Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of malignancy globally, with >600,000 mortalities
per year, and its incidence continues to increase (1). However, surgery, radiotherapy and
chemotherapy have not sufficiently improved the five-year survival
rate of patients with this fatal disease in more than three
decades. In addition, as HCC is a relatively chemoresistant tumor
and highly tolerant to cytotoxic chemotherapy, systemic cytotoxic
chemotherapy agents are rarely effective at improving the survival
of patients with advanced HCC (2,3).
Despite ongoing efforts, no effective biomarkers have yet been
identified. Therefore, the development of novel chemotherapeutic
agents and more effective therapies for the treatment of HCC are
urgently required.
Cell cycle deregulation is one of the hallmarks in
human cancer and, thus, identification of physiological targets
underlying regulatory mechanisms for cell cycle regulation is
critical in the development of novel and effective cancer therapies
(4–6). Studies have identified that members of
the never in mitosis gene A-related kinase (Nek) family are
involved in cell cycle progression (7–9). Neks
are essential for mitotic entry, possibly through regulation of the
cdc2-cyclin B axis (10). As a
member of the Nek family, Nek6 also appears to be involved in
cancer. It has been shown that the Nek6 transcript is significantly
upregulated in a series of solid tumors, including HCC (11,12).
However, the precise molecular mechanism whereby Nek6 contributes
to HCC remains unclear.
Despite previous studies which have demonstrated
that Nek6 is involved in the oncogenesis of HCC, the potential
mechanism remains ambiguous. In the present study, our aim was to
investigate the expression of Nek6 in HCC, to explore the role of
Nek6 on cell cycle regulation of HCC cells and to trace the
internal molecular mechanism.
Materials and methods
Cell lines and tissue specimens
The HCC cell lines (huh7, HepG2, Hep3B and
PLC/PRF/5) and human normal L02 cells were purchased from Shanghai
Cell Bank (Shanghai, China). The cells were incubated in Dulbecco’s
Modified Eagle’s Medium supplemented with heat-inactivated 10%
fetal bovine serum (Gibco-BRL, Carlsbad, CA, USA) at 37°C in a 5%
CO2 humidified atmosphere. The 48 pairs of HCC tissues
were collected from patients diagnosed with HCC who had undergone
liver resection at the First Affiliated Hospital of Nanjing Medical
University. The paired normal liver samples were obtained from the
same patients, from a region 3 cm away from the edge of the cancer.
Patients provided written informed consent and the experiments
involving human tissue were proceeded in conformity with the
ethical principles of research and approved by the ethics committee
of Nanjing Medical University (Nanjing, China).
Semi-quantitative reverse transcription
(RT)-polymerase chain reaction (PCR) and quantitative real-time PCR
(qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. RT was performed with 2 μg total RNA
treated with RNase-free DNase I (Takara Bio, Inc., Shiga, Japan)
and the semi-quantitative RT-PCR products were separated on 2%
agarose gel containing ethidium bromide. β-actin served as the
internal reference. The relative mRNA level was measured by qPCR
using SYBR Green I (Takara Bio, Inc.), and the mRNA level of Nek6
in each sample was normalized against β-actin. The primers used
were as follows: Forward, 5′-AAGAAGCAGAAGCGGCTCAT-3′ and reverse,
5′-ATGGATCCTCTCCGGTGACA-3′ for Nek6 (249 bp); and forward,
5′-CCTAGAAGCATTTGCGGTGG-3′ and reverse, 5′-GAGCTACGAGCTGCCTGACG-3′
for β-actin (416 bp; loading control).
Immunohistochemical staining
Nek6 protein expression in the clinical specimens of
HCC and non-HCC tissues was determined by immunohistochemistry. The
formalin-fixed samples were paraffin-embedded and sectioned (4-μm).
The slides were incubated with rabbit anti-human Nek6 polyclonal
antibody (1:100 dilution; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) at 37°C for 2 h, where the normal rabbit IgG1
monoclonal antibody (1:100; Santa Cruz Biotechnology, Inc.) was
used as a negative control (NC). This was followed by incubation
with a horseradish peroxidase-conjugated goat anti-rabbit secondary
monoclonal antibody (Dako Japan Co., Ltd., Kyoto, Japan) at 37°C
for 1 h. The signals were detected using the diaminobenzidine
substrate kit (Vector Laboratories, Burlingame, CA, USA) and
counterstaining was performed with hematoxylin.
Construction of recombinant plasmid
The cDNA for Nek6 was generated by PCR screening of
a cDNA library of HCC. The full-length PCR fragment was cloned into
the pcDNA3.1-flag expression vector using NotI/EcoRI.
