Introduction
In general, the life-time risk of suffering from
epithelial ovarian cancer (EOC) is ~1.5% in females. However, ~70%
of females with EOC are diagnosed at advanced stages, for which the
estimated long-term survival rate is ~10% (1). Neoadjuvant chemotherapy (NACT) prior
to delayed primary or interval debulking surgery subsequent to an
initial suboptimal surgery, followed by several periods of
chemotherapy, have been suggested as substitutions to primary
debulking surgery (2).
The golden criterion of EOC is the pathological
diagnosis, which shows a complete or almost complete response to
NACT (2). Therefore, accurate and
non-invasive evaluation of the tumor response to adjuvant therapy
is likely to be extremely useful. Until now, the efficacy of
therapy has been evaluated using morphological changes as
determined by imaging techniques, such as ultrasound, computed
tomography (CT), magnetic resonance imaging (MRI) and positron
emission tomography (PET) (3).
These techniques rely on changes in the size of the mass to assess
the tumor response to therapy. However, it may take several weeks
or even months to recognize the success or failure of therapy using
these methods. Furthermore, it is difficult to distinguish a
residual tumor from necrosis or fibrosis by stiffness. The serum
marker cancer antigen (CA)-125 is frequently elevated at the time
of EOC diagnosis and is commonly used to assess the response to
treatment. The reliability of the response evaluation during
chemotherapy for serous EOC may be improved by the assessment of
serum human epididymis protein 4 (4). However, Le et al (5) suggested that the CA-125 response to
NACT was not significantly predictive of progression-free
survival.
Ultrasound elastography is an emerging dynamic
imaging technique, which has been established as a promising
modality to discriminate relative tissue stiffness by surveying the
extent of deformation associated with strain under the utilization
of an external pressure. Elastography has been demonstrated to be a
useful method for ascertaining the presence of tumors and
distinguishing between different categories of abnormalities, such
as benign versus malignant lesions in breast tissue, thyroid
tissue, prostate tissue and lymph nodes (6). Furthermore, it has been applied for
the evaluation of the effect of ablative therapies, in which the
heating of tissues results in the denaturation of proteins and
consequently promotes the stiffness of ablated tissue (7). Elastography has also been acknowledged
as a practical method that can potentially identify clear
differences between the responses of malignant tissues, such as
breast cancer, to NACT (8).
Accordingly, in the present study, we hypothesized
that the evaluation of tumor stiffness by elastography has the
potential to provide additional information that is useful in
predicting the response to NACT in a clinical setting. To test this
hypothesis, the tumor stiffness in patients with advanced EOCs who
received NACT was investigated and the correlation with whether
optimal cytoreduction could be completed or not was analyzed.
Materials and methods
Patients
A total of 32 patients with International Federation
of Gynecology and Obstetrics stage III and IV EOC treated with NACT
(paclitaxel-platinum combination) and interval cytoreductive
surgery at the Department of Gynecologic Oncology, Obstetrics and
Gynecology Hospital of Fudan University (Shanghai, China) between
January 2011 and December 2012 were selected for enrolment into
this study. The study design and protocol were approved by the
Institutional Review Board of the Obstetrics and Gynecology
Hospital of Fudan University, and all patients provided written
informed consent once the nature of the procedure had been
explained fully.
Treatment
In all cases, NACT was administered following
confirmation of the cancer diagnosis by cytological or histological
examination. Prior to NACT administration, one of the 32 patients
was diagnosed histologically by laparoscopy, two were diagnosed by
laparotomy and the remaining 29 patients were diagnosed by
cytological samples obtained from abdominal paracentesis. During
interval cytoreductive surgery, histological confirmation of the
disease was also performed in all study patients.
A standard paclitaxel-platinum combination regimen
was administered as NACT. Carboplatin was administered with an area
under the curve of 5, together with paclitaxel (175
mg/m2). All patients were administered only one course
of chemotherapy for one day and all patients underwent pelvic
sonography, which included transvaginal and transabdominal imaging,
followed by pre- and post-NACT (three weeks after NACT)
elastography.
