Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
common cancers in Southern China and Southeast Asia, where the
incidence rate ranges from 20 to 50 per 100,000 and is
approximately 100-fold higher than that in the Western world
(1,2). The five-year survival rate for
patients with early-stage disease is ≤95%; however, for patients
with stage III and IV disease, the five-year overall survival rate
declines to ~70% (3,4). The majority of cases of NPC often
present at an advanced stage at the time of diagnosis, due to its
deep location and vague symptoms. Therefore, a screening protocol
for the early diagnosis of NPC is urgently required, and may
contribute to improving the treatment outcome.
Detection of Epstein-Barr virus (EBV) DNA and
antibodies against EBV antigens, such as viral capsid antigen
immunoglobulin A (VCA-IgA), is the method currently used for the
serological diagnosis of NPC; however, specificity and sensitivity
of these methods are considered unsatisfactory (5–9). A
large number of studies describe the presence of autoantibodies to
tumor-associated antigens (TAAs) in serum samples from patients
with a variety of types of cancer, including NPC (10–15).
Changes in the levels of gene expression and aberrant expression of
tissue-restricted gene products are thought to mainly account for
the humoral immune response to TAAs, which functions to remove
precancerous lesions during the early events of carcinogenesis
(16–19). Autoantibodies have been found to
precede manifestations of symptomatic cancer, and detection of
autoantibodies may be useful in types of cancer where there are
high-risk populations (20). Thus,
identification of novel autoantibody biomarkers may lead to early
diagnosis or prediction of disease progression in patients with
NPC.
Cancer-testis antigens (CTAs), a group of tumor
antigens, are encoded by genes that are normally expressed only in
the human germline, but are also expressed in various tumor types.
CTAs are widely explored as both a diagnostic marker and a
therapeutic target in malignant lesions (21). As one of the most immunogenic CTAs,
the NY-ESO-1 antigen was originally found in esophageal cancer by
serological recombinant cDNA expression cloning. The aberrant
expression of NY-ESO-1 has been observed in a variety of neoplasms.
NY-ESO-1 elicits both humoral and cellular immune responses in
patients with NY-ESO-1-expressing tumors (22). It has been estimated that 10–50% of
patients with NY-ESO-1-expressing tumors develop antibody responses
(23). Autoantibodies against
NY-ESO-1 present in a broad variety of cancer types, such as lung,
breast and prostate cancer, providing a possibility of early
detection (10,24,25).
However, to the best of our knowledge, the potential value of
autoantibodies against NY-ESO-1 as biomarker in the evaluation of
NPC has not yet been studied.
Combined analysis of VCA-IgA and NY-ESO-1
autoantibodies may increase the ability to detect NPC. In the
present study, the diagnostic value of serum autoantibodies against
NY-ESO-1 in NPC were investigated, and the possible correlation
between NY-ESO-1 autoantibodies and clinical parameters was
explored. Additionally, assay of VCA-IgA, a serological marker in
clinical use for NPC, was conducted.
Materials and methods
Study population
The study comprised 112 patients with NPC at the
Department of Radiation Oncology, The Cancer Hospital of Shantou
University Medical College (Shantou, China) between December 2012
and July 2013. NPC was defined on the basis of a routine diagnostic
workup comprised of nasopharyngoscopy and radiological imaging
techniques [computed tomography (CT), magnetic resonance imaging
(MRI) and ultrasonography], and was biopsy-proven in all poorly
differentiated squamous carcinoma types. Tumor stage was defined
according to the seventh edition of the UICC/AJCC staging system
for nasopharyngeal carcinoma (26).
The control group consisted of 138 healthy volunteers with no
previous malignant disease. The patient group and the control group
were matched as closely as possible, in terms of age and gender
(Table I).
 | Table ICharacteristics of the study
population. |
Table I
Characteristics of the study
population.
| NPC patients | Normal controls |
|---|
| Number | 112 | 138 |
| Gender |
| Male | 86 | 90 |
| Female | 26 | 48 |
| Mean age ± SD
(years) | 49±10 | 50±9 |
| Age range
(years) | 28–76 | 40–71 |
| T stage |
| T1 | 11 | - |
| T2 | 36 | - |
| T3 | 35 | - |
| T4 | 30 | - |
| N stage |
| N0 | 11 | - |
| N1 | 41 | - |
| N2 | 53 | - |
| N3 | 7 | - |
| M stage |
| M0 | 108 | - |
| M1 | 4 | - |
| Overall stage |
| I | 1 | - |
| II | 22 | - |
| III | 53 | - |
| IV | 36 | - |
Patients were all newly diagnosed. Peripheral blood
samples from normal controls and NPC patients, obtained at the time
of diagnosis and prior to any therapeutic procedures, were
centrifuged at 1,250 × g for 5 min at room temperature, and stored
at −80°C until use. Prior to the use of these clinical materials
for investigation, approval for the study from the institutional
ethics review committee of Shantou University Medical College and
written informed consent from all patients were obtained. This
study was conducted in accordance with the principles set out in
the Declaration of Helsinki.