DNA sequencing confirmed the successful construction of the
plasmid, and the plasmid for transfection was prepared using a
TIANprep mini plasmid kit [Tiangen Biotech (Beijing) Co., Ltd.,
Beijing, China]. Lipofectamine 2000 transfection reagent
(Invitrogen Life Technologies) was used to perform the
transfections according to the manufacturer’s instructions.
Small interfering RNA (siRNA) and short
hairpin RNA (shRNA) preparation
For siRNA transfection, oligonucleotides targeting
Nek6 were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China). The siNek6 sequences used were as follows:
5′-GAUCGAGCAGUGUGACUACdTdT and 5′-GCUCGGUGACCUUGGUCUGdTdT. For
shRNA preparation, a synthesized DNA nucleotide fragment encoding
shRNA for the knockdown of endogenous Nek6 was inserted into pSUPER
(OligoEngine, Seattle, WA, USA). The same vector (pSUPER-shNC) with
irrelevant nucleotides not targeting any annotated human genes was
used as a NC.
Cell proliferation and soft agar
assay
Cell viability was measured using the Cell Counting
Kit-8 (Dojindo Laboratories, Kunamoto, Japan) according to the
manufacturer’s instructions. For the soft agar assay, 2,000 cells
were cultured in 24-well culture plates containing 1% base agar and
0.5% top agar. The colony morphology was recorded and colony
numbers were counted and calculated for each well following the
21-day study period.
Cell cycle distribution assay
The transfected cells at the logarithmic growth