All procedures were performed by a single
radiologist with 10 years of experience in transvaginal and
transabdominal sonography who had specialized in elastography for
the last three years. All patients underwent imaging with an Acuson
S2000 system (Siemens Medical Solutions, Mountain View, CA, USA)
using a transvaginal 7-MHz probe and a transabdominal 4–5-MHz
probe. First, with conventional transvaginal and transabdominal
sonography, the tumor was located and assessed for its size and
overall sonographic appearance. Three vessels were measured and the
mean pulsatility and resistive indices were calculated. The section
of the ovarian tumor with the largest solid component was selected
for analysis. In each scanning plane selected for documentation
with conventional sonography, transabdominal elastography was also
performed immediately after acquisition of the conventional
grayscale sonogram. Next, the real-time elastogram and grayscale
sonogram were displayed simultaneously in the dual mode. The region
of interest in the elastogram was set to include sufficient
surrounding mass tissue. The tissue elasticity information was
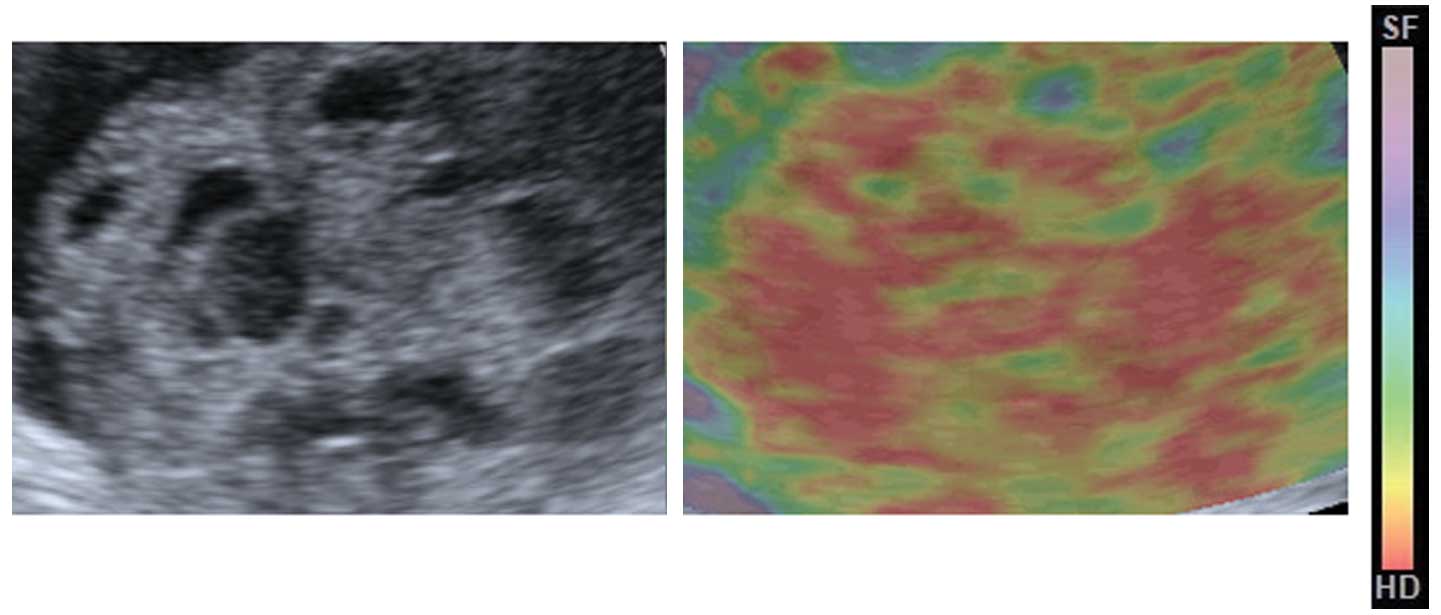
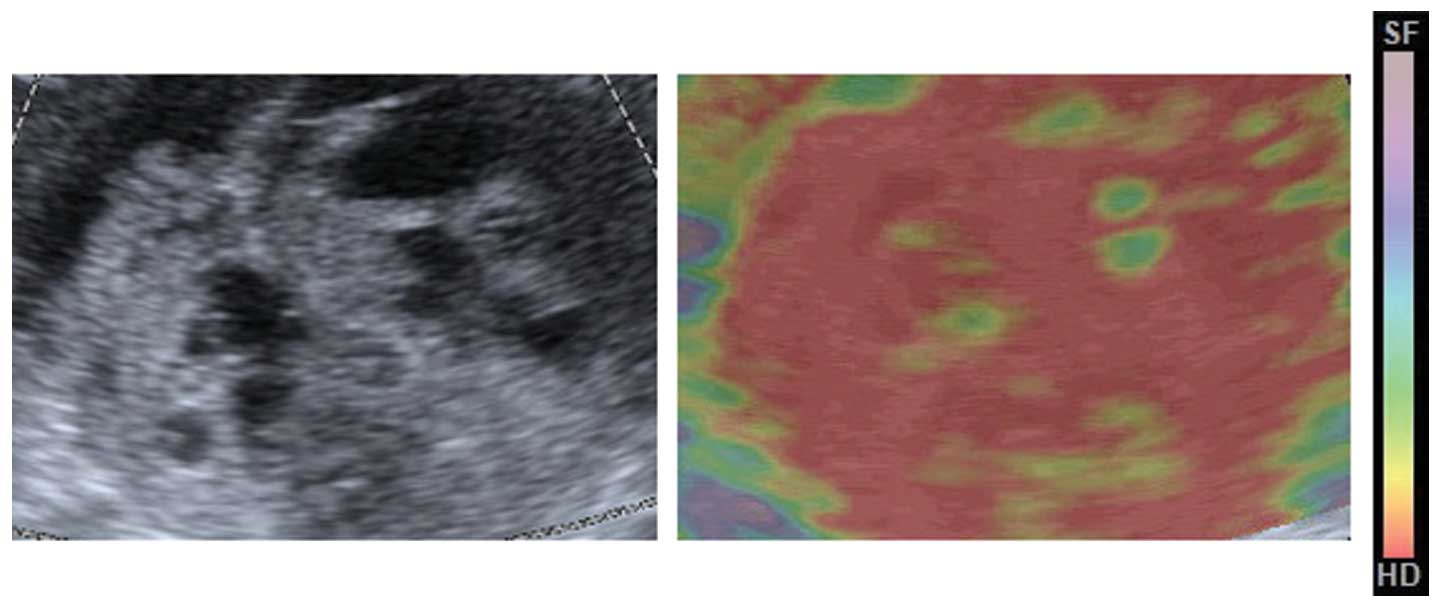
superimposed over the sonogram and displayed in color, with green
indicating medium tissue stiffness, red indicating hard tissue and
blue indicating soft tissue, as confirmed by previously published
studies that had used the Acuson S2000 system (9).
Imaging analysis
The elastograms were evaluated using four-point
scale from a study of neck masses (Table I) (10,11).
Although the machines used in the studies varied, the principle of
imaging tissue stiffness was consistent. Due to the lack of
universally accepted criteria for scoring elastograms of ovarian
