Introduction
Pancreatic cancer is one of the most common
malignant tumors of the digestive system due to its latent onset,
difficulty in surgical resection, poor prognosis and high mortality
rate, for which the only effective treatment at present is surgical
resection. Due to the lack of efficient methods for the early
diagnosis of pancreatic cancer, as well as its highly malignant
nature and potential for metastatic progression during its early
stages, only 10% of all pancreatic cancer patients present with
resectable disease at diagnosis (1). Autophagy is a process that facilitates
self-digestion and degradation of proteins and organelles in
eukaryotic cells, which affects the incidence of tumors,
neurodegeneration and myocardial hypertrophy (2). Previous studies have reported that
autophagy also leads to cell death, a nonapoptotic form of
programmed cell death, which is termed autophagic or type II cell
death to distinguish it from apoptosis (3,4). LC3
and p62 are molecular markers of autophagy, in which the ratio of
LC3-II to LC3-I increases, while the expression of p62 decreases
(5). The PI3K/Akt signaling pathway
has an important role in the regulation of autophagy (6); however, the specific signaling
mechanism underlying autophagy has yet to be elucidated. Thus,
PI3K/Akt signaling molecules may have potential therapeutic targets
in pancreatic cancer (7).
In recent years, a number of studies have aimed to
extract and screen the active components from traditional Chinese
herbs in order to develop more effective drugs for cancer treatment
(8,9). Shikonin, a naphthoquinone derived from
the roots of lithospermum, has been reported to inhibit the
proliferation of tumor cells in breast, liver and lung cancer, as
well as induce cell death through apoptosis and cytoclasis
(10–12). However, the effect of shikonin on
autophagy has yet to be elucidated. The present study aimed to
investigate the effect of shikonin on autophagy in BXPC-3 human
pancreatic cancer cells, as well as its underlying mechanism.
Materials and methods
Reagents
Shikonin was purchased from the Guangzhou Institute
for Drug Control (Guangzhou, China). The chemical structure of
shikonin is shown in Fig. 1. All
other chemicals and reagents were purchased from Sigma-Aldrich (St.
Louis, MO, USA), unless specified otherwise.
Cell culture
The BXPC-3 pancreatic cancer cell line was obtained
from the Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China) and cultured in RPMI-1640
medium supplemented with 10% fetal calf serum (HyClone
Laboratories, Inc., Logan, UT, USA), penicillin and streptomycin at
37°C in 5% CO2. Cells were regularly passaged to
maintain exponential growth.
Cell proliferation assay
Proliferation of the BXPC-3 cells was analyzed using
the Cell Counting Kit-8 (CCK8; Tongren Shanghai Co, Shanghai,
China) according to the manufacturer’s instructions. In brief, the
BXPC-3 cells were washed, counted and seeded at a density of
4×105 cells/ml in each well on 96-well plates. After 6
h, specific concentrations of shikonin (0, 1, 2.5 and 5 μmol/l)
were added to the cells. At 24 and 48 h after treatment, CCK8
solution was added and incubated for 4 h. Cell viability was
determined using a spectrophotometer (NanoDrop 1000, Thermo Fisher
Scientific, Waltham, MA, USA) at an absorbance of 450 nm. The
viability of the cells was calculated according to the following
formula: Cell viability (%) = 100 − ((mean absorbance of untreated
group − mean absorbance of treatment group)/mean absorbance of
untreated group ×100). Results were calculated based on three
individual experiments performed in triplicate.
Western blot analysis
Cells were collected and lysed using
radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The cell lysates were
collected following centrifugation and the protein concentration
was quantified using a bovine serum albumin (BSA) method. Equal
quantities of protein were loaded and separated using 10% SDS-PAGE,
then transferred to nitrocellulose membranes (Millipore
Corporation, Billerica, MA, USA). The membranes were blocked with
3% BSA for 2 h, then incubated with polyclonal rabbit anti-mouse
LC3, polyclonal rabbit anti-mouse p62, polyclonal rabbit anti-mouse
PI3K, polyclonal rabbit anti-human p-PI3K, polyclonal rabbit
anti-rat Akt, polyclonal rabbit anti-rat p-Akt and polyclonal
rabbit anti-rat β-actin antibodies (Santa Cruz Biotechnology, Inc.)
at 4°C overnight. Subsequent to washing three times with
Tris-buffered saline and Tween 20, the membranes were incubated
with corresponding horseradish peroxidase-conjugated polyclonal
goat anti-rabbit secondary antibodies (Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature. The membranes were developed
using an enhanced chemiluminescence kit (Santa Cruz Biotechnology,
Inc.) and exposed to X-ray film. β-actin was used as a loading
control. The density of the bands on the membrane was analyzed
using an image analyzer (LabWorks Software, Upland, CA, USA).