phase were harvested and single-cell suspensions containing
1×106 cells were permeabilized with 70% ethanol. The
cells were then labeled with 50 μg/ml of propidium iodide and
treated with 250 μg/ml of RNase at 4°C for 30 min. Analysis was
performed using FACS Calibur (BD Biosciences, San Jose, CA, USA)
and analyzed using Cell Quest software (BD Biosciences). Data are
presented as the mean ± standard deviation (SD) from at least three
independent experiments.
Immunoblotting analysis
Western blot analysis was performed according to the
manufacturer’s recommended instructions (ImmunoCruz™ IP/WB Optima A
System, Santa Cruz Biotechnology, Inc.). Briefly, cell extracts
were prepared in lysis buffer [25 mmol/l Tris (pH 6.8), 1% SDS, 5
mmol/l EDTA and protease inhibitor cocktail (1:00); Sigma-Aldrich].
The blot was incubated with blocking solution (5% non-fat milk and
0.1% Tween 20 in phosphate-buffered saline) for 2 h at room
temperature. Polyclonal rabbit anti-human Nek6, cdc2 and cyclin B
(1:200; Santa Cruz Biotechnology, Inc.) antibodies were used in
this study.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5 software (GraphPad, La Jolla, CA, USA). Data are presented
as the mean ± standard deviation, and were evaluated for
statistical significance using unpaired Student’s t-test or one-way
analysis of variance, where P<0.05 was considered to indicate a
statistically significant difference.
Results