cancer in the published literature and the novel nature of this
study, for easy analysis of the images, the elastograms were graded
on the simplified four-point scale, evaluating the stiffness of the
solid areas surrounded by fluid in the mass (Table I). This scale was adapted from a
previous thyroid ultrasound elastographic study (11). This scale was used as a standard in
our prior study and was found to be feasible for serous ovarian
cancer (9).
 | Table IElasticity scoring systems for
cervical lymph nodes. |
Table I
Elasticity scoring systems for
cervical lymph nodes.
| Elastographic
scorea | Elastographic
appearance |
|---|
| 1 (soft) | Predominantly purple,
green or yellow, with <10% displaying red. The node is
indistinguishable from the surrounding tissues |
| 2 (moderately
soft) | Predominantly yellow
or green, with red areas comprising between 10 and 50%. The node is
partially delineated from the surrounding tissues |
| 3 (moderately
stiff) | Predominantly red,
with yellow or green areas comprising between 10 and 50%. The node
is partially delineated from the surrounding tissues |
| 4 (stiff) | Predominantly red,
with <10% appearing yellow or green. The node is distinguishable
from the surrounding tissues |
The elastograms were evaluated on the basis of their
conventional sonographic presentation. All data collection and
imaging analysis were performed prospectively by another
radiologist who also had 10 years of experience in sonography and
had specialized in elastography for the last three years. In
addition, the radiologist was blinded to the final histological
diagnoses. All patients underwent surgery, and the pathological
diagnoses were categorized as of ovarian origin based on the
conventional criteria of the two pathologists.