NY-ESO-1 protein expression and
purification
The full-length cDNA for NY-ESO-1 was cloned in the
pDEST17 (Invitrogen Life Technologies, Carlsbad, CA, USA)
expression vector. The resulting recombinant plasmids were verified
by sequencing prior to expression trials. To obtain the recombinant
proteins, the expression host E. coli Rosetta (DE3)
(Novagen, Darmstadt, Germany) was transformed with the recombinant
plasmid. Selection of transformed colonies was performed on LB-agar
plates containing 100 μg/ml ampicillin [Sangon Biotech (Shanghai)
Co., Ltd., Shanghai, China). Cells carrying the recombinant plasmid
were inoculated in 5 ml LB medium supplemented with 100 μg/ml
ampicillin, and cultured overnight at 37°C with rotary shaking
(~250 × g). Subsequently, the cell culture was transferred to 2 l
of fresh LB medium supplemented with 100 μg/ml ampicillin. When the
optical density (OD) at 600 nm reached 0.4–0.6, IPTG (Merck,
Darmstadt, Germany) was added to a final concentration of 0.4 mM to
induce the expression of recombinant protein. The cells were grown
at 30°C and, after ~3 h, were harvested by centrifugation at 14,800
× g for 10 min at 4°C. The bacterial pellet was resuspended in 1X
phosphate-buffered saline (PBS; 2.7 mM KCl, 4.3 mM
Na2HPO4, 1.8 mM KH2PO4,
137 mM NaCl; pH 7.4) buffer supplemented with 8 M urea and 1 mM
phenylmethylsulfonyl fluoride. The cell fractions were purified
under denaturing conditions and were refolded in vitro while
immobilized on a Ni2+-NTA-Sepharose (Novagen) column.
Following incubation, the column was washed with wash buffer A (1X
PBS, 8 M urea; pH 8.0) followed by wash buffer B (1X PBS). The
refolded proteins were eluted with elution buffer (PBS, 500 mM
imidazole; pH 7.4) and dialyzed twice against 4 l of 50% glycerol
in PBS. Protein concentrations were determined by bicinchoninic
acid (BCA) protein assay, using the BCA Protein Assay kit (Thermo
Scientific, Pierce Biotechnology, Rockford, IL, USA), and bovine
serum albumin (BSA; Thermo Fisher Scientific, Boston, MA, USA) was
used as a standard. The purity of the recombinant protein was
assessed by Coomassie Blue staining (Thermo Fisher Scientific),
following SDS-PAGE.
Measurement of serum autoantibodies
against NY-ESO-1
Autoantibodies against NY-ESO-1 were measured by
enzyme-linked immunosorbent assay (ELISA). Purified recombinant
antigen, NY-ESO-1, was diluted in 50 mM bicarbonate buffer (pH 9.6)
to a final concentration of 0.1 μg/ml. The antigen dilutions were
dispensed into 96-well microtiter plates (100 μl/well; Haotian
Biotechnology Co., Ltd., Haimen, China) and incubated overnight at
4°C. The plates were washed three times with phosphate-buffered
saline containing 0.05% Tween 20 [PBST; Sangon Biotech (Shanghai)
Co., Ltd.], and then blocked with a blocking buffer (PBST
containing 1% BSA) at 37°C for 1 h, followed by three washes with
PBST. Serum samples and quality control samples (QCS, a pooled
plasma sample collected randomly from 50 patients with NPC), were
diluted 1/110 in blocking buffer, then were incubated at 37°C for 1
h, as were appropriate control rabbit polyclonal anti-human
NY-ESO-1 antibodies (Immunosoft, Zhoushan, China) specific for
capture proteins. After washing four times with PBST, polyclonal
horseradish peroxidase (HRP)-conjugated goat anti-human IgG or
anti-rabbit IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) were used as secondary antibodies at the dilution recommended
by the manufacturer. After a 60-min incubation, the plates were
washed, and pre-prepared 3,3′,5,5′-tetramethylbenzidine (Intec
Products, Inc., Xiamen, China) and hydrogen peroxide (Intec
Products, Inc.) were added. Color formation was allowed to proceed
for 15 min, before termination with 0.5 M
H2SO4. The absorbance of each well was read
at 450 nm and referenced to 630 nm by a MK3 microplate reader
(Thermo Fisher Scientific).