Statistical analysis
Results are presented as the mean ± standard
deviation. Statistical analyses were performed using Student’s
t-test or one- or two-way analysis of variance followed by Tukey’s
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of shikonin on BXPC-3 cell
viability
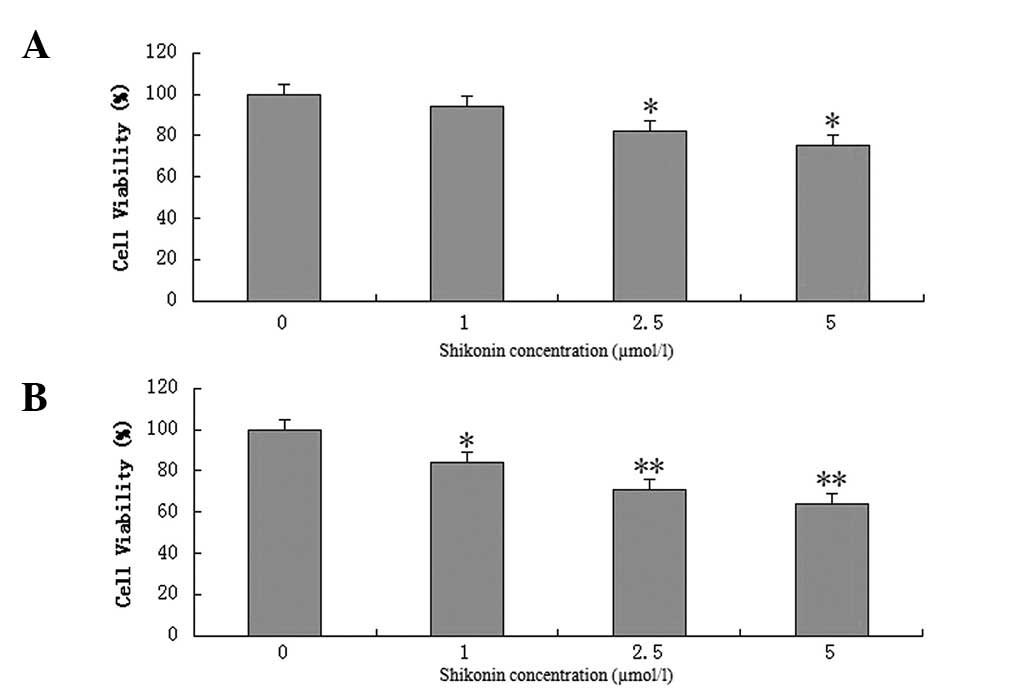
BXPC-3 cells were exposed to various concentrations
of shikonin (1, 2.5 and 5 μmol/l) for 24 and 48 h. Shikonin was
found to have a dose-dependent effect on BXPC-3 cell viability
(Fig. 2). After 24 h, 1 μmol/l
shikonin was not observed to inhibit breast cancer cell growth;
however, when the concentration was increased to 2.5 and 5 μmol/l,
cancer cell survival was found to significantly decrease. After 48
h, 1, 2.5 and 5 μmol/l shikonin were observed to significantly
inhibit BXPC-3 cell viability, and cancer cell survival decreased
in a dose-dependent manner.
Western blot analysis
The protein expression of LC3 and p62 was assessed
using western blot analysis. In the cells treated with 2.5 and 5
μmol/l shikonin for 24 h, the protein expression of LC3-II/LC3-I
was observed to increase, while that of p62 was found to decrease,
compared with the serum-free positive control cells (Fig. 3A). After 48 h, the expression of
LC3-II/LC3-I was observed to increase in the cells treated with 1,
2.5 and 5 μmol/l shikonin, while the expression of p62 was found to
decrease (Fig. 3B).
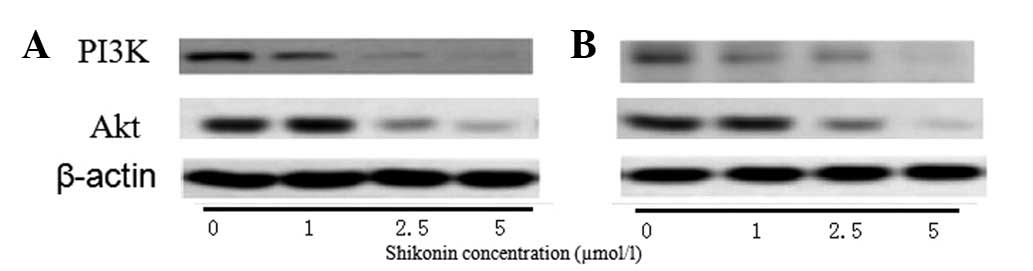
After 24 h (Fig.
