) | 2 (5.6) |
| AFP, n (%) |
| >400 ng/ml | 16 (44.4) |
| ≤400 ng/ml | 20 (55.6) |
| Stage, n (%) |
| 0 | 2 (5.6) |
| A | 25 (69.4) |
| B | 8 (22.2) |
| C | 1 (2.8) |
| D | 0 (0.0) |
Mutation analysis of key genes in the
RAS/RAF and PI3K/PTEN pathways
We analyzed hotspot-containing gene fragments of key
genes in RAS/RAF and PI3K/PTEN pathways using PCR amplification
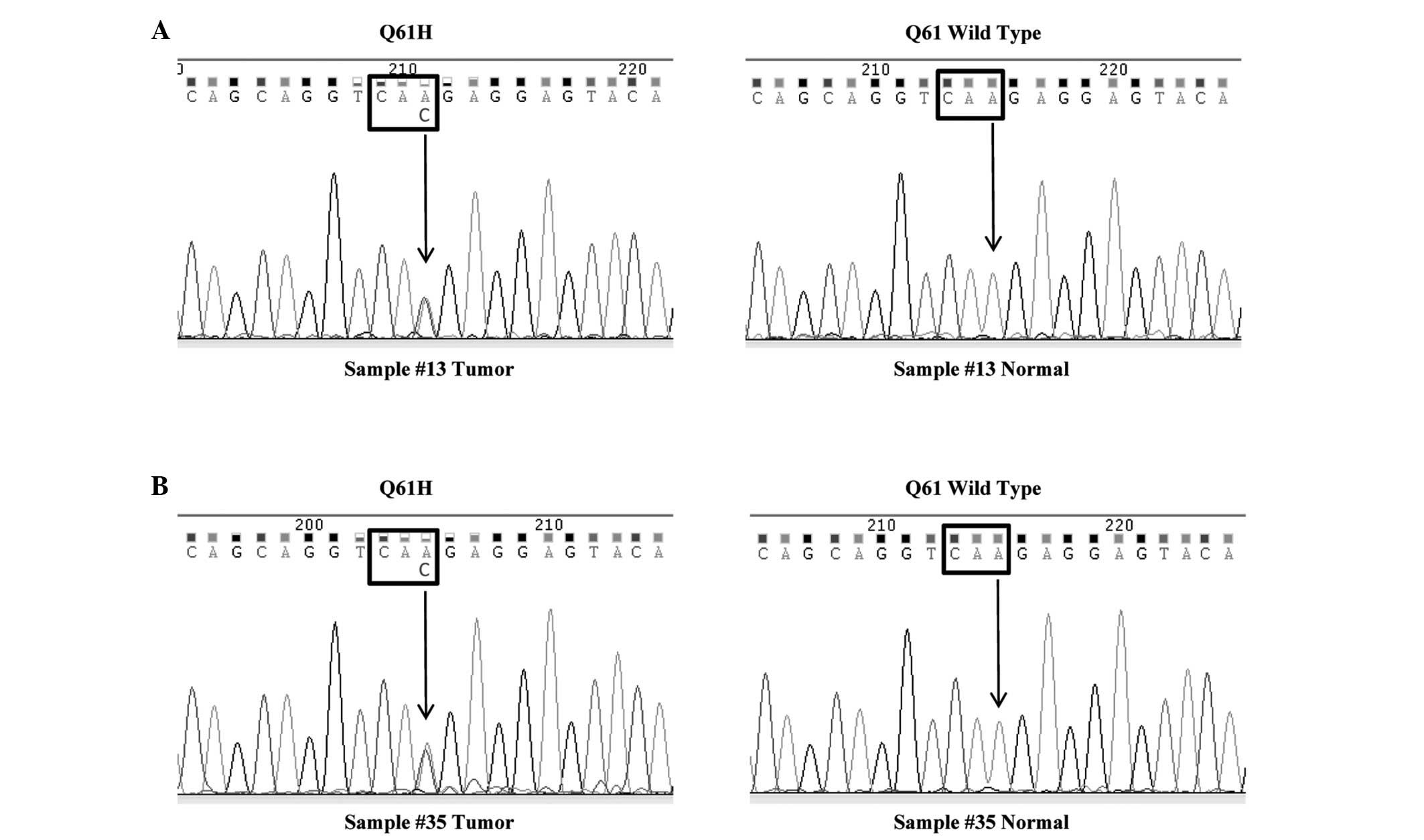
followed by direct sequencing. The hotspots were listed in Table I. In all, two samples (Sample #13
and #35) had point mutations in codon 61 (Q61H) of the KRAS
gene and the mutation rate was 5.6% (Fig. 1). In the two cases, codon 61 was
altered from CAA, coding for Gln, to CAC, coding for His. To
confirm the two mutations occurred at the somatic level, we tested
codon 61 mutation status in non-tumorous tissues from the two
patients. The results showed that codon 61 was wild-type in normal
tissues from Sample #13 and #35 (Fig.