All cancer and normal samples were interspersed on
the plates and run in duplicate. QCSs were run to ensure quality
control monitoring of the assay runs by using Levey-Jennings plots.
With the purpose of minimizing an intra-assay deviation, the ratio
of the difference between duplicated sample OD values to their sum
was used to assess the precision of the assay. If the ratio was
>10%, the test of this sample was considered invalid and the
sample was repeated.
ELISA for EBV VCA-IgA
Concentrations of VCA-IgA in all samples were
determined in duplicate by ELISA using the EB virus VCA-IgA
detection kit (Berer Bioengineering, Beijing, China). The
experiments were conducted according to the manufacturer’s
instructions. Briefly, one well of blank control, two wells of
negative controls and two wells of positive controls were included
on each plate, then each serum sample was added to the plates (100
μl in each well) at a 1:10 dilution and allowed to incubate at 37°C
for 30 min. After washing five times, 100 μl of HRP-conjugated
anti-human IgA antibody was added into each well. Following
incubation at 37°C for 30 min, the plates were then washed. Color
development was conducted by the addition of 100 μl
tetramethylbenzidine substrate solution and incubation at 37°C for
15 min. When the reaction had stopped, absorbance of test sera was
immediately read on the microplate reader (Thermo Fisher
Scientific), using 450 nm as the primary wavelength and 630 nm as
the secondary wavelength.
Statistical analysis
All analyses were performed using SPSS, version 17.0
(SPSS, Inc., Chicago, IL, USA) or GraphPad Prism software (GraphPad
Software, Inc., La Jolla, CA, USA). The number and proportion of
positive samples are presented with the exact 95% confidence
interval (CI) for binomial proportions (27). The comparison of NY-ESO-1
autoantibody and VCA-IgA between sera of normal controls and NPC
