Introduction
Myofibroblastoma is a benign and rare mesenchymal
neoplasm composed of spindle cells in clusters and fascicles, with
interspersed bands of hyalinized collagen (1,2). The
majority of the masses are located in the breast, however, the
number of extramammary myofibroblastoma cases being reported is
increasing (2–6). Only three cases of intracranial
myofibroblastoma have been reported (7–9).
Histopathological and immunohistochemical staining is crucial to
determine a diagnosis of intracranial myofibroblastoma. Tumor
resectioning may be a useful treatment. The present study describes
the case of a female patient with meningeal myofibroblastoma. In
this report we describe the clinical and pathological features of
this rare tumor and discuss the differential diagnoses. To the best
of our knowledge, this is the first study to show the presence of
myofibroblastoma in the left frontal lobe. Patient provided written
informed consent.
Case report
A 47-year-old female with a history of ovarian cyst
resection presented to The First Affiliated Hospital (College of
Medicine, Zheijian University, Hangzhou, Zhejiang, China) with
paroxysmal mild headaches that had been apparent for 4 years. The
headaches had increased in intensity over the past 6 days. Computed
tomography (CT) revealed a low-density mass lesion in the frontal
lobe. The physical examination was normal. The analysis of tumor
markers showed no abnormal findings. A cerebro-spinal fluid
examination disclosed a slightly elevated level of protein at 0.50
g/l (normal, 0.15–0.45 g/l), with no other abnormal findings.
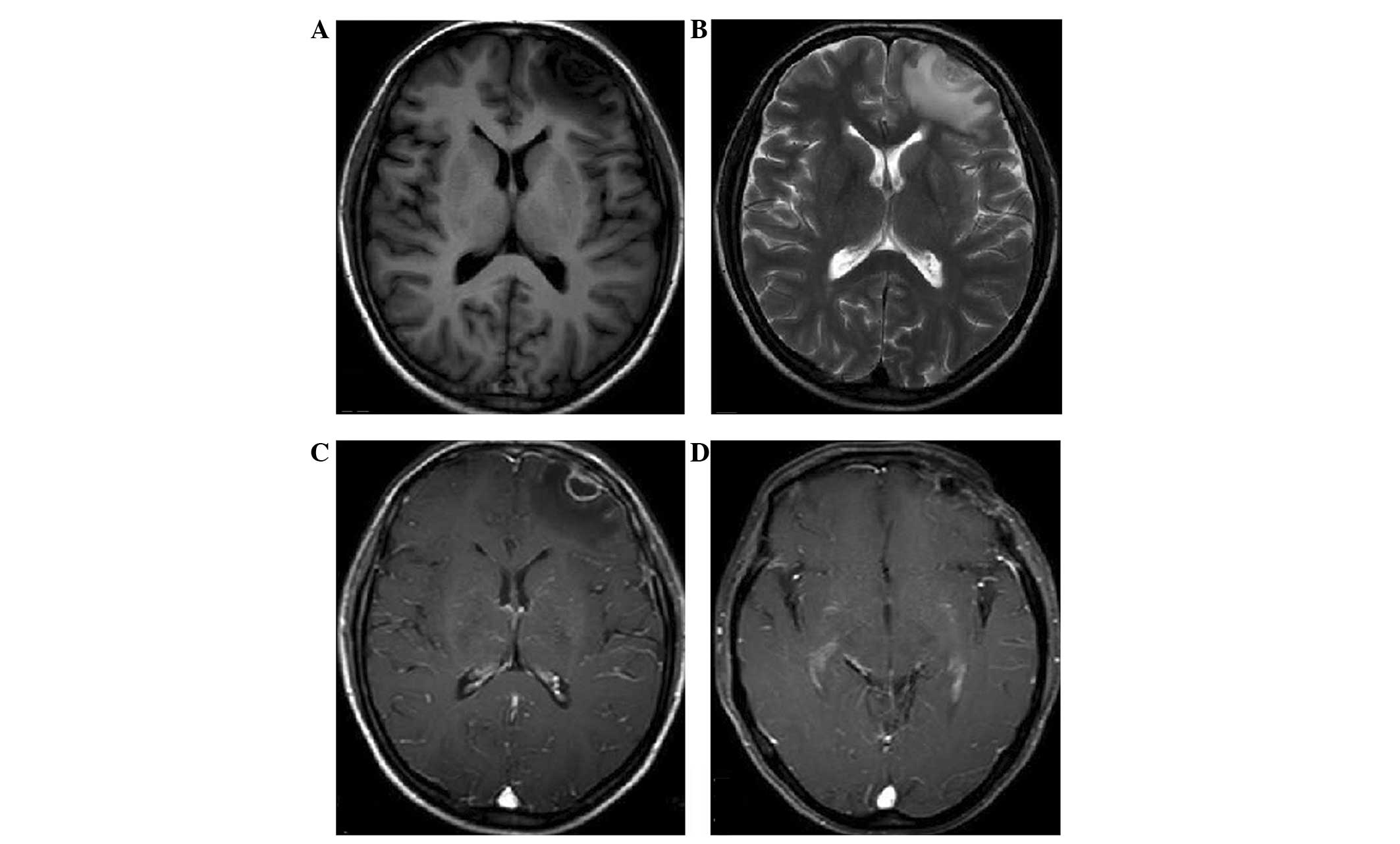
