Introduction
Gastrointestinal stromal tumours (GISTs), although
rare tumours overall, are the most common type of mesenchymal
tumour of the GI tract. Approximately 85–90% of GISTs are
associated with gain-of-function KIT gene mutations, which lead to
constitutive activation of KIT kinase activity and to uncontrolled
cell proliferation. A notably smaller proportion (5%) is associated
with analogous mutations in PDGFRα and <10% contain no
identified receptor tyrosine-kinase mutations (termed wild-type
GISTs) (1–3).
Traditional cytotoxic treatments, although active in
other subtypes of sarcoma, are ineffective in GISTs. Elucidating
the GIST molecular pathophysiology as a mutation-driven process has
enabled the development of targeted kinase-inhibitor therapies,
which have revolutionised treatment strategies and clinical
outcomes for patients with advanced GISTs (4,5).
Imatinib mesylate, an oral selective inhibitor of
the kinase activities of KIT and PDGFRα, was the first targeted
therapy to demonstrate dramatic efficacy on GISTs. Prior to
imatinib, the median overall survival (mOS) of metastatic GIST
patients was 19 months (6,7). However, ~4% of patients are intolerant
to imatinib therapy, ~15% show primary resistance to imatinib and
>80% eventually develop a secondary or acquired resistance
following a median treatment time of approximately two years.
Resistance most commonly develops as a result of secondary KIT
mutations in clonally expanded cancer cells (8).
Sunitinib malate is the only approved second-line
treatment option for advanced GIST patients who are resistant or
intolerant to imatinib. Sunitinib is an oral multitargeted receptor
tyrosine-kinase inhibitor (TKI) of KIT, PDGFRα, all three isoforms
of the vascular endothelial growth factor receptors (VEGFR-1, -2
and -3) and various other tyrosine-kinase receptors. It
consequently targets the primary kinases that are implicated in
GIST pathogenesis as well as those involved in tumour-associated
angiogenesis (9).
Results of a randomised, placebo-controlled, phase
III study of a regimen of 50 mg/day sunitinib during an
intermittent dosing schedule of 4-weeks-on treatment followed by
2-weeks-off treatment (a 4w/2w schedule) demonstrated significant
efficacy and safety in patients with advanced GISTs following PD or
those with an intolerance to imatinib (10). The median time to tumour progression
was more than four times longer with sunitinib compared with a
placebo treatment (27.3 vs. 6.4 weeks; P<0.0001) and a
significant difference in OS, favouring sunitinib [hazard ratio
(HR), 0.49; P=0.007] was shown.
Long-term survival data of this trial was subjected
to a novel type of statistical analysis; the rank-preserving
structural failure time method, which accounts for bias that is
introduced by patients crossing over from a placebo to an active
treatment. This analysis demonstrated the long-term OS benefit that
was provided by sunitinib compared with a placebo (74.7 vs. 36.0
weeks; HR, 0.46; P<0.0001) (11). These results led to multinational
approval of sunitinib in this patient population; those who have an
intolerance to imatinib and/or those showing PD.
Furthermore, an open-label phase II study was
conducted on a large number of patients with sunitinib administered
at a lower dose on a continuous daily dosing schedule (37.5 mg/day
without off-treatment periods), which demonstrated that this type
of administration provided a safe and effective dosing option
without additional accumulation across cycles, and no novel or
unexpected adverse events (AEs) were reported (12).
However, sunitinib is associated with AEs, which are
generally mild to moderate, which may lead to a dose reduction,
interruption or suspension of therapy, in the long term.
The most common AEs are fatigue, diarrhoea, nausea
and vomiting, skin and hair discolouration, stomatitis, hand-foot
syndrome, hypertension (HTN) and hypothyroidism. At the time of
data cut-off in the placebo-controlled study, treatment-associated
AEs of any severity grade and of serious AEs, were reported in 83
and 20% of patients, respectively. Twenty-eight per cent of
patients interrupted their treatment, 11% required a dose reduction
and 9% discontinued treatment due to the AEs experienced in the
study (10); similar results were
reported in the next expanded access studies (13).
In addition, a pharmacokinetic/pharmacodynamic
meta-analysis was performed in order to investigate the association
between clinical endpoints and sunitinib exposure in patients with
advanced solid tumours, including 454 patients affected by GISTs
(14). This demonstrated longer
time-to-progression and OS, and a trend towards a higher
probability of a decrease in tumour size or halting of tumour
growth in patients with the greatest exposure to sunitinib. These
analyses stressed the importance of maintaining patients on a 50-mg
dose, thus avoiding unscheduled dosing interruptions or ‘jerky’
consumption of sunitinib. Therefore, the effective management of
AEs is crucial to obtain consistent compliance, and achieve and
maintain optimal clinical efficacy (15,16).
With the aim to improve patients’ adherence and
reduce sunitinib-associated side effects, numerous studies using
alternative doses or schedules of sunitinib have been conducted.
For example, certain patients who were affected by metastatic renal
cell carcinoma (mRCC) and for whom sunitinib represented the
first-line therapy, were changed from the standard 4w/2w schedule
to a new 2w/1w or 7-day-on treatment followed by 3-day-off
treatment schedule (7d/3d) (17,18).