was conducted by the use of the nonparametric Mann-Whitney U test.
The cutoff value for the VCA-IgA assay was calculated according to
the manufacturer’s recommendations. Receiver operating
characteristic (ROC) curve analysis was performed to determine the
cutoff value of the autoantibody assay. In addition, an area under
the ROC curve (AUC) with 95% CI was calculated for both NY-ESO-1
autoantibody and VCA-IgA. χ2 tests were carried out to
identify correlations between the positivity of the different
markers and clinical parameters. In all tests, two-sided P<0.05
was considered to indicate a statistically significant
difference.
Results
Serum levels of autoantibodies against
NY-ESO-1 and VCA-IgA between NPC patients and normal controls
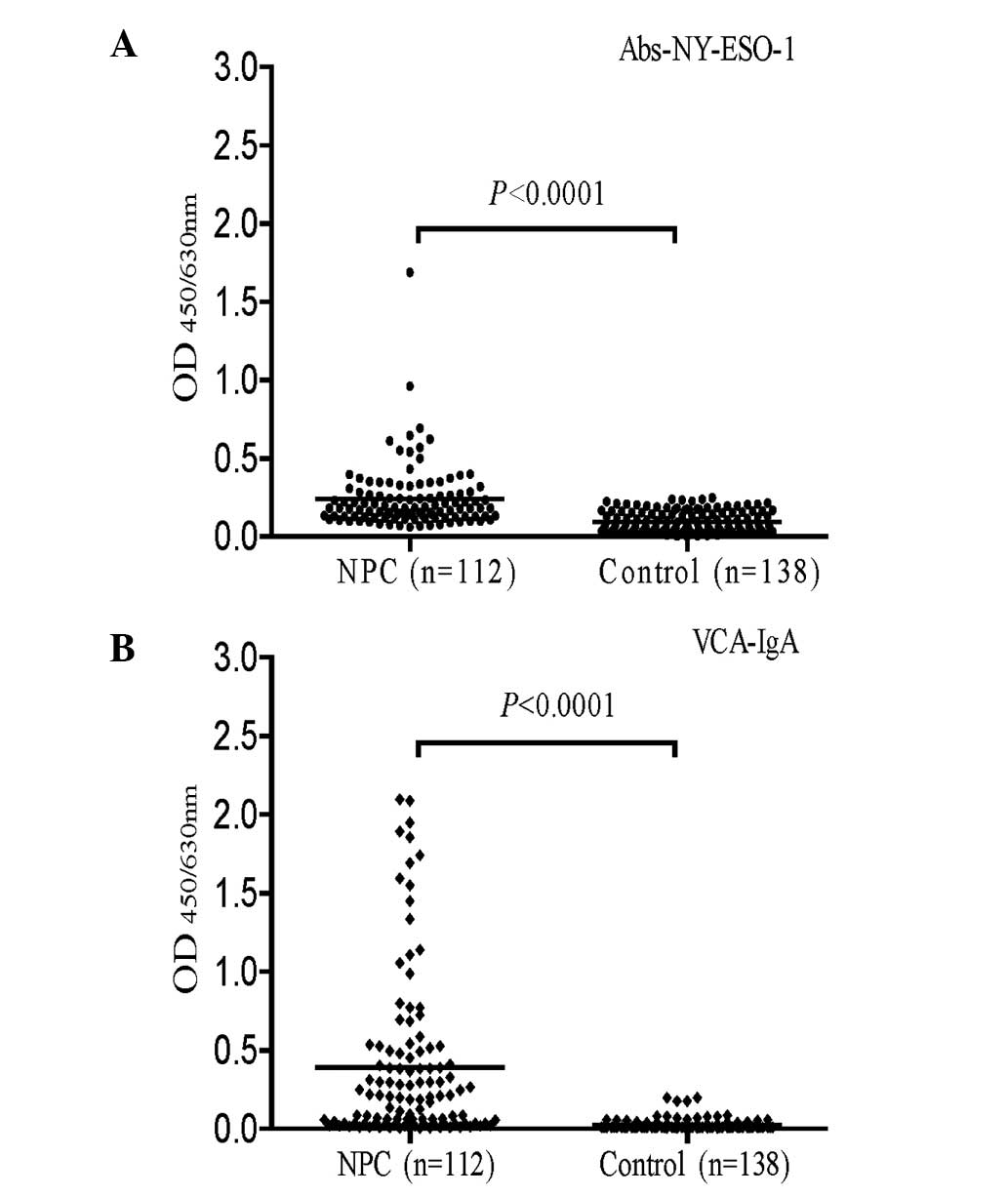
The mean OD450/630 ± standard deviation
of serum autoantibodies against NY-ESO-1 was 0.241±0.206 in the 112
NPC patients and 0.095±0.067 in the 138 normal controls. The serum
levels of autoantibodies against NY-ESO-1 were significantly higher
in the NPC patients than in the normal controls (P<0.0001;
Fig. 1A). The levels of VCA-IgA
were also significantly higher in patients with NPC than in the
normal controls (0.390±0.524 versus 0.024±0.033; P<0.0001;
Fig. 1B).
Evaluation of autoantibodies against
NY-ESO-1 as diagnostic marker
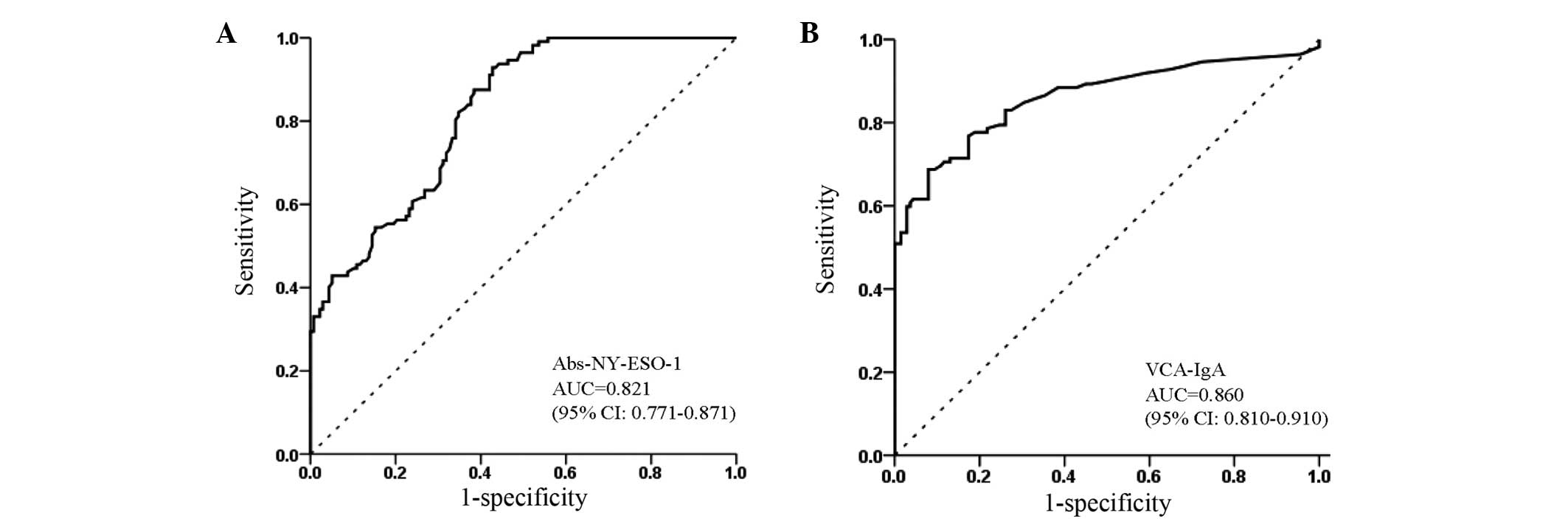
The diagnostic value of autoantibodies against
NY-ESO-1 was evaluated using the ROC analysis. According to the ROC
curve, the optimal cutoff value of autoantibodies against NY-ESO-1
for NPC was 0.210, providing a sensitivity of 42.9% (95% CI,
33.7–52.6%) and a specificity of 94.9% (95% CI, 89.4–97.8%)
(Table II). ROC curve analysis
showed that VCA-IgA alone (AUC, 0.860; 95% CI, 0.810–0.910;
Fig. 2B) was marginally more
effective than autoantibodies alone against NY-ESO-1 (AUC=0.819,
95% CI, 0.770–0.869; Fig. 2A).