1). The two patients harboring KRAS mutation were male.
Patient no. 13 was 49 years old, had HBV infection and stage A HCC,
and an AFP level of 3.4 ng/ml. Patient no. 35 was 69 years old, had
stage B HCC and was negative for HBV infection, with an AFP level
of 1.95 ng/ml. No other mutations in the HRAS, NRAS,
BRAF, PIK3CA, PIK3R1 and PTEN genes
were identified.
Discussion
Targeting the RAS/RAF and PI3K/PTEN pathways are
novel therapeutic strategies that may be exploited for the
treatment of HCC (8). As the
RAF-kinase inhibitor sorafenib has been demonstrated to be
effective in the treatment of HCC, BRAF mutations have
become a favored target in HCC treatment recently (25). However, the somatic mutation
prevalence and distribution of the key genes in the two pathways
remain largely unknown in Chinese patients with HCC. Therefore, the
present study set out to examine the frequency of hotspot mutations
of the KRAS, NRAS, HRAS, BRAF,
PIK3CA, PIK3R1 and PTEN genes in 36 human HCC
tissues from Chinese patients. Only KRAS somatic mutations
were identified, with a mutation rate of 5.6%.
The incidence of KRAS mutations has been
found in 80% of advanced pancreatic cancer (26), 45% of cholangiocarcinoma (27) and 32% of colorectal cancer (28) patients. COSMIC database has shown
that mutations in codons 12, 13 and 61 of the KRAS gene are
known hotspots in various types of cancer. The frequency and
distribution of KRAS mutation in HCC from several previous
studies are summarized in Table
III. The majority of these studies have shown that KRAS
gene mutations occur infrequently (<10%) in HCC. The codon 12
accounts for the majority of KRAS mutations detected (~70%),
whereas mutations affecting codon 13 and codon 61 account for the
remaining 30%. One third of the twelve studies did not evaluate the
KRAS codon 61 mutation status, which may cause bias in the
distribution of the KRAS mutation. Three whole exome
sequencing studies conducted mutational screening in all
KRAS exons and found that the mutations were clustered in
the hotspots (29–31). In the current study, mutations were
detected in codons 12, 13 and 61 of the KRAS gene, and two
out of 36 (5.6%) HCCs harbored KRAS mutations in codon 61.
Therefore, KRAS gene mutations may participate in
hepatocellular carcinogenesis.
 | Table IIIReported point mutations in codons
12, 13, and 61 of KRAS in hepatocellular carcinomas. |
Table III
Reported point mutations in codons
12, 13, and 61 of KRAS in hepatocellular carcinomas.
| Author (ref) | Population | No. of
patients | Codon 12 | Codon 13 | Codon 61 | Frequency (%) |
|---|
| Zuo et al
(36) | Chinese | 64 | 2 | 1 | NA | 4.7 |
| Huang et al
(31) | Chinese | 10 | 0 | 0 | 0 | 0.0 |
| Taketomi et
al (33) | Japanese | 61 | 0 | 1 | 0 | 1.6 |
| Tsuda et al
(43) | Japanese | 30 | 1 | 0 | 0 | 3.3 |
| Taniguchi et
al (44) | Japanese | 15 | 0 | 0 | NA | 0.0 |
| Fujimoto et
al (29) | Japanese | 27 | 0 | 0 | 0 | 0.0 |
| Tada et al
(45) | Japanese | 12 | 0 | 0 | 0 | 0.0 |
| Bose et al
(46) | Indian | 30 | 2 | 0 | 0 | 6.7 |
| Tannapfel et
al (27) | German | 25 | 0 | 0 | NA | 0.0 |
| Weihrauch et
al (47) | German | 20 | 3 | 0 | NA | 15.0 |
| Challen et
al (32) | British | 19 | 0 | NA | 1 | 5.3 |
| Guichard et
al (30) | French | 149 | 1 | 0 | 1 | 1.3 |
| Colombino et
al (37) | Italian | 65 | 1 | 0 | 0 | 1.5 |
The present study also investigated the hotspot
mutations in NRAS and HRAS, but found no mutation in
the two genes. Few studies have focused on the mutation incidence
of these two RAS family members in Chinese patients with
HCC. A whole exome sequencing study identified no mutation in these
two genes in a Chinese population (31). Challen et al found that the
frequency of NRAS mutations was 15.8%, but did not identify
HRAS mutations, in British patients with HCC (32). Taketomi et al reported
neither NRAS nor HRAS mutations were detected in
Japanese HCC cases (33). Thus, the
mutational activation of NRAS and HRAS genes is an
uncommon event in the pathogenesis of HCCs.