Following NACT, all patients underwent interval
cytoreductive surgery regardless of their responses to NACT. The
optimality was defined according to the Gynecologic Oncology Group
definition in which optimal cytoreduction was defined as <1 cm
of the maximal residual tumor size (1). The pathological diagnoses were
categorized as of ovarian origin based on the conventional criteria
of two pathologists post-operatively. Cases not confirmed as
high-grade serous ovarian carcinoma (HGSC) were excluded.
Statistical analysis
The SPSS version 11.0 for Windows software package
(SPSS, Inc., Chicago, IL, USA) was used for statistical data
analysis. Data are presented as the mean ± standard deviation.
Student’s t-test was applied to determine whether tumor sizes,
CA-125 levels and resistive and pulsatility indices differed
between the pre- and post-NACT groups. Fisher’s exact test was
performed to determine whether the elasticity scores varied between
the two groups. The diagnostic sensitivity, specificity, accuracy,
positive predictive value (PPV) and negative predictive value (NPV)
were also calculated.
Results
Of the 32 patients studied, eight (25%) were
excluded by histopathological analysis for diagnoses not pertinent
to this study, including case of one fallopian tube cancer, two
mucinous carcinomas, two cases of clear cell cancer, one metastatic
carcinoma (peritoneum), and two tumors of undetermined origin. The
remaining cases (24/32; 75%) were all of HGSC.
The sonographic and serum marker characteristics of
the lesions pre- and post-NACT are shown in Table II. No statistically significant
differences were identified between the mean tumor diameters and
the CA-125 levels pre- and post-NACT. The resistive and pulsatility
indices were also not significantly different pre- and post-NACT.
However, the mean elasticity score was statistically higher for the
post-NACT lesions than for the pre-NACT lesions (3.13±0.57 vs.
2.04±0.51, respectively; P<0.001).
 | Table IICharacterization of pre- and post-NACT
using conventional sonography and serum marker. |
Table II
Characterization of pre- and post-NACT
using conventional sonography and serum marker.
| Index | Pre-NACT | Post-NACT | P-value |
|---|
| Mean diameter
(cm) | 10.27±6.56 | 11.56±5.22 | 0.61 |
| CA-125 (U/ml) | 675.32±87.78 | 703.96±79.81 | 0.76 |
| Resistance index | 0.34±0.24 | 0.31±0.34 | 0.57 |
| Pulsatility
index | 0.82±0.43 | 0.76±0.35 | 0.71 |
| Mean elasticity
score | 2.04±0.51 | 3.13±0.57 | <0.001 |
The median elasticity score for the pre-NACT lesions
on the four-point scale was 2, and the score for the post-NACT
lesions was 4. The distributions of the elasticity scores for pre-
are post-NACT are shown in Table
III. Of the pre-NACT lesions, 79.2% were scored as 1 or 2, and
20.8% were scored as 3. Of the post-NACT lesions, 66.7% were scored
as 3 and 4, and 33.3% were scored as 1 and 2 (Figs. 1 and 2). Cases of post-NACT with scores of 3 and
4 had a higher optimal cytoreduction rate than the cases with
scores of 1 and 2 (93.8 vs 25.0%, respectively; P<0.001). When
the post-NACT elasticity scores of 3 and 4 were used for the
prediction of optimal cytoreduction, elastography had 88.2%
sensitivity, 85.7% specificity, a 93.8% PPV, a 75.0% NPV and 87.5%
accuracy.
 | Table IIIElasticity scores in pre- and
post-NACT lesions (n=24). |
Table III
Elasticity scores in pre- and
post-NACT lesions (n=24).
| Elasticity score | Pre-NACT, n | Post-NACT, n | Optimal
cytoreduction, n (%) |
|---|
| 1 | 4 | 2 | 0 |
| 2 | 15 | 6 | 2 (25.0)a |
| 3 | 5 | 3 | 3 |
| 4 | 0 | 13 | 12 (93.8)b |
| Total | 24 | 24 | 17 |
Discussion
EOCs have the highest mortality rates in
gynecological oncology, occurring in the advanced stage and having
spread beyond the ovary at the time of diagnosis due to its rapid
growth. EOCs are rarely detected at an early stage and always
exhibit p53, BRCA, WT1 and p16 mutations, as well as high (>10%)
Ki67 levels (1). Defined as the
chemotherapy modality prior to primary surgery, NACT is an
alternative method to primary optimal cytoreduction for the
management of advanced EOC (12).