Nek6 is frequently upregulated in
HCC
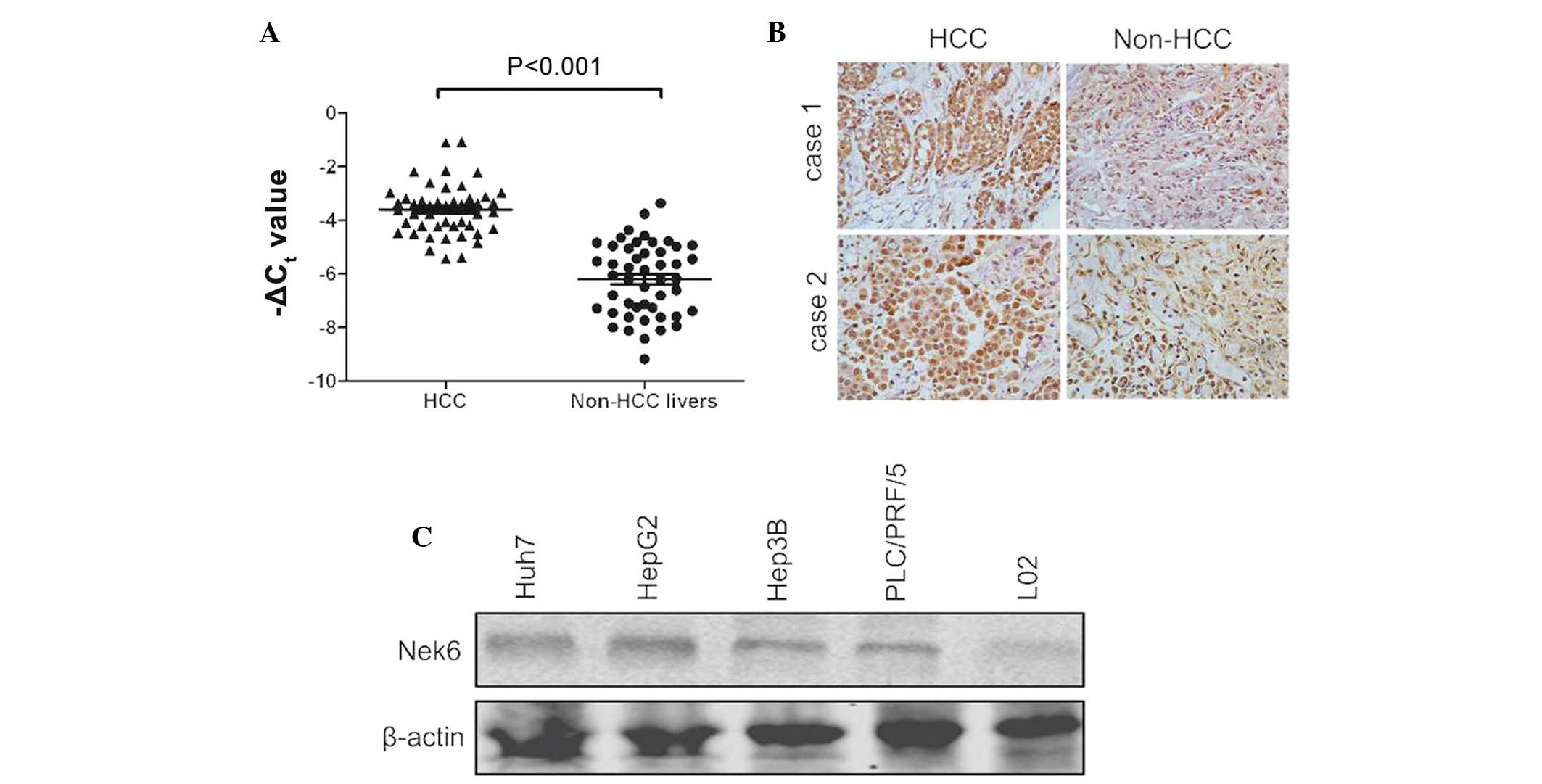
Nek6 was found to be significantly upregulated in 38
(79.1%) of the 48 HCC specimens, whereas the transcript of the gene
was rarely detected in adjacent non-cancerous livers, using a
semi-quantitative RT-PCR assay (Fig.
1A). To confirm the upregulation of the gene, Nek6 was also
evaluated in the 20 pairs of HCC and non-HCC livers through
immunohistochemistry. The results showed that the protein levels of
Nek6 were evidently increased in HCC when compared with the
adjacent non-cancerous livers (Fig.
1B). Nek6 was also markedly expressed in several HCC cell
lines, including Huh7, HepG2, Hep3B and PLC/PRF/5 cells (Fig. 1C). Overall, these findings indicated
that the upregulation of Nek6 may be a significant event in the
oncogenesis of HCC.
Overexpression of Nek6 promotes cellular
proliferation and colony formation
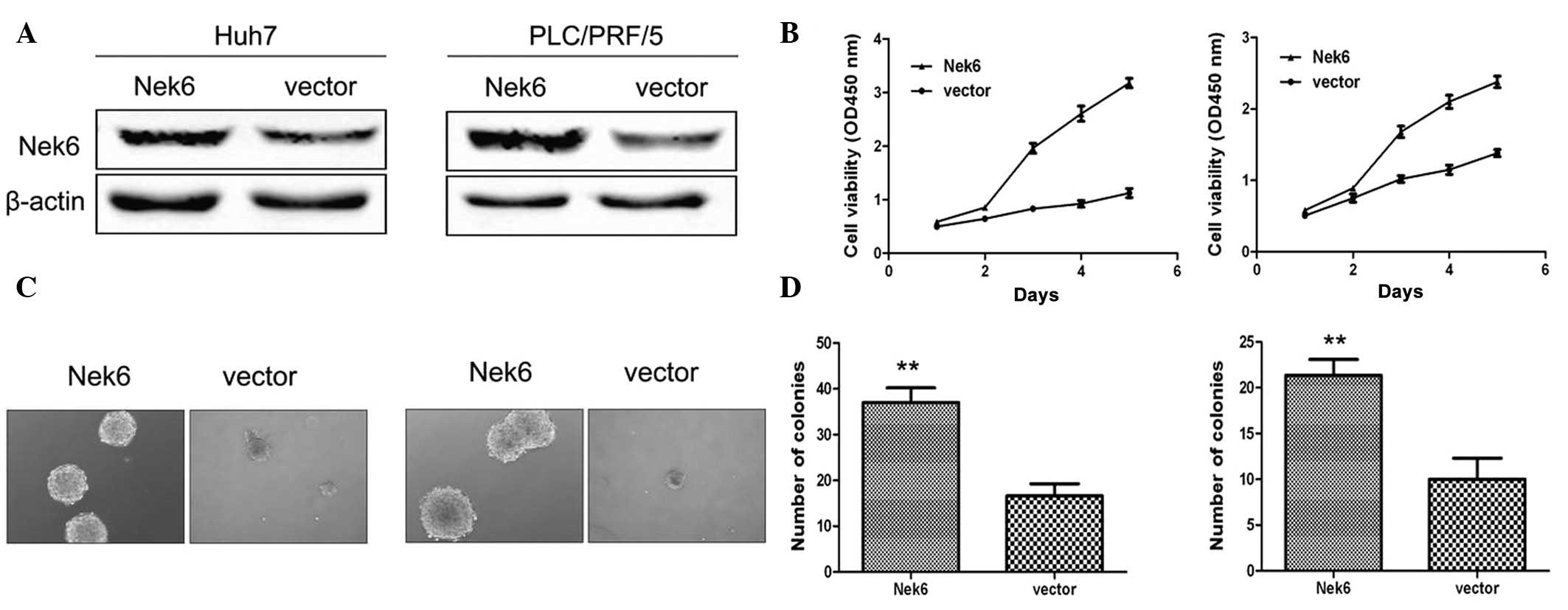
To reveal whether the dysregulated Nek6 may
contribute to hepatocarcinogenesis, Huh7 and PLC/PRF/5 cells, which
express relatively low levels of Nek6 (Fig. 1C), were transfected with pcDNA
encoding Nek6 (Fig. 2A). As
compared with the control, exogenous Nek6 promoted the significant
cell proliferation of the HCC cells (Fig. 2B). Similarly, ectopic Nek6 exhibited
a significantly enhanced effect on the anchorage-independent growth
ability of Huh7 and PLC/PRF/5 cells (Fig. 2C and D). These collective results