According to the manufacturer’s instructions, the recommended
clinical cutoff value of VCA-IgA was 0.150. The sensitivity and
specificity of VCA-IgA were 55.4% (95% CI, 45.7–64.7%) and 95.7%
(95% CI, 90.4–98.2%), respectively. The efficacy of combination of
autoantibodies against NY-ESO-1 and VCA-IgA is presented in
Table II. Use of the combination
of autoantibodies against NY-ESO-1 and VCA-IgA provided an enhanced
sensitivity of 80.4% (95% CI, 71.6–87.0%) and a specificity of
90.6% (95% CI, 84.1–94.7%). Predictive values and likelihood ratios
for autoantibodies against NY-ESO-1, VCA-IgA and both markers
combined in the diagnosis of NPC are shown in Table II. These results indicate that
NY-ESO-1 autoantibodies may be a potentially useful serum biomarker
for NPC, particularly when used in conjunction with VCA-IgA.
 | Table IIResults for measurement of
Abs-NY-ESO-1, VCA-IgA and both markers combined in the diagnosis of
NPC. |
Table II
Results for measurement of
Abs-NY-ESO-1, VCA-IgA and both markers combined in the diagnosis of
NPC.
| NPC vs. NC | Abs-NY-ESO-1 | VCA-IgA | Abs-NY-ESO-1 +
VCA-IgA |
|---|
| Sensitivity (%) | 42.9 (33.7–52.6) | 55.4 (45.7–64.7) | 80.4 (71.6–87.0) |
| Specificity (%) | 94.9 (89.4–97.8) | 95.7 (90.4–98.2) | 90.6 (84.1–94.7) |
| PPV (%) | 87.3 (74.9–94.3) | 91.2 (81.1–96.4) | 87.4 (79.0–92.0) |
| NPV (%) | 67.2 (60.0–73.6) | 72.5 (65.3–78.7) | 85.0 (78.0–90.2) |
| Positive LR | 8.45
(3.98–17.94) | 12.72
(5.72–28.34) | 8.53
(5.04–14.43) |
| Negative LR | 0.60 (0.51–0.71) | 0.47 (0.38–0.57) | 0.22 (0.15–0.32) |
In this study, 23 patients with NPC had early-stage
disease (AJCC stage I and II). The positive rates of autoantibodies
against NY-ESO-1 and VCA-IgA in NPC patients with early-stage
disease were 47.8% (95% CI, 27.4–68.9%) and 39.1% (95% CI,
20.5–61.2%), respectively, which were significantly higher than
those in the normal controls (P<0.0001; Table III). This indicates that detection
of autoantibodies against NY-ESO-1 enables discrimination between
early-stage NPC and normal controls.
 | Table IIIPositive rates of Abs-NY-ESO-1,
VCA-IgA and both markers combined between early stage NPC and
normal controls. |
Table III
Positive rates of Abs-NY-ESO-1,
VCA-IgA and both markers combined between early stage NPC and
normal controls.
| | Abs-NY-ESO-1 | VCA-IgA |
Abs-NY-ESO-1+VCA-IgA |
|---|
| |
|
|
|
|---|
| n | Positive (%, 95%
CI) | P-value | Positive (%, 95%
CI) | P-value | Positive (%, 95%
CI) | P-value |
|---|
| Early-stage
(I+II) | 23 | 11 (47.8,
27.4–68.9) | P<0.0001 | 9 (39.1,
20.5–61.2) | P<0.0001 | 17 (73.9,
51.3–88.9) | P<0.0001 |
| Normal | 138 | 7 (5.1,
2.2–10.6) | | 6 (4.3, 1.8–9.6) | | 13 (9.4,
5.3–15.9) | |
Correlations between positive rates of
autoantibodies against NY-ESO-1, VCA-IgA and clinicopathological
variables
Analysis of serum samples in NPC patients showed
that autoantibodies against NY-ESO-1 did not significantly differ
with age, gender, T stage, N stage or overall stage (Table IV). The correlations of VCA-IgA and
both markers combined with clinicopathological characteristics in
NPC patients were also evaluated. However, there was no correlation
with any of the variables (Table
IV).