The most significant potential benefit of NACT is increasing the
percentage of patients who are subsequent candidates for achieving
optimal cytoreduction. In patients with advanced ovarian cancer,
optimal cytoreduction can be selected by evaluating the response to
NACT by ultrasound, CT, MRI and PET, as well as by analysis of the
levels of CA-125 (13).
Elastography, a novel functional imaging technique,
may allow the earlier identification of a response, with higher
accuracy. At present, the main role of elastography is to
discriminate between cancer and benign lesions associated with
conventional ultrasonography and to decrease the administration of
unnecessary puncture biopsies, which is based on the tissue
stiffness (14). The diagnosis
depends on the principle that cancer is relatively hard compared
with non-cancerous lesions, however, relatively soft cancers do
also exist.
Hayashi et al (15) reported that the mean breast
elastography scores were significantly lower for the clinical and
pathologic complete response groups than for the other groups.
Tumor stiffness assessed by elastography was potentially predictive
for the response to NACT. Evaluated by elastography, tissue
stiffness may be confirmed as a clinically significant tumor
feature, which cannot be gained by other functional imaging
techniques (15). Similarly, in an
additional study, Falou et al (8) identified an early clinically response
in patients during the process of NACT by elastography. These
results indicated that elastography may potentially be applied as
an early predictor of tumor treatment response in patients with
cancer (8).
The present study demonstrated that elastography may
be useful in predicting the outcome of neoadjuvant therapy in
patients with advanced HGSC. In our prior study (9), two different types of serous ovarian
cancer were evaluated that showed different stiffness ranges on
elastography. A tendency for HGSC to be less stiff than low-grade
serous carcinoma (LGSC) was identified, which may be elucidated by
the fact that the predominantly solid tissue of HGSC developed
necrosis rapidly. Conversely, LGSC grew relatively slowly so that
its solid lesions were less flexible and more stiff (9). Recent studies have confirmed that
chemotherapy based on platinum for patients with the p53 mutation
has higher sensitivity, in contrast to previous theories (16,17).
Indeed, owing to the loss of the capability to repair DNA, rapidly
proliferating cells of HGSC are more sensitive to platinum
(16). Chemotherapy has been found
to control the development of cell necrosis in force, and the
stiffness of HGSC post-NACT is likely to develop toward that of
LGSC (17). As a result, the mean
elasticity score of the pre-NACT lesions was lower than that of the
post-NACT lesions in the present study. This indicated that
techniques with functions rather than anatomy imaging may provide
improved precision in monitoring response, as functional changes
are predicted to develop prior to morphological changes.
In the current study, it was also confirmed that
cases with scores of 3 and 4 post-NACT had higher optimal
cytoreduction rates than cases with scores of 1 and 2. Since all
patients were administered only one course of chemotherapy, this
confirmed that elastography is a sensitive tool for the evaluation
of NACT in patients with HGSC.
There were certain limitations to the current study.
First, the sample size was small, and the aim of this study was not
to compare elastography against conventional sonography, CT and
MRI, but to evaluate its potential role as a novel tool.
Furthermore, due to a lack of universally accepted criteria for
scoring elastograms of ovarian cancer in the published literature,
a simplified four-point scale was used based on a previous thyroid
elastographic study (11) similar
to our prior study (9). In
addition, the section of the ovarian tumor with the largest solid
component was selected for analysis and did not represent all of
the lesions. Finally, all the patients exhibited HGSCs, and further
investigation is required to analyze whether the results can be
applied to other EOCs.
To the best of our knowledge, this is the first
study to investigate the application of elastography in the
evaluation of NACT in HGSC. Despite its small sample size and
retrospective design, several conclusions can be drawn from the
study. First, elastography is a more sensitive tool for the
evaluation of NACT in patients with HGSC compared with CT and MRI.
Furthermore, elastography may aid gynecologists in choosing the
optimal cytoreduction. We hypothesize that elastography is likely
to become a standard tool for the assessment of the treatment
response to NACT in HGSC based on the results of future
studies.
Acknowledgements
This study was supported by a grant from the Science
and Technology Commission of Shanghai Municipality (grant no.
134119a8200).