suggested that Nek6 overexpression is significant in promoting the
cell growth and colony formation of HCC cells.
Knockdown of Nek6 inhibits cellular
proliferation and colony formation
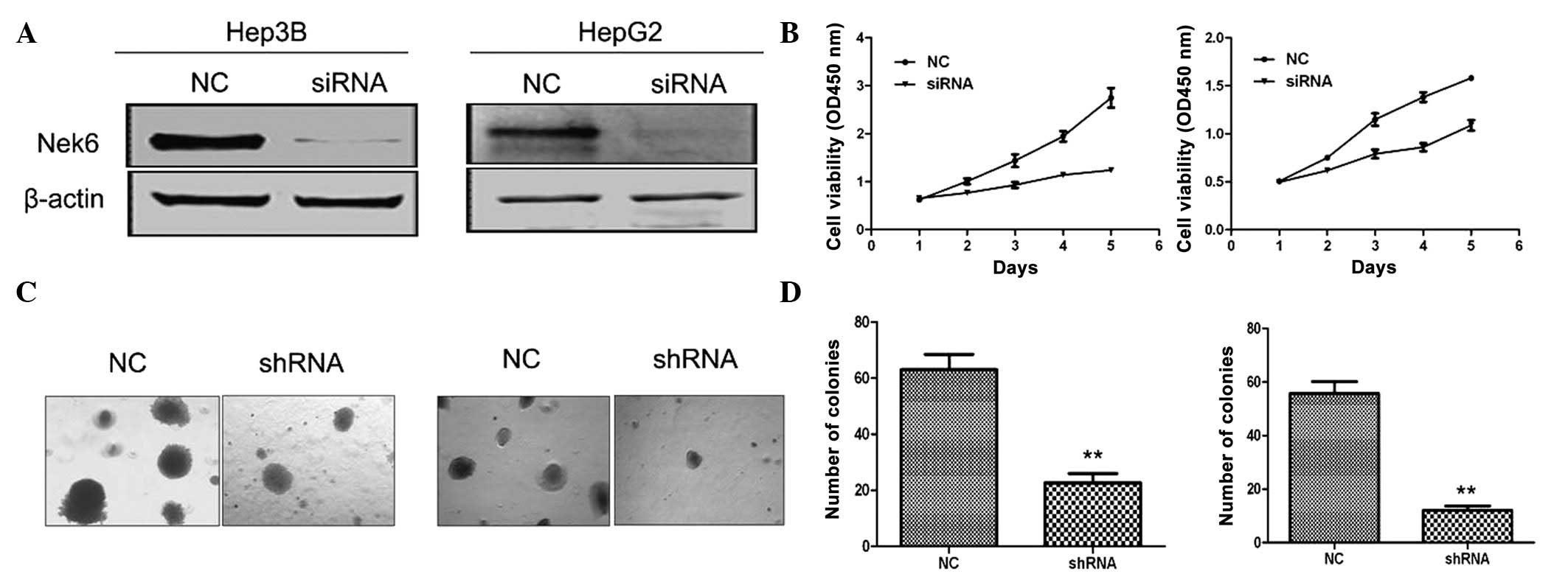
To further evaluate the contribution of the
upregulation of Nek6 to the oncogenesis of HCC, the siRNA was
designed and chemically synthesized for the knockdown of Nek6. To
test the efficacy of the siRNA, it was transiently transfected into
Hep3B and HepG2 cells, which express relatively high levels of Nek6
(Fig. 1C). The results indicated
that the siRNA significantly knocked down the endogenous Nek6, when
compared with siRNA-NC (Fig. 3A).
Notably, the siRNA evidently inhibited the cell growth of Hep3B and
HepG2 cells when compared with the siRNA-NC (Fig. 3B). Furthermore, the shRNA evidently
inhibited the colony formation of Hep3B and HepG2 cells in soft
agar (Fig. 3C and D). The results
suggested that the upregulation of Nek6 may contribute to the
hepatocarcinogenesis. Overall, these findings suggested that
endogenous Nek6 may be essential for maintaining the cellular
proliferation and colony formation of HCC cells.
Nek6 influences cell cycle
progression
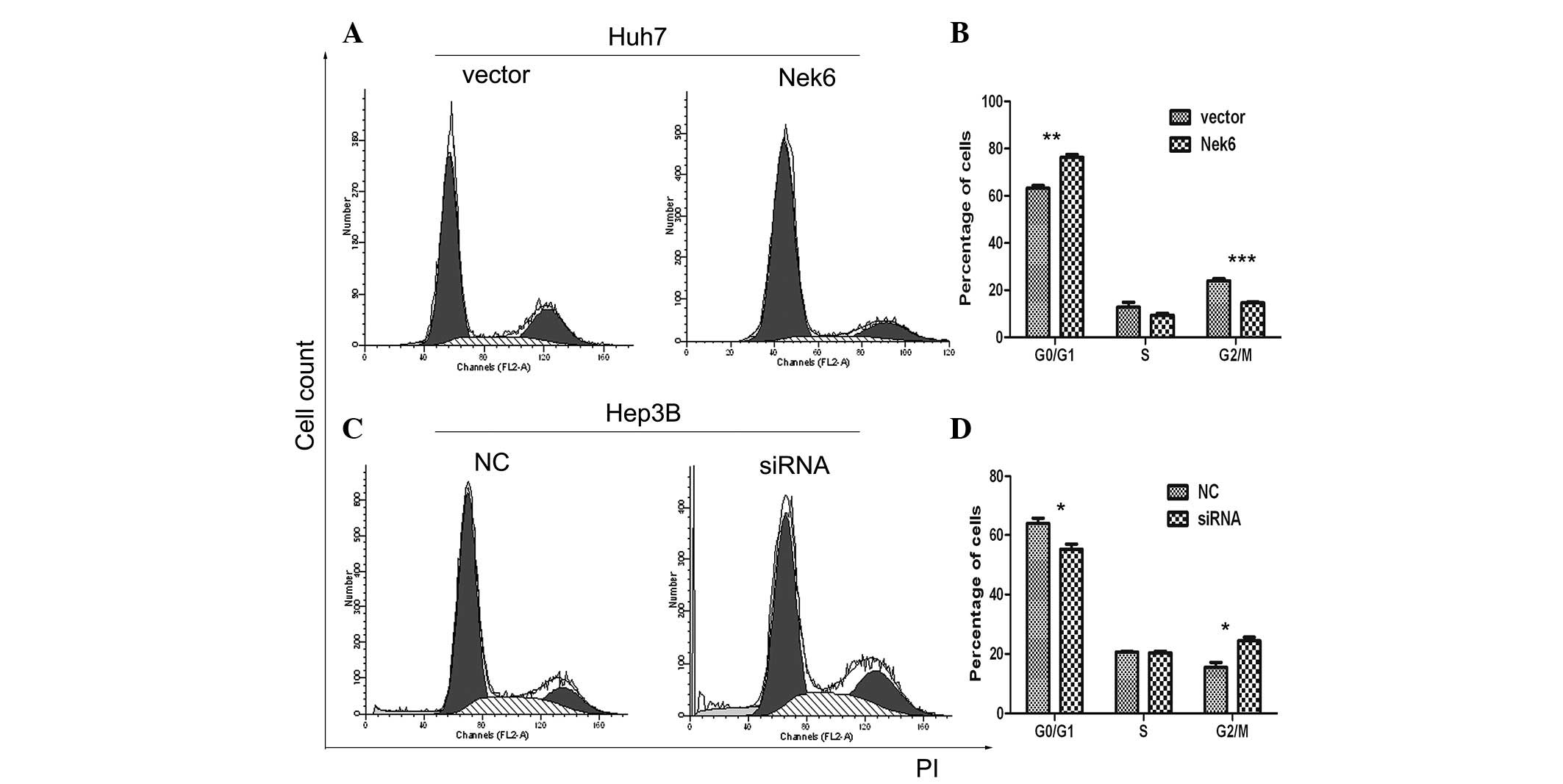
To evaluate the function of Nek6 on cell cycle
progression, flow cytometry was performed to detect the cell cycle
distribution of Huh7 and Hep3B cells. The enforced Nek6 pushed Huh7