BRAF mutations can abnormally activate
downstream signaling pathways in HCC and act as indicator of
cetuximab resistance in patients with colon cancer (34,35).
BRAF mutations are believed to be rare in HCCs. Previously,
no BRAF mutations were identified in German and Chinese
populations (27,36). However, Colombino et al
detected that the BRAF gene was highly mutated in ~23% of
Italian HCC cases (37). In the
current series, no BRAF mutations were observed, indicating
that BRAF mutation does not play a major role in abnormal
activation of RAS/RAF signaling pathway.
PIK3CA, PIK3R1 and PTEN are key
genes in the PI3K/PTEN pathway (8).
In the current study, it was found that mutations were absent in
the three genes. Previously, PIK3CA was observed to be
frequently mutated in Korean and Italian patients with HCC, with
mutation rates of 35.6 and 28%, respectively (15,37).
However, Tanaka et al did not identify PIK3CA
mutations in Japanese patients with HCC, and Riener et al
reported that the PIK3CA mutation incidence was 2% in Swiss
patients with HCC (38,39). In two studies in Chinese patients
with HCC, the mutation rates were 1.6 and 1.1% (36,40),
which were similar to those of the present study. The conflicting
data may be due to the different genetic backgrounds of the
populations, HBV infection status and smaller sample size in the
current study. PIK3R1 mutation has been found to occur
infrequently in numerous cancer types, including ovarian and colon
cancer (19), and the present study
showed a low frequency of alteration of PIK3R1 in HCC.
Inactivation of PTEN in HCC may be largely due to frequent
loss of heterozygosity of the PTEN allele; the frequency was
identified to be ≤44.4% (41). Wang
et al investigated PTEN mutations in exons 5 and 8,
but failed to detect any (42),
which was in agreement with the results of the present study.
Mutations in the PIK3CA, PIK3R1 and PTEN genes
rarely occur in HCC, suggesting that somatic point mutations of
these three genes may not play an important role in HCC in the
Chinese population. However, further research is necessary to
confirm these results in larger sample size.
In summary, the present study investigated the
prevalence of KRAS, NRAS, HRAS, BRAF,
PIK3CA, PIK3R1 and PTEN mutations in 57
hotspot mutations. Two cases of KRAS mutation were
identified among 36 HCC cases. The findings indicated that point
mutations in the KRAS gene, but not mutations in the
NRAS, HRAS, BRAF, PIK3CA, PIK3R1
and PTEN genes, at the somatic level contribute to the
abnormal activation of the RAS/RAF and PI3K/PTEN pathways in HCC.
Considering the low frequency of key genes in the RAS/RAF and
PI3K/PTEN signaling pathways, other mechanisms to activate the
RAS/RAF and PI3K/PTEN pathways, such as gene amplification,
deletion, and aberrant methylation, may be involved in the
development and progression of HCC.
Acknowledgements
This study was supported by grants from the Key
Research Program of the Chinese Academy of Sciences (grant no.
KSZD-EW-Z-019), the National Nature Science Foundation (grant nos.
31101261, 81302507 and 81302809), the Ministry of Science and
Technology of China (grant no. 2014AA020524), the Shanghai
Institutes for Biological Sciences, Chinese Academy of Sciences
(grant nos. 2012KIP308 and 2012KIP515) and the Food Safety Research
Center and Key Laboratory of Food Safety Research of INS, SIBS,
CAS. Dr. Peizhan Chen was partially supported by the SA-SIBS
scholarship program.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.
|
|
2
|
McGlynn KA and London WT: The global
epidemiology of hepatocellular carcinoma: present and future. Clin
Liver Dis. 15:223–243. vii–x. 2011.
|
|
3
|
Owonikoko TK and Khuri FR: Targeting the
PI3K/AKT/mTOR pathway: biomarkers of success and tribulation. Am
Soc Clin Oncol Educ Book. 2013.DOI:
10.1200/EdBook_AM.2013.33.e395
|
|
4
|
McCubrey JA, Steelman LS, Abrams SL, et
al: Targeting survival cascades induced by activation of
Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for
effective leukemia therapy. Leukemia. 22:708–722. 2008.
|
|
5
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
|
|
6
|
Zheng Y, Li J, Johnson DL and Ou JH:
Regulation of hepatitis B virus replication by the
ras-mitogen-activated protein kinase signaling pathway. J Virol.