Magnetic resonance imaging (MRI) disclosed a well-circumscribed
mass in the left frontal lobe (Fig.
1). The clear boundary and surrounding extruded brain tissue
indicated that the mass had undergone expansive growth. The mass
appeared as hypointense on T1-weighted images and was of mixed
intensity on T2-weighted images (Fig.
1A and B). Gadolinium-diethylene triamine pentaacetic acid
(DTPA)-enhanced T1-weighted images revealed that the mass was
heterogeneously enhanced, with ring-like enhancement of the
boundary. The cerebral dura on the base of the lesion was also
believed to be contrast-enhanced, with thickening of the left
frontal bone, which may have been a result of reactive
hypervascularity or tumoral invasion of the dura (Fig. 1C). The preliminary diagnosis of the
mass was of a meningioma.
A resection of the mass was performed; the mass
appeared to be dusty pink color and had a resilient structure, with
abundant blood supply. Significant adhesion to the surrounding
brain tissue was present, together with severe edema surrounding
the mass. The base of the mass was attached to the convex dura,
with focal localization on the dura of the frontal basal section.
Diagnosis of the frozen section performed at the time of surgery
disclosed a suspected myofibroblastoma. The patient’s headache had
resolved at 2 weeks post-surgery. CT revealed postoperative
manifestation and no evidence of lesion recurrence. Anti-epilepsy
therapy was recommended when the patient was discharged from the
hospital. The patient recovered well, with no evidence of mass
recurrence on MRI to date at 24-months post-surgery (Fig. 1D).