cells to enter into the G0/G1 phase in
advance (Fig. 4C and D);
conversely, siRNA targeting endogenous Nek6 led to significant
G2/M phase arrest, or delayed the entry into the
G0/G1 phase in Hep3B cells (Fig. 4C and D) when compared with the
siRNA-NC, which was used as control. These results supported the
theory that Nek6 may contribute to the cell proliferation of HCC
via promoting the progression of the G2/M phase in the
cell cycle.
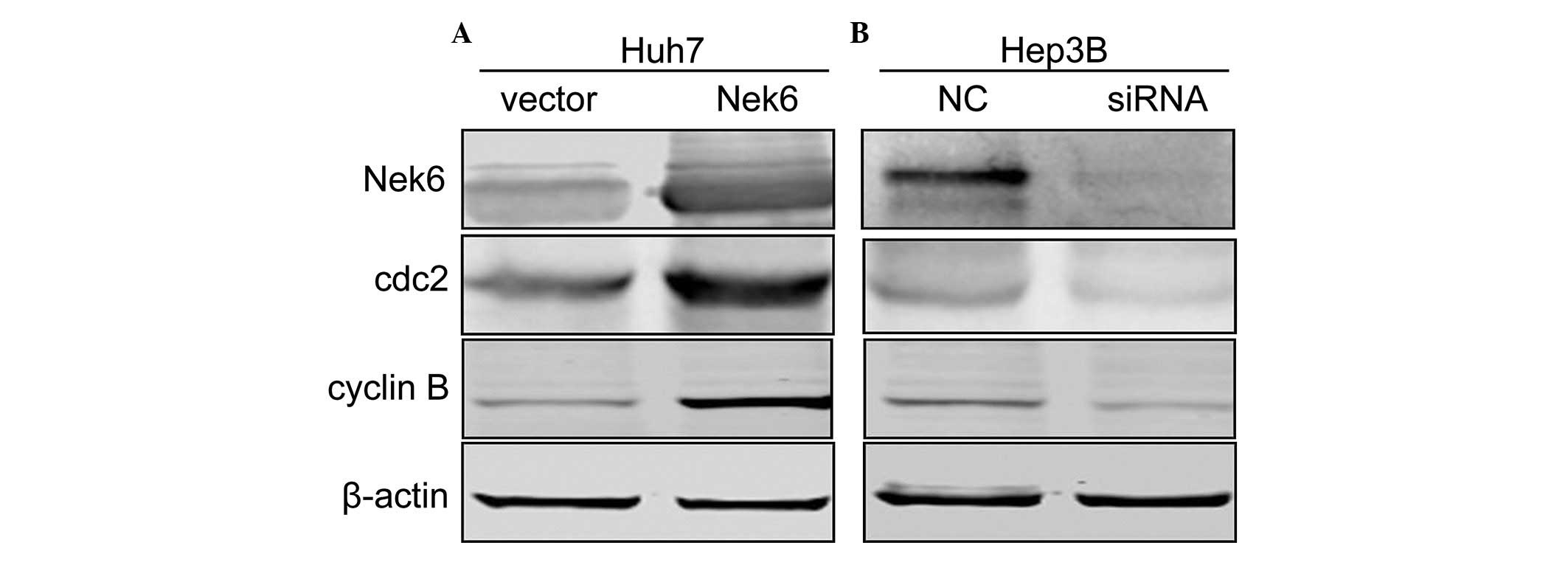
Nek6 mediates cyclin B transcription
through cdc2 modulation
To explore the molecular mechanisms by which Nek6
overexpression contributes to the promotion of the G2/M
to G0/G1 transition, specific known important
factors were analyzed, including cyclin B, D1 and E, by
immunoblotting assay. For Nek6 overexpression, only cyclin B was
significantly upregulated in Huh7 cells; by contrast, as Nek6 had
been knocked down by siRNA, cyclin B was evidently decreased in
Hep3B cells (Fig. 5). This
suggested that cyclin B, a key factor responsible for
G2/M cell cycle phase progression, may be crucial in the
promotion of the G2/M to G0/G1
transition that is triggered by Nek6 overexpression. To address the
mechanisms responsible for the enhanced cyclin B transcription, the
upstream regulatory elements of cyclin B were analyzed. The protein
level of cyclin-dependent protein kinase, cdk1/cdc2, was examined
using western blot analysis following vector/siRNA transfection.