 | Table IVAssociation of positive rates of
Abs-NY-ESO-1, VCA-IgA and both markers combined with
clinicopathologic characteristics in NPC patients. |
Table IV
Association of positive rates of
Abs-NY-ESO-1, VCA-IgA and both markers combined with
clinicopathologic characteristics in NPC patients.
| | Abs-NY-ESO-1 | VCA-IgA |
Abs-NY-ESO-1+VCA-IgA |
|---|
| |
|
|
|
|---|
| n | Positive (%, 95%
CI) | P-value | Positive (%, 95%
CI) | P-value | Positive (%, 95%
CI) | P-value |
|---|
| Gender |
| Male | 86 | 36 (41.9,
31.5–53.0) | 0.698 | 49 (57.0,
45.9–67.5) | 0.531 | 69 (80.2,
70.0–87.7) | 0.952 |
| Female | 26 | 12 (46.2,
27.1–66.3) | | 13 (50.0,
30.4–69.6) | | 21 (80.8,
60.0–92.7) | |
| Age, years |
| ≤50 | 53 | 23 (43.4,
30.1–57.6) | 0.913 | 28 (52.8,
38.8–66.5) | 0.610 | 40 (75.5,
61.4–85.8) | 0.217 |
| >50 | 59 | 25 (42.4,
29.8–55.9) | | 34 (57.6,
44.1–70.2) | | 50 (84.7,
72.5–92.4) | |
| T stage |
| T1+T2 | 47 | 22 (46.8,
32.4–61.8) | 0.472 | 21 (44.7,
30.5–59.8) | 0.053 | 36 (76.6,
61.6–87.2) | 0.394 |
| T3+T4 | 65 | 26 (40.0,
28.3–52.9) | | 41 (63.1,
50.2–74.4) | | 54 (83.1,
71.3–90.9) | |
| N stage |
| N0+N1 | 52 | 26 (50.0,
36.0–64.0) | 0.155 | 24 (46.2,
32.5–60.4) | 0.068 | 40 (76.9,
62.8–87.0) | 0.394 |
| N2+N3 | 60 | 22 (36.7,
24.9–50.2) | | 38 (63.3,
49.8–75.1) | | 50 (83.3,
71.0–91.3) | |
| Overall stage |
| I+II
(early-stage) | 23 | 11 (47.8,
27.4–68.9) | 0.589 | 9 (39.1,
20.5–61.2) | 0.079 | 17 (73.9,
51.3–88.9) | 0.563 |
|
III+IV(advanced-stage) | 89 | 37 (41.6,
31.2–52.5) | | 53 (59.6,
48.6–69.7) | | 73 (82.0,
72.1–89.1) | |
Discussion
NY-ESO-1 was discovered based on its capacity to
incite a humoral immune response in cancer patients. The elevated
levels of the serum autoantibodies against NY-ESO-1 have been
reported in various types of cancer (10,24,25).
The present study demonstrated that the detection of autoantibodies
against NY-ESO-1 in the peripheral blood has potential diagnostic
value for NPC. The AUC for autoantibodies against NY-ESO-1 in NPC
patients was 0.821 (95% CI, 0.771–0.871), with a sensitivity of
42.9% (95% CI, 33.7–52.6%) and specificity of 94.9% (95% CI,
89.4–97.8%). The diagnostic accuracy of autoantibodies against
NY-ESO-1 in NPC was greater than those previously detected in
breast cancer, lung cancer and neuroblastoma (10,11,24,28,29).
Detection of NPC patients at the early stage is
essential for optimal prognosis (3,4). A
promising approach for the early detection of cancer is to measure
circulating antibodies to TAAs in patient serum other than markers
from cancer cells (19). Previous
investigations showed that autoantibodies can be detectable as
early as five years before radiographic detection on incidence
screening in lung cancer, and can be detected in the asymptomatic
stage of breast cancer up to five years before the onset of disease
(30,31). Our study also indicates that the
induction of autoantibodies against NY-ESO-1 occurs early in the
process of carcinogenesis, which is consistent with previous
studies on other types of cancer (10,24,25,32). A
higher proportion of patients with early-stage NPC had positive
results for autoantibodies against NY-ESO-1 than for VCA-IgA
(Table III). Although statistical
analysis showed that the correlation of positivity of
autoantibodies against NY-ESO-1 was not significant between early-
and advanced-stage patients, there appeared to be a higher
incidence of autoantibodies in patients with early-stage tumors
than in the advanced disease group. These results indicate that
autoantibodies against NY-ESO-1 may be a promising marker for the
early detection of NPC. However, the number of the NPC patients
with early-stage disease in this study was relatively small. Thus,
it is necessary to further validate these results using a large
cohort of early-stage NPC patient samples.