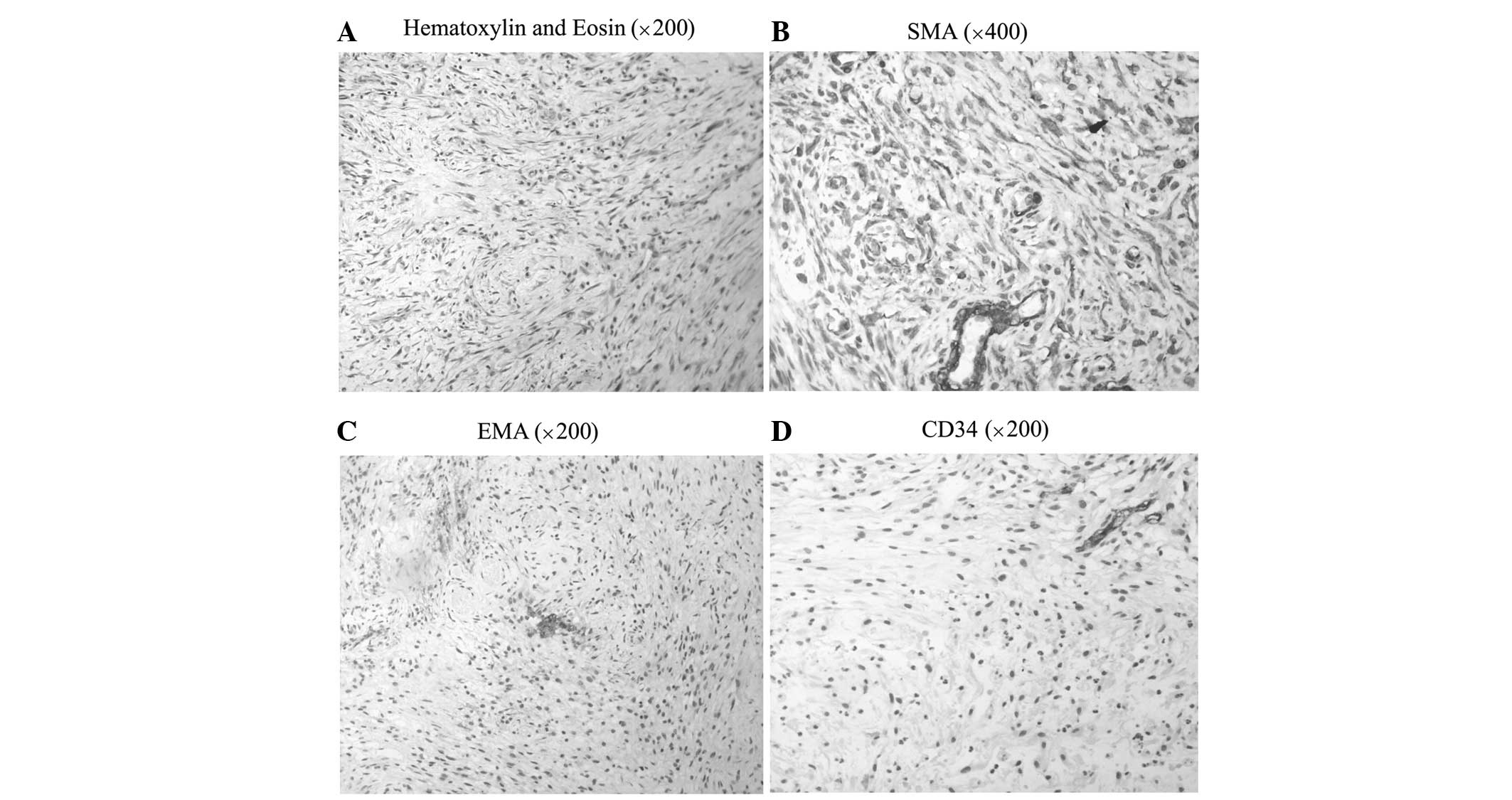
The histopathological findings revealed abundant
fascicular clusters of spindle- and oval-shaped cells, which were
arranged in interlaced or swirled patterns. Elongated to
oval-shaped nuclei with inconspicuous nucleoli were another
distinguishing feature. Finely-dispersed chromatin and
poorly-defined cytoplasm were also observed. Mitotic figures were
rare. Mucinous degeneration and necrosis were observed in the
eosinophilic area, while an occasional lymphocyte and neutrophil
were seen between tumor cells (Fig.
2A).
Immunohistochemical staining demonstrated that the
majority of the tumor cells were strongly positive for smooth
muscle actin (SMA; Fig. 2B), the
Ki-67 index was >10% and only a few cells were positive for
epithelial membrane antigen (EMA; Fig.
2C). However, the tumor cells were negative for cluster of
differentiation (CD)117, CD34 (Fig.
2C), S-100 and desmin. All of the pathological evidence
supported the diagnosis of myofibroblastoma.