Notably, the protein levels of cdc2 were increased more rapidly in
response to Nek6 overexpression (Fig.
5A); however, Nek6 RNAi cells showed clear decreases in cdc2
levels following siRNA transfection (Fig. 5B). These collective results support
the hypothesis that Nek6 mediates cyclin B expression in a positive
manner through regulation of the transcription of cdc2.
Discussion
Human Neks have been identified to contribute to
cell cycle progression and to be dysregulated in cancer tissues. To
date, 11 Neks have been identified in the human genome. Among them,
Nek1 (13), 2 (14,15),
10 (16) and 11 (17) are required for G2/M
arrest. Furthermore, Nek1 is overexpressed in cholangiocarcinoma
tumors and MCF7 cells (18,19). Nek8 is upregulated in primary human
breast tumors (20), and the
ectopic overexpression of Nek10 has been found in breast cancer
(21). In HCC, only Nek3 (22) and Nek6 (11,23)
have been reported to be upregulated in cancerous tissues.
Nek6 is a serine/threonine kinase belonging to the
Nek family, which is significantly involved in mitotic cell cycle
progression (9). In a subsequent
study, Nek6 was also found to suppress anticancer drug-induced
premature senescence (24).
Furthermore, it has been shown that Nek6 is able to stimulate
tumorigenesis in vitro and in vivo (25,26).
However, the intrinsic functions of Nek6 on tumorigenesis and cell
cycle progression in HCC are not well known.
The results of the present study, in conjunction
with previous reports (11,23), suggest that the expression of Nek6
is upregulated in HCC tissues compared with the benign normal
tissue, which showed low Nek6 expression. The results of the
current study also revealed that the enforced Nek6 promoted the
proliferation and colony formation of Huh7 and PLC/PRF/5 cells. In
addition, Nek6 may be essential for maintaining the hallmark of
human HCC cells. Nek6 knockdown also inhibited the cellular
proliferation and anchorage-independent growth of Hep3B and HepG2
cells. These results indicated that the upregulation of Nek6 may
contribute to oncogenesis and the progression of HCC. To further
understand the role of Nek6 in HCC, the cell cycle distribution of
HCC cells and the different levels of Nek6 expression were
examined. In this study, the ectopic Nek6 promoted the transition
from G2/M to G0/G1 phase of the
cell cycle, whereas Nek6 knockdown delayed the transition through
G2/M arrest. Furthermore, Nek6 was found to function as
a transactivator of cyclin B via upregulation of the cdc2 level in
HCC cells. Cyclin B is a well-documented important regulator that
promotes the progression of the G2/M phase. This study
proposed a possible mechanism in which Nek6 overexpression enhances
the upregulation of cdc2, which may in turn activate the cell cycle
regulator, cyclin B, in HCC cells in a dominant-positive manner.
Subsequently, cyclin B overexpression can promote cell cycle
progression and confer specific hallmarks of tumor cells with
anchorage-independent growth and tumorigenicity in vitro. To
the best of our knowledge, this study is the first to uncover the
upregulation of Nek6 as an enhancer for cell cycle progression and
hepatocarcinogenesis via the promotion of cyclin B expression.
However, the complexity of Nek6 contribution to HCC requires
further investigation.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.
|
|
2
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008.
|
|
3
|
Thomas M: Molecular targeted therapy for
hepatocellular carcinoma. J Gastroenterol. 44(Suppl 19): S136–S141.