In the current study, VCA-IgA was demonstrated to be
a useful diagnostic biomarker of NPC, which is in agreement with a
study by Chang et al (33).
However, this assay used alone is not sensitive enough for the
purpose of primary screening. Autoantibodies against NY-ESO-1 have
a marginally lower AUC value than the VCA-IgA marker; however, it
is a molecular marker rather than a traditional EBV marker for
diagnosis of NPC. Notably, the combined use of autoantibodies
against NY-ESO-1 with a classical EBV marker increased its
diagnostic sensitivity (Table II).
As a screening test, high sensitivity should be a prerequisite in
order that potential patients are not missed. In this case, to
increase the sensitivity and marginally reduce the specificity as a
tradeoff can be justified. The combination of autoantibodies
against NY-ESO-1 and VCA-IgA resulted in a high sensitivity of
80.4% (95% CI, 71.6–87.0%), while maintaining a specificity of
90.6% (95% CI, 84.1–94.7%), which demonstrated greater diagnostic
efficacy compared with the classic VCA-IgA test alone (Table II). These results suggest
autoantibodies against NY-ESO-1 may be a good supplement to VCA-IgA
for NPC primary screening.
This study is the first to suggest that
autoantibodies against NY-ESO-1 may represent a potential non-EBV
serum marker for the diagnosis of NPC, particularly in early-stage
NPC patients. Moreover, the results revealed that the combined
detection of autoantibodies against NY-ESO-1 and VCA-IgA could
increase the sensitivity with modest specificity for the
serological screening and diagnosis of NPC.
Acknowledgements
This study was supported by grants from the National
High Technology Research and Development Program of China (grant
nos. 2012AA02A503 and 2012AA02A209).
References
|
1
|
Razak AR, Siu LL, Liu FF, et al:
Nasopharyngeal carcinoma: the next challenges. Eur J Cancer.
46:1967–1978. 2010.
|
|
2
|
Yu MC and Yuan JM: Epidemiology of
nasopharyngeal carcinoma. Semin Cancer Biol. 12:421–429. 2002.
|
|
3
|
Cao XP, Lu TX, Ye WJ and Cui NJ:
Prospective study on long-term efficacy of external plus
intracavitary radiotherapy on stage I–II nasopharyngeal carcinoma.
Ai Zheng. 26:204–207. 2007.(In Chinese).
|
|
4
|
Chen Y, Sun Y, Liang SB, et al: Progress
report of a randomized trial comparing long-term survival and late
toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy
versus radiotherapy alone in patients with stage III to IVB
nasopharyngeal carcinoma from endemic regions of China. Cancer.
119:2230–2238. 2013.
|
|
5
|
Chan KH, Gu YL, Ng F, et al: EBV specific
antibody-based and DNA-based assays in serologic diagnosis of
nasopharyngeal carcinoma. Int J Cancer. 105:706–709. 2003.
|
|
6
|
Low WK, Leong JL, Goh YH and Fong KW:
Diagnostic value of Epstein-Barr viral serology in nasopharyngeal
carcinoma. Otolaryngol Head Neck Surg. 123:505–507. 2000.
|
|
7
|
Tsang RK, Vlantis AC, Ho RW, et al:
Sensitivity and specificity of Epstein-Barr virus IGA titer in the
diagnosis of nasopharyngeal carcinoma: a three-year institutional
review. Head Neck. 26:598–602. 2004.
|
|
8
|
Gallagher A, Armstrong AA, MacKenzie J, et
al: Detection of Epstein-Barr virus (EBV) genomes in the serum of
patients with EBV-associated Hodgkin’s disease. Int J Cancer.
84:442–448. 1999.
|
|
9
|
Lei KI, Chan LY, Chan WY, Johnson PJ and
Lo YM: Diagnostic and prognostic implications of circulating
cell-free Epstein-Barr virus DNA in natural killer/T-cell lymphoma.
Clin Cancer Res. 8:29–34. 2002.
|
|
10
|
Chapman C, Murray A, Chakrabarti J, et al:
Autoantibodies in breast cancer: their use as an aid to early
diagnosis. Ann Oncol. 18:868–873. 2007.
|
|
11
|
Boyle P, Chapman CJ, Holdenrieder S, et
al: Clinical validation of an autoantibody test for lung cancer.