Discussion
Studies on myofibroblastoma in the central nervous
system are extremely rare. To the best of our knowledge, only three
cases of intracranial myofibroblastoma have previously been
reported (7–9). The details of these case studies are
summarized in Table I. From these
data, there is a trend towards females being more likely to suffer
from intracranial myofibroblastoma. The age in the studies varies
between 9 and 70 years. All intracranial myofibroblastomas have
definite or suspected attachment to the dura.
 | Table IInformation on reported intracranial
myofibroblastoma cases. |
Table I
Information on reported intracranial
myofibroblastoma cases.
| First author, year
(ref.) | Age, years | Symptoms | Location of the
lesion | Size, cm |
|---|
| Carneiro et
al, 1989 (3) | 9 | Diplopia, convergent
strabismus | Meninges overlying
the parietal lobe | 1.2 |
| | right ocular
protrusion | No data | 3.5 |
| Prayson et al,
1993 (4) | 70 | Headache, visual
change | Posterior falx below
the sagittal sinus | 2×3×4 |
| | | right occipital
lobe | 2×4×6 |
| | | Adjacent to the
superior sagittal sinus | No data |
| Shinojima et
al, 2002 (5) | 34 | Headache, visual
disturbance | Suprasellar
region | 2.5×2×3 |
| Present study | 47 | Headache | Frontal lobe | 1.7×1.5×2 |
Myofibroblastoma is a well-circumscribed benign
tumor. Histopathological findings demonstrate that the tumor is
composed of spindle cells in clusters and fascicles, with thick
interspersed hyalinized collagen bands. It has features of
fibroblasts and smooth muscle cells, with frequent mitoses. The
cells are also characterized by elongated to oval-shaped nuclei,
irregular nuclear contours, finely-dispersed chromatin and
poorly-defined cytoplasm (1,2,7,9,10).
Immunohistochemical staining has shown that the tumor cells are
strongly positive for SMA and vimentin and weakly positive for
desmin and CD34 (8,9). However, the reactivity to factor
VIII-related antigen (a marker of endothelial cells), EMA, MAK-6
(cytokeratin; a marker of epithelial cells and meningeal cells) and
glial fibrillary acidic protein are negative (8). Ultrastructural examination discloses
that the mass is composed of myofibroblastic cells and fibroblastic
cells. The cytoplasm of the myofibroblastic cells contains abundant
dilated rough endoplasmic reticulum (rER), while the cytoplasm of
the fibroblastic cells contains actin-like microfilaments, with
dense bodies, and abundant rER (9).
The clinical manifestations of intracranial
myofibroblastoma are similar to meningioma and include intracranial
hypertension, skull destruction and the presence of systematic
symptoms. Headaches caused by intracranial hypertension subsequent
to the effects of a mass are extremely common. The masses may grow
slowly, as all patients tend to have a long medical history prior
to their admittance to hospital. Intratumoral hemorrhage may also
be a feature of the mass (9).
CT and MRI are useful imaging methods in diagnosing
myofibroblastoma, as it is well-circumscribed on each of these
techniques. The mass can appear as a low- or mixed low- and
high-density mass on CT (9). In the
present study, the mass was hypointense on T1-weighted images and
was of mixed intensity on T2-weighted images (Fig. 1A and B). This result is different to
that in the study by Shinojima et al (9), which showed that the mass was
isointense on T1- and hypointense on T2-weighted MI. This
difference may be due to intratumoral hemorrhage in the previous
case. The mass showed heterogeneous contrast enhancement on
gadolinium-DTPA-enhanced T1-weighted images in the present study;
this has also been demonstrated in the two previous cases (8,9). One
notable point was that the cerebral dura on the base of the lesion
was also contrast-enhanced, with thickening of the left frontal
bone in the present patient. This is similar to the dural tail
sign, which indicates that it may be a result of the invasion of
dural vessels by tumor cells and packing at the point of tumor
attachment, reactive hypervascularity or tumoral invasion of the
dura (11). The ring-like enhanced
boundary in the current patient was mostly likely the meninges, due
to the continuity between the boundary and the meninges. All the
myofibroblastomas in the previous three cases, plus that in the
present study, had definite or suspected attachments to the dura,
which indicated their origination from the meninges, possibly from
modified fibroblasts or pre-existing myofibroblasts (9).