Abbreviations:
|
NACT
|
neoadjuvant chemotherapy
|
|
HGSC
|
high-grade serous ovarian
carcinoma
|
|
CA-125
|
cancer antigen 125
|
|
NPV
|
negative predictive value
|
|
PPV
|
positive predictive value
|
|
EOC
|
epithelial ovarian cancer
|
References
|
1
|
Ozols RF: Systemic therapy for ovarian
cancer: current status and new treatments. Semin Oncol. 33(2 Suppl
6): S3–S11. 2006.
|
|
2
|
Chan YM, Ng TY, Ngan HY and Wong LC:
Quality of life in women with neoadjuvant chemotherapy for advanced
ovarian cancer: a prospective longitudinal study. Gynecol Oncol.
88:9–16. 2003.
|
|
3
|
Rockall A, Munari A and Avril N: New ways
of assessing ovarian cancer response: metabolic imaging and beyond.
Cancer Imaging. 12:310–314. 2012.
|
|
4
|
Hynninen J, Auranen A, Dean K, et al:
Serum HE4 profile during primary chemotherapy of epithelial ovarian
cancer. Int J Gynecol Cancer. 21:1573–1578. 2011.
|
|
5
|
Le T, Hopkins L, Faught W and
Fung-Kee-Fung M: The lack of significance of Ca125 response in
epithelial ovarian cancer patients treated with neoadjuvant
chemotherapy and delayed primary surgical debulking. Gynecol Oncol.
105:712–715. 2007.
|
|
6
|
Garra BS: Elastography: current status,
future prospects, and making it work for you. Ultrasound Q.
27:177–186. 2011.
|
|
7
|
Kolokythas O, Gauthier T, Fernandez AT, et
al: Ultrasound-based elastography: a novel approach to assess radio
frequency ablation of liver masses performed with expandable
ablation probes: a feasibility study. J Ultrasound Med. 27:935–946.
2008.
|
|
8
|
Falou O, Sadeghi-Naini A, Prematilake S,
et al: Evaluation of neoadjuvant chemotherapy response in women
with locally advanced breast cancer using ultrasound elastography.
Transl Oncol. 6:17–24. 2013.
|
|
9
|
Xie M, Zhang X, Zhan J and Hua K:
Application of real-time elastography based on ultrasound in
discrimination of low- and high-grade serous ovarian carcinoma. J
Ultrasound Med. 32:257–262. 2013.
|
|
10
|
Itoh A, Ueno E, Tohno E, et al: Breast
disease: clinical application of US elastography for diagnosis.
Radiology. 239:341–350. 2006.
|
|
11
|
Hong Y, Liu X, Li Z, et al: Real-time
ultrasound elastography in the differential diagnosis of benign and
malignant thyroid nodules. J Ultrasound Med. 28:861–867. 2009.
|
|
12
|
Vergote I, Tropé CG, Amant F, et al;
European Organization for Research and Treatment of
Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group.
Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV
ovarian cancer. N Engl J Med. 363:943–953. 2010.
|
|
13
|
Bristow RE, Eisenhauer EL, Santillan A and
Chi DS: Delaying the primary surgical effort for advanced ovarian
cancer: a systematic review of neoadjuvant chemotherapy and
interval cytoreduction. Gynecol Oncol. 104:480–490. 2007.
|
|
14
|
Gong X, Xu Q, Xu Z, et al: Real-time
elastography for the differentiation of benign and malignant breast
lesions: a meta-analysis. Breast Cancer Res Treat. 130:11–18.
2011.
|
|
15
|
Hayashi M, Yamamoto Y, Ibusuki M, et al:
Evaluation of tumor stiffness by elastography is predictive for
pathologic complete response to neoadjuvant chemotherapy in
patients with breast cancer. Ann Surg Oncol. 19:3042–3049.
2012.
|
|
16
|
Kurman RJ and Shih IeM: Molecular
pathogenesis and extraovarian origin of epithelial ovarian cancer -
shifting the paradigm. Hum Pathol. 42:918–931. 2011.
|
|
17
|
Lederman JA, Marth C, Carey MS, et al;
Gynecologic Cancer InterGroup. Role of molecular agents and
targeted therapy in clinical trials for women with ovarian cancer.
Int J Gynecol Cancer. 21:763–770. 2011.
|