Ann Oncol. 22:383–389. 2011.
|
|
12
|
Chang W, Wu L, Cao F, et al: Development
of autoantibody signatures as biomarkers for early detection of
colorectal carcinoma. Clin Cancer Res. 17:5715–5724. 2011.
|
|
13
|
Zayakin P, Ancāns G, Siliņa K, et al:
Tumor-associated autoantibody signature for the early detection of
gastric cancer. Int J Cancer. 132:137–147. 2013.
|
|
14
|
Liu H, Zhang J, Wang S, et al: Screening
of autoantibodies as potential biomarkers for hepatocellular
carcinoma by using T7 phase display system. Cancer Epidemiol.
36:82–88. 2012.
|
|
15
|
Tong YQ, Liu B, Huang J, et al: BMI-1
autoantibody in serum as a new potential biomarker of
nasopharyngeal carcinoma. Cancer Biol Ther. 7:340–344. 2008.
|
|
16
|
Brass N, Rácz A, Bauer C, et al: Role of
amplified genes in the production of autoantibodies. Blood.
93:2158–2166. 1999.
|
|
17
|
Jäger D, Stockert E, Güre AO, et al:
Identification of a tissue-specific putative transcription factor
in breast tissue by serological screening of a breast cancer
library. Cancer Res. 61:2055–2061. 2001.
|
|
18
|
Tan EM: Autoantibodies as reporters
identifying aberrant cellular mechanisms in tumorigenesis. J Clin
Invest. 108:1411–1415. 2001.
|
|
19
|
Finn OJ: Immune response as a biomarker
for cancer detection and a lot more. N Engl J Med. 353:1288–1290.
2005.
|
|
20
|
Qiu J, Choi G, Li L, et al: Occurrence of
autoantibodies to annexin I, 14-3-3 theta and LAMR1 in
prediagnostic lung cancer sera. J Clin Oncol. 26:5060–5066.
2008.
|
|
21
|
Robbins PF, Morgan RA, Feldman SA, et al:
Tumor regression in patients with metastatic synovial cell sarcoma
and melanoma using genetically engineered lymphocytes reactive with
NY-ESO-1. J Clin Oncol. 29:917–924. 2011.
|
|
22
|
Jäger E, Nagata Y, Gnjatic S, et al:
Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral
and cellular immune responses. Proc Natl Acad Sci USA.
97:4760–4765. 2000.
|
|
23
|
Gnjatic S, Nishikawa H, Jungbluth AA, et
al: NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer
Res. 95:1–30. 2006.
|
|
24
|
Chapman CJ, Murray A, McElveen JE, et al:
Autoantibodies in lung cancer: possibilities for early detection
and subsequent cure. Thorax. 63:228–233. 2008.
|
|
25
|
Gati A, Lajmi N, Derouiche A, et al:
NY-ESO-1 expression and immunogenicity in prostate cancer patients.
Tunis Med. 89:779–783. 2011.
|
|
26
|
Chen L, Mao YP, Xie FY, et al: The seventh
edition of the UICC/AJCC staging system for nasopharyngeal
carcinoma is prognostically useful for patients treated with
intensity-modulated radiotherapy from an endemic area in China.
Radiother Oncol. 104:331–337. 2012.
|
|
27
|
Fang Qiang-Ji, Hu Liang-Ping and Zhao
Nai-Qing: Binomial distribution. Statistical Methods for Biomedical
Research. 1st edition. Higher Education Press; Beijing: pp.
462007
|
|
28
|
Chapman CJ, Thorpe AJ, Murray A, et al:
Immunobiomarkers in small cell lung cancer: potential early cancer
signals. Clin Cancer Res. 17:1474–1480. 2011.
|
|
29
|
Rodolfo M, Luksch R, Stockert E, et al:
Antigen-specific immunity in neuroblastoma patients: antibody and
T-cell recognition of NY-ESO-1 tumor antigen. Cancer Res.
63:6948–6955. 2003.
|
|
30
|
Zhong L, Coe SP, Stromberg AJ, et al:
Profiling tumor-associated antibodies for early detection of
non-small cell lung cancer. J Thorac Oncol. 1:513–519. 2006.
|
|
31
|
Fernández Madrid F: Autoantibodies in
breast cancer sera: candidate biomarkers and reporters of
tumorigenesis. Cancer Lett. 230:187–198. 2005.
|
|
32
|
Türeci O, Mack U, Luxemburger U, et al:
Humoral immune responses of lung cancer patients against tumor
antigen NY-ESO-1. Cancer Lett. 236:64–71. 2006.
|
|
33
|
Chang KP, Hao SP, Chang JH, et al:
Macrophage inflammatory protein-3alpha is a novel serum marker for
nasopharyngeal carcinoma detection and prediction of treatment
outcomes. Clin Cancer Res. 14:6979–6987. 2008.
|