The differential diagnosis of meningeal
myofibroblastoma includes other spindle cell neoplasms of the
meninges, such as solitary fibrous tumors (SFTs), fibrous
meningiomas and hemangiopericytomas.
SFTs are rare tumors that can also occur in the
meninges. Histopathological findings demonstrate the presence of
numerous monomorphic spindle- or oval-shaped cells and diffuse
intercellular reticulin fibers. These findings are similar to those
of myofibroblastoma. However, unlike for myofibroblastoma,
branching hemangiopericytoma-like vessels and rare mitotic figures
are characteristics that are also present (12,13).
Immunohistochemical analysis has shown that SFTs are strongly and
diffusely positive for CD34, vimentin, B-cell lymphoma-2 and CD99,
but negative for SMA, EMA or S-100 protein (14–17).
No cells with the features of smooth muscle cells are found under
ultrastructural examination (15).
Fibrous meningioma is another type of meningioma.
Unlike myofibroblastoma, fibrous meningiomas are
glycogen-containing tumors. Additionally, a storiform pattern,
psammoma body and collagen calcification are defining
characteristics (12,18). Immunohistochemical analysis shows
that these tumors are positive for vimentin (100%), focal EMA
(80%), S-100 protein (80%) and collagen IV (25%). CD34 staining is
patchy and weak (60%) (18,19).
Meningeal hemangiopericytomas (HPCs) are also
meningeal neoplasms, and are composed of oval- to spindle-shaped
cells, with dense intercellular reticulin fibers. However, unlike
myofibroblastomas, HPCs are prone to multiple recurrences and
eventual metastasis. Another difference can be found in the
existence of numerous small blood vessels (12,19,20).
HPC is characteristically positive for vimentin, factor XIIIa and
Leu-7, and CD34 staining is patchy and weakly positive. Focal
desmin and cytokeratin positivity is occasional, with negative EMA
and S-100 staining (12,19,21).
On CT and MRI, the tumors are characterized by irregular borders
rather than the well-defined borders of a myofibroblastoma
(20).
The prognosis of meningeal myofibroblastoma is
optimistic. The masses are slow-growing and the histopathology
findings show no evidence of malignancy. In the present patient,
the resection of the tumor proved to be a successful treatment and
no recurrence was found, similar to the two previous cases
(7,9).
The present patient exhibited a rare type of
meningeal neoplasm. Histopathological and immunohistochemical
staining is crucial to identify the diagnosis of a
myofibroblastoma. Further study is required to identify the origin
of the tumor and the association between the tumor and other
meningeal neoplasms, such as SFT.
Abbreviations:
|
MRI
|
magnetic resonance imaging
|
|
CT
|
computed tomography
|
|
CD
|
cluster of differentiation
|
|
SMA
|
smooth muscle actin
|
|
EMA
|
epithelial membrane antigen
|
|
DTPA
|
diethylene triamine pentaacetic
acid
|
|
rER
|
rough endoplasmic reticulum
|
|
SFT
|
solitary fibrous tumor
|
|
HPC
|
hemangiopericytoma
|
References
|
1
|
Powari M, Srinivasan R and Radotra BD:
Myofibroblastoma of the male breast: a diagnostic problem on
fine-needle aspiration cytology. Diagn Cytopathol. 26:290–293.
2002.
|
|
2
|
Salomão DR, Crotty TB and Nascimento AG:
Myofibroblastoma and solitary fibrous tumour of the breast:
histopathologic and immunohistochemical studies. Breast. 10:49–54.
2001.
|
|
3
|
Alguacil-Garcia A: Intranodal
myofibroblastoma in a submandibular lymph node. A case report. Am J
Clin Pathol. 97:69–72. 1992.
|
|
4
|
Meister P, Wöckel W, Schmidt D and Trupka
A: Pulmonary myofibroblastic nodules with “amianthoid features”.