77:7707–7712. 2003.
|
|
7
|
Liu H, Xu J, Zhou L, et al: Hepatitis B
virus large surface antigen promotes liver carcinogenesis by
activating the Src/PI3K/Akt pathway. Cancer Res. 71:7547–7557.
2011.
|
|
8
|
Steelman LS, Chappell WH, Abrams SL, et
al: Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in
controlling growth and sensitivity to therapy-implications for
cancer and aging. Aging (Albany NY). 3:192–222. 2011.
|
|
9
|
Fernández-Medarde A and Santos E: Ras in
cancer and developmental diseases. Genes Cancer. 2:344–358.
2011.
|
|
10
|
Fransén K, Klintenäs M, Osterström A,
Dimberg J, Monstein HJ and Söderkvist P: Mutation analysis of the
BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas.
Carcinogenesis. 25:527–533. 2004.
|
|
11
|
Libra M, Malaponte G, Navolanic PM, et al:
Analysis of BRAF mutation in primary and metastatic melanoma. Cell
Cycle. 4:1382–1384. 2005.
|
|
12
|
Fukushima T, Suzuki S, Mashiko M, et al:
BRAF mutations in papillary carcinomas of the thyroid. Oncogene.
22:6455–6457. 2003.
|
|
13
|
Roymans D and Slegers H:
Phosphatidylinositol 3-kinases in tumor progression. Eur J Biochem.
268:487–498. 2001.
|
|
14
|
Samuels Y, Wang Z, Bardelli A, et al: High
frequency of mutations of the PIK3CA gene in human cancers.
Science. 304:5542004.
|
|
15
|
Lee JW, Soung YH, Kim SY, et al: PIK3CA
gene is frequently mutated in breast carcinomas and hepatocellular
carcinomas. Oncogene. 24:1477–1480. 2005.
|
|
16
|
Shayesteh L, Lu Y, Kuo WL, et al: PIK3CA
is implicated as an oncogene in ovarian cancer. Nat Genet.
21:99–102. 1999.
|
|
17
|
Ligresti G, Militello L, Steelman LS, et
al: PIK3CA mutations in human solid tumors: role in sensitivity to
various therapeutic approaches. Cell Cycle. 8:1352–1358. 2009.
|
|
18
|
Urick ME, Rudd ML, Godwin AK, Sgroi D,
Merino M and Bell DW: PIK3R1 (p85alpha) is somatically mutated at
high frequency in primary endometrial cancer. Cancer Res.
71:4061–4067. 2011.
|
|
19
|
Philp AJ, Campbell IG, Leet C, et al: The
phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in
human ovarian and colon tumors. Cancer Res. 61:7426–7429. 2001.
|
|
20
|
Stambolic V, Suzuki A, de la Pompa JL, et
al: Negative regulation of PKB/Akt-dependent cell survival by the
tumor suppressor PTEN. Cell. 95:29–39. 1998.
|
|
21
|
Tamguney T and Stokoe D: New insights into
PTEN. J Cell Sci. 120:4071–4079. 2007.
|
|
22
|
Chappell WH, Steelman LS, Long JM, et al:
Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and
importance to inhibiting these pathways in human health.
Oncotarget. 2:135–164. 2011.
|
|
23
|
Llovet JM, Bru C and Bruix J: Prognosis of
hepatocellular carcinoma: the BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999.
|
|
24
|
Forbes SA, Bindal N, Bamford S, et al:
COSMIC: mining complete cancer genomes in the Catalogue of Somatic
Mutations in Cancer. Nucleic Acids Res. 39:D945–D950. 2011.
|
|
25
|
Zhu AX: Development of sorafenib and other
molecularly targeted agents in hepatocellular carcinoma. Cancer.
112:250–259. 2008.
|
|
26
|
De La O JP and Murtaugh LC: Notch and Kras
in pancreatic cancer: at the crossroads of mutation,
differentiation and signaling. Cell Cycle. 8:1860–1864. 2009.
|
|
27
|
Tannapfel A, Sommerer F, Benicke M, et al:
Mutations of the BRAF gene in cholangiocarcinoma but not in
hepatocellular carcinoma. Gut. 52:706–712. 2003.
|
|
28
|
Balschun K, Haag J, Wenke AK, von
Schonfels W, Schwarz NT and Rocken C: KRAS, NRAS, PIK3CA exon 20,
and BRAF genotypes in synchronous and metachronous primary
colorectal cancers diagnostic and therapeutic implications. J Mol
Diagn. 13:436–445. 2011.