The results demonstrated that treatment using alternative schedules
was associated with significantly decreased toxicity in patients
who had initially experienced a grade 3 or greater toxicity on the
4w/2w schedule, as well as enabled a marked extension of the
treatment duration.
A phase I trial in GIST patients evaluated the
feasibility of administering 50 mg sunitinib for 2 weeks followed
by a 1-week-off period (19). The
pharmacokinetic data demonstrated that the 2w/1w schedule provided
prolonged sunitinib exposure compared with the 4w/2w schedule,
without significant accumulation of sunitinib between courses, and
that the 2w/1w schedule was better tolerated, with only minor dose
adjustments or modifications required.
In the majority of countries, sunitinib represents
the only approved therapeutic option (after imatinib) for patients
that are affected by GISTs. For this reason, continuing sunitinib
treatment, even following progressive disease (PD), has been
proposed as a promising alternative. In an open-label retrospective
study, 704 patients were dichotomized based on whether sunitinib
treatment was continued or terminated following PD (20). The study demonstrated that the
patients who continued on sunitinib exhibited an improved clinical
outcome compared with those who terminated it (mOS, 22.8 vs. 13.2
months). The results of the abovementioned study supported the
adoption of this strategy in clinical practice.
Various additional inhibitors of KIT and PDGFRα
kinases have been developed. However, despite promising results in
imatinib/sunitinib-resistant disease control in early phase trials,
to date, none but one (regorafenib, a novel, oral multikinase
inhibitor) have shown benefits in prospective phase III trials. In
fact, the Food and Drug Administration only recently expanded the
use of regorafenib to GIST as a result of the GRID trial results
(21).
In the present study, the experiences of patients
affected by GIST are described, referring specifically to the
management of treatment, with the aim of discussing optimization of
the treatment duration and patient outcome. In particular, two
clinical cases of patients who were affected by GIST and treated
with alternative schedules of sunitinib are presented, in addition
to the case of a patient whose therapy with sunitinib has been
continued following PD using complementary loco-regional treatment,
thus obtaining a prolonged clinical benefit. Finally, a hypothesis
explaining these encouraging results is provided along with a
comparison between our data and those of other possible treatment
options that have been reported in the literature.
Materials and methods
Patients
Between December 2001 and June 2013, 67 patients
affected by advanced GIST were treated at the Ematology and Medical
Oncology Unit ‘L&A Seràgnoli’, S.Orsola-Malpighi Hospital,
University of Bologna (Bologna, Italy) with sunitinib following PD
or intolerance to imatinib. The primary treatment schedule adopted
in our centre is 37.5 mg/day sunitinib continuously. All 67
patients were retrospectively analyzed; 64 were treated following
the standard guidelines and three required personalized treatment
management. In the majority of cases, sunitinib demonstrated
efficacy and safety profiles similar to those reported in the
previous literature (10,12,13).
Patients presented with predominantly transient or self-limiting
side effects, primarily managed with dose delay, temporary dose
reduction or standard supportive medical treatments. However, in
three cases, it was necessary to adopt tailored strategies. As the
aim of the present study was to analyse alternative schedules and
integrated treatment options, the present study focused mainly on
these three patients. Their clinical history will be described
individually as it may significantly facilitate clinical practice.
All patients provided consent.
Results
In 64 out of the 67 patients, sunitinib treatment
was discontinued in favour of other standard or experimental
treatment options in order to prevent severe toxicities or PD. Such
options include the following: Rechallenge with imatinib,
nilotinib, sorafenib, regorafenib or the best supportive care.
Conversely, in three cases out of the 67, treatment with sunitinib
was prolonged despite intolerance or PD through the adoption of
personalized measures or treatment adjustment (Table I). The clinical management of these
three patients is reported below.
 | Table ICharacteristics of three patients who
continued sunitinib despite disease progression or intolerance,
through adoption of an AS or an an IS. |
Table I
Characteristics of three patients who
continued sunitinib despite disease progression or intolerance,
through adoption of an AS or an an IS.