2009.
|
|
4
|
Malumbres M and Barbacid M: Cell cycle
kinases in cancer. Curr Opin Genet Dev. 17:60–65. 2007.
|
|
5
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996.
|
|
6
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000.
|
|
7
|
Fry AM and Nigg EA: Characterization of
mammalian NIMA-related kinases. Methods Enzymol. 283:270–282.
1997.
|
|
8
|
Quarmby LM and Mahjoub MR: Caught Nek-ing:
cilia and centrioles. J Cell Sci. 118:5161–5169. 2005.
|
|
9
|
O’Regan L, Blot J and Fry AM: Mitotic
regulation by NIMA-related kinases. Cell Div. 2:252007.
|
|
10
|
Wu L, Osmani SA and Mirabito PM: A role
for NIMA in the nuclear localization of cyclin B in Aspergillus
nidulans. J Cell Biol. 141:1575–1587. 1998.
|
|
11
|
Chen J, Li L, Zhang Y, et al: Interaction
of Pin1 with Nek6 and characterization of their expression
correlation in Chinese hepatocellular carcinoma patients. Biochem
Biophys Res Commun. 341:1059–1065. 2006.
|
|
12
|
Capra M, Nuciforo PG, Confalonieri S, et
al: Frequent alterations in the expression of serine/threonine
kinases in human cancers. Cancer Res. 66:8147–8154. 2006.
|
|
13
|
Polci R, Peng A, Chen PL, Riley DJ and
Chen Y: NIMA-related protein kinase 1 is involved early in the
ionizing radiation-induced DNA damage response. Cancer Res.
64:8800–8803. 2004.
|
|
14
|
Faragher AJ and Fry AM: Nek2A kinase
stimulates centrosome disjunction and is required for formation of
bipolar mitotic spindles. Mol Biol Cell. 14:2876–2889. 2003.
|
|
15
|
Lou Y, Yao J, Zereshki A, et al: NEK2A
interacts with MAD1 and possibly functions as a novel integrator of
the spindle checkpoint signaling. J Biol Chem. 279:20049–20057.
2004.
|
|
16
|
Melixetian M, Klein DK, Sørensen CS and
Helin K: NEK11 regulates CDC25A degradation and the IR-induced G2/M
checkpoint. Nat Cell Biol. 11:1247–1253. 2009.
|
|
17
|
Moniz LS and Stambolic V: Nek10 mediates
G2/M cell cycle arrest and MEK autoactivation in response to UV
irradiation. Mol Cell Biol. 31:30–42. 2011.
|
|
18
|
Kokuryo T, Yamamoto T, Oda K, et al:
Profiling of gene expression associated with hepatolithiasis by
complementary DNA expression array. Int J Oncol. 22:175–179.
2003.
|
|
19
|
Tsunoda N, Kokuryo T, Oda K, et al: Nek2
as a novel molecular target for the treatment of breast carcinoma.
Cancer Sci. 100:111–116. 2009.
|
|
20
|
Bowers AJ and Boylan JF: Nek8, a NIMA
family kinase member, is overexpressed in primary human breast
tumors. Gene. 328:135–142. 2004.
|
|
21
|
Ahmed S, Thomas G, Ghoussaini M, et al:
Newly discovered breast cancer susceptibility loci on 3p24 and
17q23.2. Nat Genet. 41:585–590. 2009.
|
|
22
|
Hernández M and Almeida TA: Is there any
association between nek3 and cancers with frequent 13q14 deletion?
Cancer Invest. 24:682–688. 2006.
|
|
23
|
Cao X, Xia Y, Yang J, et al: Clinical and
biological significance of never in mitosis gene A-related kinase 6
(NEK6) expression in hepatic cell cancer. Pathol Oncol Res.
18:201–207. 2012.
|
|
24
|
Jee HJ, Kim HJ, Kim AJ, Song N, Kim M and
Yun J: Nek6 suppresses the premature senescence of human cancer
cells induced by camptothecin and doxorubicin treatment. Biochem
Biophys Res. 408:669–673. 2011.
|
|
25
|
Jeon YJ, Lee KY, Cho YY, et al: Role of
NEK6 in tumor promoter-induced transformation in JB6 C141 mouse
skin epidermal cells. J Biol Chem. 285:28126–28133. 2010.
|
|
26
|
Nassirpour R, Shao L, Flanagan P, et al:
Nek6 mediates human cancer cell transformation and is a potential
cancer therapeutic target. Mol Cancer Res. 8:717–728. 2010.
|