|
|
29
|
Fujimoto A, Totoki Y, Abe T, et al:
Whole-genome sequencing of liver cancers identifies etiological
influences on mutation patterns and recurrent mutations in
chromatin regulators. Nat Genet. 44:760–764. 2012.
|
|
30
|
Guichard C, Amaddeo G, Imbeaud S, et al:
Integrated analysis of somatic mutations and focal copy-number
changes identifies key genes and pathways in hepatocellular
carcinoma. Nat Genet. 44:694–698. 2012.
|
|
31
|
Huang J, Deng Q, Wang Q, et al: Exome
sequencing of hepatitis B virus-associated hepatocellular
carcinoma. Nat Genet. 44:1117–1121. 2012.
|
|
32
|
Challen C, Guo K, Collier JD, Cavanagh D
and Bassendine MF: Infrequent point mutations in codons 12 and 61
of ras oncogenes in human hepatocellular carcinomas. J Hepatol.
14:342–346. 1992.
|
|
33
|
Taketomi A, Shirabe K, Muto J, et al: A
rare point mutation in the Ras oncogene in hepatocellular
carcinoma. Surg Today. 43:289–292. 2013.
|
|
34
|
Huynh H, Nguyen TT, Chow KH, Tan PH, Soo
KC and Tran E: Over-expression of the mitogen-activated protein
kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its
role in tumor progression and apoptosis. BMC Gastroenterol.
3:192003.
|
|
35
|
Di Nicolantonio F, Martini M, Molinari F,
et al: Wild-type BRAF is required for response to panitumumab or
cetuximab in metastatic colorectal cancer. J Clin Oncol.
26:5705–5712. 2008.
|
|
36
|
Zuo Q, Huang H, Shi M, et al: Multivariate
analysis of several molecular markers and clinicopathological
features in postoperative prognosis of hepatocellular carcinoma.
Anat Rec (Hoboken). 295:423–431. 2012.
|
|
37
|
Colombino M, Sperlongano P, Izzo F, et al:
BRAF and PIK3CA genes are somatically mutated in hepatocellular
carcinoma among patients from South Italy. Cell Death Dis.
3:e2592012.
|
|
38
|
Tanaka Y, Kanai F, Tada M, et al: Absence
of PIK3CA hotspot mutations in hepatocellular carcinoma in Japanese
patients. Oncogene. 25:2950–2952. 2006.
|
|
39
|
Riener MO, Bawohl M, Clavien PA and Jochum
W: Rare PIK3CA hotspot mutations in carcinomas of the biliary
tract. Genes Chromosomes Cancer. 47:363–367. 2008.
|
|
40
|
Li X, Zhang Q, He W, et al: Low frequency
of PIK3CA gene mutations in hepatocellular carcinoma in Chinese
population. Pathol Oncol Res. 18:57–60. 2012.
|
|
41
|
Bae JJ, Rho JW, Lee TJ, et al: Loss of
heterozygosity on chromosome 10q23 and mutation of the phosphatase
and tensin homolog deleted from chromosome 10 tumor suppressor gene
in Korean hepatocellular carcinoma patients. Oncol Rep.
18:1007–1013. 2007.
|
|
42
|
Wang L, Wang WL, Zhang Y, Guo SP, Zhang J
and Li QL: Epigenetic and genetic alterations of PTEN in
hepatocellular carcinoma. Hepatol Res. 37:389–396. 2007.
|
|
43
|
Tsuda H, Hirohashi S, Shimosato Y, Ino Y,
Yoshida T and Terada M: Low incidence of point mutation of c-Ki-ras
and N-ras oncogenes in human hepatocellular carcinoma. Jpn J Cancer
Res. 80:196–199. 1989.
|
|
44
|
Taniguchi K, Yamada T, Sasaki Y and Kato
K: Genetic and epigenetic characteristics of human multiple
hepatocellular carcinoma. BMC Cancer. 10:5302010.
|
|
45
|
Tada M, Omata M and Ohto M: Analysis of
ras gene mutations in human hepatic malignant tumors by polymerase
chain reaction and direct sequencing. Cancer Res. 50:1121–1124.
1990.
|
|
46
|
Bose S, Sakhuja P, Bezawada L, et al:
Hepatocellular carcinoma with persistent hepatitis B virus
infection shows unusual downregulation of Ras expression and
differential response to Ras mediated signaling. J Gastroenterol
Hepatol. 26:135–144. 2011.
|
|
47
|
Weihrauch M, Benicke M, Lehnert G,
Wittekind C, Wrbitzky R and Tannapfel A: Frequent k- ras -2
mutations and p16(INK4A)methylation in hepatocellular carcinomas in
workers exposed to vinyl chloride. Br J Cancer. 84:982–989.
2001.
|