4A), 1 μmol/l shikonin was not found to affect the expression
of PI3K and Akt; however, at 48 h (Fig.
4B), the expression of PI3K and Akt were observed to decrease
compared with that in the control cells. In the cells treated with
2.5 and 5 μmol/l, the expression of PI3K and Akt was found to
decrease after 24 and 48 h in a dose-dependent manner.
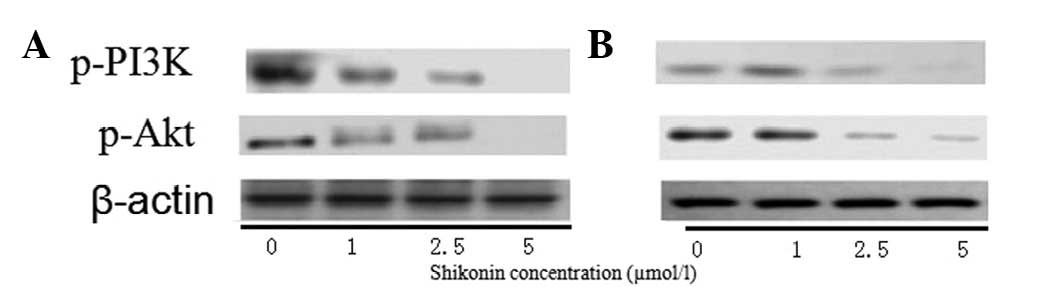
The protein expression of p-PI3K and p-Akt was also
assessed using western blot analysis, which showed that shikonin
affected the expression of p-PI3K and p-Akt in a dose-dependent
manner. After 24 h (Fig. 5A), no
significant difference was observed in the expression of p-PI3K and
p-Akt in the cells treated with 1 μmol/l shikonin compared with
that in the control cells. However, in the cells treated with 2.5
and 5 μmol/l shikonin, the expression of p-PI3K and p-Akt was found
to be decreased compared with that in the control cells. After 48 h
(Fig. 5B), compared with the
control group, the expression of p-PI3K and p-Akt was observed to
be decreased in all three treatment groups.
Discussion
Pancreatic cancer is a highly prevalent malignant
tumor of the digestive tract, which has a low survival rate. In
general, the median survival time is three to five months and the
five-year survival rate is <5% (13). Autophagy has been proposed to be an
important survival pathway associated with the prevention and
treatment of tumors (14,15). Autophagy is a highly conserved
biological process in which a number of macromolecules participate
in the degeneration and recycling of organelles. Autophagy is rare
under normal conditions, but significantly increases during certain
situations, including hunger, growth factor deficiency, oxygen
deficiency, intracellular stress and growth signal stimulation. LC3
and SQSTM1/p62 are important proteins in autophagy, during which
cytoplasmic-pattern LC3 (LC3-I) is converted to autophagosomal
membrane LC3 (LC3-II), leading to increased LC3-II levels. However,
p62 expression is generally decreased during autophagy (16). The PI3K/Akt/mammalian target of
rapamycin (mTOR) signaling pathway is a key regulator of
physiological cellular processes that are associated with
proliferation, differentiation, apoptosis, motility, metabolism and
autophagy (17). During hunger and
oxygen deficiency, the PI3K/Akt signaling pathway has been reported
to negatively regulate autophagy through mediating the expression
of mTOR (18).
Shikonin, a naphthoquinone derived from the roots of
lithospermum or cultured through plant tissue cultivation methods,
inhibits breast, liver, rectum, mouth, bladder and skin cancer
cells (19). The present study
aimed to investigate the effect of varying concentrations of
shikonin on the viability and autophagy of BXPC-3 pancreatic cancer
cells. Shikonin was found to reduce BXPC-3 cell viability through
increasing the expression of LC3-II/LC3-I and decreasing the
expression of SQSTM1/p62 to promote BXPC-3 cell autophagy. In
addition, the expression of PI3K and Akt, as well as the levels of
p-PI3K and p-Akt were observed to decrease. These findings suggest
that shikonin promotes autophagy through inhibiting the expression
and phosphorylation of PI3K and Akt, as well as through inhibiting
viability in BXPC-3 cells. A previous study on the antitumor
effects of shikonin reported its role in accelerating apoptosis and
necrocytosis (20). To the best of
our knowledge, the present study is the first study to show that
shikonin promotes autophagy in tumor cells, which may significantly
enhance its potential as an antitumor agent. However, the
underlying molecular mechanism requires further investigation, as
the promotion of autophagy is a complex process.