Pathol Res Pract. 187:906–911. 1991.
|
|
5
|
Herrera GA, Johnson WW, Lockard VG and
Walker BL: Soft tissue myofibroblastomas. Mod Pathol. 4:571–577.
1991.
|
|
6
|
Sahin AA, Ro JY, Ordoñez NG, et al:
Myofibroblastoma of the tongue. An immunohistochemical,
ultrastructural, and flow cytometric study. Am J Clin Pathol.
94:773–777. 1990.
|
|
7
|
Carneiro F, Gonçalves V and Simões MS:
Myofibroblastoma of the meninges. Ultrastruct Pathol. 13:599–605.
1989.
|
|
8
|
Prayson RA, Estes ML, McMahon JT, Kalfas I
and Sebek BA: Meningeal myofibroblastoma. Am J Surg Pathol.
17:931–936. 1993.
|
|
9
|
Shinojima N, Ohta K, Yano S, et al:
Myofibroblastoma in the suprasellar region. Case report. J
Neurosurg. 97:1203–1207. 2002.
|
|
10
|
Laskin WB, Fetsch JF and Tavassoli FA:
Superficial cervicovaginal myofibroblastoma: fourteen cases of a
distinctive mesenchymal tumor arising from the specialized
subepithelial stroma of the lower female genital tract. Hum Pathol.
32:715–725. 2001.
|
|
11
|
Sotoudeh H and Yazdi HR: A review on dural
tail sign. World J Radiol. 2:188–192. 2010.
|
|
12
|
Suzuki SO, Fukui M, Nishio S and Iwaki T:
Clinicopathological features of solitary fibrous tumor of the
meninges: An immunohistochemical reappraisal of cases previously
diagnosed to be fibrous meningioma or hemangiopericytoma. Pathol
Int. 50:808–817. 2000.
|
|
13
|
Yilmaz C, Kabatas S, Ozen OI, et al:
Solitary fibrous tumor. J Clin Neurosci. 16:1578–1581. 2009.
|
|
14
|
Martorell M, Pérez-Vallés A, Gozalbo F, et
al: Solitary fibrous tumor of the thigh with epithelioid features:
a case report. Diagn Pathol. 2:192007.
|
|
15
|
Prayson RA, McMahon JT and Barnett GH:
Solitary fibrous tumor of the meninges. Case report and review of
the literature. J Neurosurg. 86:1049–1052. 1997.
|
|
16
|
Song Z, Yu C, Song X, Wei L and Liu A:
Primary solitary fibrous tumor of the thyroid - report of a case
and review of the literature. J Cancer. 2:206–209. 2011.
|
|
17
|
Tihan T, Viglione M, Rosenblum MK, Olivi A
and Burger PC: Solitary fibrous tumors in the central nervous
system. A clinicopathologic review of 18 cases and comparison to
meningeal hemangiopericytomas. Arch Pathol Lab Med. 127:432–439.
2003.
|
|
18
|
Carneiro SS, Scheithauer BW, Nascimento
AG, Hirose T and Davis DH: Solitary fibrous tumor of the meninges:
a lesion distinct from fibrous meningioma. A clinicopathologic and
immunohistochemical study. Am J Clin Pathol. 106:217–224. 1996.
|
|
19
|
Perry A, Scheithauer BW and Nascimento AG:
The immunophenotypic spectrum of meningeal hemangiopericytoma: a
comparison with fibrous meningioma and solitary fibrous tumor of
meninges. Am J Surg Pathol. 21:1354–1360. 1997.
|
|
20
|
Alén JF, Lobato RD, Gómez PA, et al:
Intracranial hemangiopericytoma: study of 12 cases. Acta Neurochir
(Wien). 143:575–586. 2001.
|
|
21
|
Winek RR, Scheithauer BW and Wick MR:
Meningioma, meningeal hemangiopericytoma (angioblastic meningioma),
peripheral hemangiopericytoma, and acoustic schwannoma. A
comparative immunohistochemical study. Am J Surg Pathol.
13:251–261. 1989.
|