References
|
1
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004.
|
|
2
|
Lorin S, Hamaï A, Mehrpour M and Codogno
P: Autophagy regulation and its role in cancer. Semin Cancer Biol.
23:361–379. 2013.
|
|
3
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008.
|
|
4
|
Levine B and Yuan J: Autophagy in cell
death: an innocent convict? J Clin Invest. 115:2679–2688. 2005.
|
|
5
|
Klionsky DJ, Abeliovich H, Agostinis P, et
al: Guidelines for the use and interpretation of assays for
monitoring autophagy in higher eukaryotes. Autophagy. 4:151–175.
2008.
|
|
6
|
Hsieh MJ, Tsai TL, Hsieh YS, et al:
Dioscin-induced autophagy mitigates cell apoptosis through
modulation of PI3K/Akt and ERK and JNK signaling pathways in human
lung cancer cell lines. Arch Toxicol. 87:1927–1937. 2013.
|
|
7
|
Prasad R, Vaid M and Katiyar SK: Grape
proanthocyanidin inhibit pancreatic cancer cell growth in vitro and
in vivo through induction of apoptosis and by targeting the
PI3K/Akt pathway. PLoS One. 7:e430642012.
|
|
8
|
Wang CY, Bai XY and Wang CH: Traditional
chinese medicine: a treasured natural resource of anticancer drug
research and development. Am J Chin Med. 42:543–559. 2014.
|
|
9
|
Liu Z, Chen S, Cai J, et al: Traditional
Chinese medicine syndrome-related herbal prescriptions in treatment
of malignant tumors. J Tradit Chin Med. 33:19–26. 2913.
|
|
10
|
Gong K and Li W: Shikonin, a Chinese
plant-derived naphthoquinone, induces apoptosis in hepatocellular
carcinoma cells through reactive oxygen species: A potential new
treatment for hepatocellular carcinoma. Free Radic Biol Med.
51:2259–2271. 2011.
|
|
11
|
Yao Y and Zhou Q: A novel antiestrogen
agent Shikonin inhibits estrogen-dependent gene transcription in
human breast cancer cells. Breast Cancer Res Treat. 121:233–240.
2010.
|
|
12
|
Wiench B, Eichhorn T, Paulsen M and
Efferth T: Shikonin directly targets mitochondria and causes
mitochondrial dysfunction in cancer cells. Evid Based Complement
Alternat Med. 2012:7260252012.
|
|
13
|
Sabater L, Calvete J, Aparisi L, et al:
Pancreatic and periampullary tumors: morbidity, mortality,
functional results and long-term survival. Cir Esp. 86:159–166.
2009.(In Spanish).
|
|
14
|
Yue Z, Jin S, Yang C, et al: Beclin 1, an
autophagy gene essential for early embryonic development, is a
haploinsufficient tumor suppressor. Proc Natl Acad Sci USA.
100:15077–15082. 2003.
|
|
15
|
Yang ZJ, Chee CE, Huang S and Sinicrope F:
Autophagy modulation for cancer therapy. Cancer Biol Ther.
11:169–176. 2011.
|
|
16
|
Bjørkøy G, Lamark T, Brech A, et al:
p62/SQSTM1 forms protein aggregates degraded by autophagy and has a
protective effect on huntingtin-induced cell death. J Cell Biol.
171:603–614. 2005.
|
|
17
|
Martelli AM, Evangelisti C, Follo MY, et
al: Targeting the phosphatidylinositol 3-kinase/Akt/mammalian
target of rapamycin signaling network in cancer stem cells. Curr
Med Chem. 18:2715–2726. 2011.
|
|
18
|
Singh BN, Kumar D, Shankar S and
Srivastava RK: Rottlerin induces autophagy which leads to apoptotic
cell death through inhibition of PI3K/Akt/mTOR pathway in human
pancreatic cancer stem cells. Biochem Pharmacol. 84:1154–1163.
2012.
|
|
19
|
Wu H, Xie J, Pan Q, et al: Anticancer
agent shikonin is an incompetent inducer of cancer drug resistance.
PLoS One. 8:e527062013.
|
|
20
|
Yang H, Zhou P, Huang H, et al: Shikonin
exerts antitumor activity via proteasome inhibition and cell death
induction in vitro and in vivo. Int J Cancer. 124:2450–2459.
2009.
|