| | | | Duration of therapy,
months (regimen) |
|---|
| | | |
|
|---|
| Case | Agea, years | Gender | Risk category at
diagnosis | Imatinib: 400
mg/day | Imatinib: 800
mg/day | Sunitinib: SS | Sunitinib: AS or
IS |
|---|
| 1 | 85 | F | Metastatic | 28 | 5 | 7 | 32 (25
mg/day)
7 (25 mg 1d/1db) |
| 2 | 66 | F | Low | 12 | 1 | 7 | 36 (25 mg/day) |
| 3 | 61 | M | Moderate | 6 | 0 | 53 | 30 (post RFA) |
Case 1
In October 2006, an 82-year-old female presented
with an acute episode of severe anaemia. A large gastric lesion
associated with multiple liver and bone metastases was detected by
a computed tomography (CT)-scan. Due to the persistence of anaemia,
the patient underwent a partial gastric resection and histological
examination revealed a GIST. In addition, mutational analysis
revealed a common KIT exon 11 mutation (p.V559D). In November 2006,
the patient commenced first-line imatinib therapy at the standard
dose of 400 mg/day and achieved a prolonged, stable disease. In
March 2009, a new abdominal lesion of the left iliac fossa was
identified. Thus, the patient was administered 800 mg/day imatinib,
however, the treatment was prematurely interrupted due to severe
bilateral pleural effusion. Therefore, in August 2009 the patient
was enrolled in an A6181078 trial and commenced second-line therapy
with sunitinib at 37.5 mg/day, which was reduced to 25 mg due to
persistent leukopenia and neutropenia. Bone marrow toxicity also
resulted in numerous treatment interruptions with a duration
ranging from a few days up to a month. However, the disease
remained stable until November 2012, when a mild dimensional
increase of the known abdominal lesion was observed. However, due
to the clinical benefit and the marginal focal progression, the
same therapy was continued with a modified sunitinib schedule
incorporating an intermittent administration (1d/1d) at a reduced
dose of 25 mg in order to overcome bone marrow toxicity. In April
2013, on the final CT scan evaluation, the disease was identified
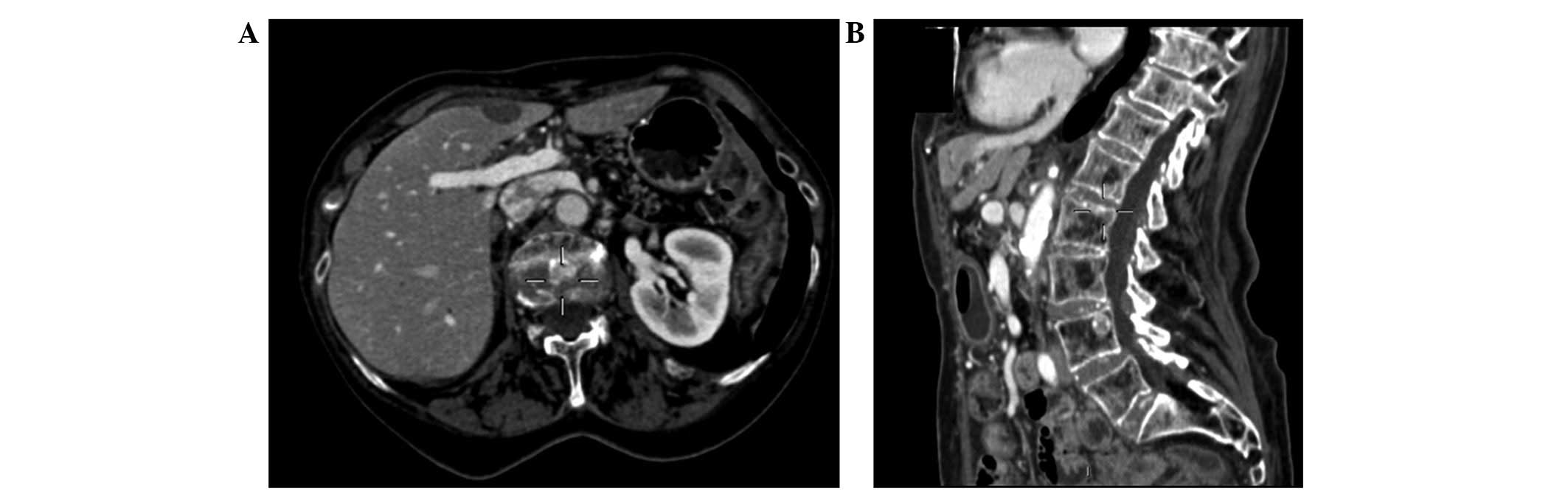
to be stable (Fig. 1).
Case 2
In November 1997, a 54-year-old female underwent
surgical resection of a digiunal GIST. During the follow-up
programme ~11 years later ultrasonography revealed a
retro-pancreatic mass with multiple liver lesions. The diagnosis of
GIST was determined by a CT-guided liver biopsy. Tumour genotyping
revealed a mutation on exon 18 of the PDGFRα gene, excluding the
D842V mutation. In September 2008, treatment with 400 mg/day
imatinib was initiated. CT scan and contrast enhanced
ultrasonography (CEUS) showed stable disease until September 2009,
when CEUS demonstrated a mild increase in the size of the hepatic
lesions. Thus, the dose was altered to 800 mg/day. An additional
CEUS that was performed one month later indicated further mild
progression. Hence, in November 2009, the patient commenced a
second-line treatment of 37.5 mg/day sunitinib. Side effects caused
by the treatment were characterized by diarrhoea and grade 3 HTN
according to the National Cancer Institute Common Terminology
Criteria for Adverse Events version 4.0 (22). Although
angiotensin-converting-enzyme-inhibitors, beta-blockers,
calcium-antagonists and diuretics were administered, the patient’s
blood pressure remained particularly difficult to control. Thus,
the sunitinib schedule was modified to 25 mg/day continuously,
which resulted in the improved control of AEs. Any attempt to
reintroduce the standard dose of 37.5 mg/day was characterized by a
recurrence of diarrhoea and blood pressure instability. Therefore,
since June 2010, the patient has continuously been treated with an
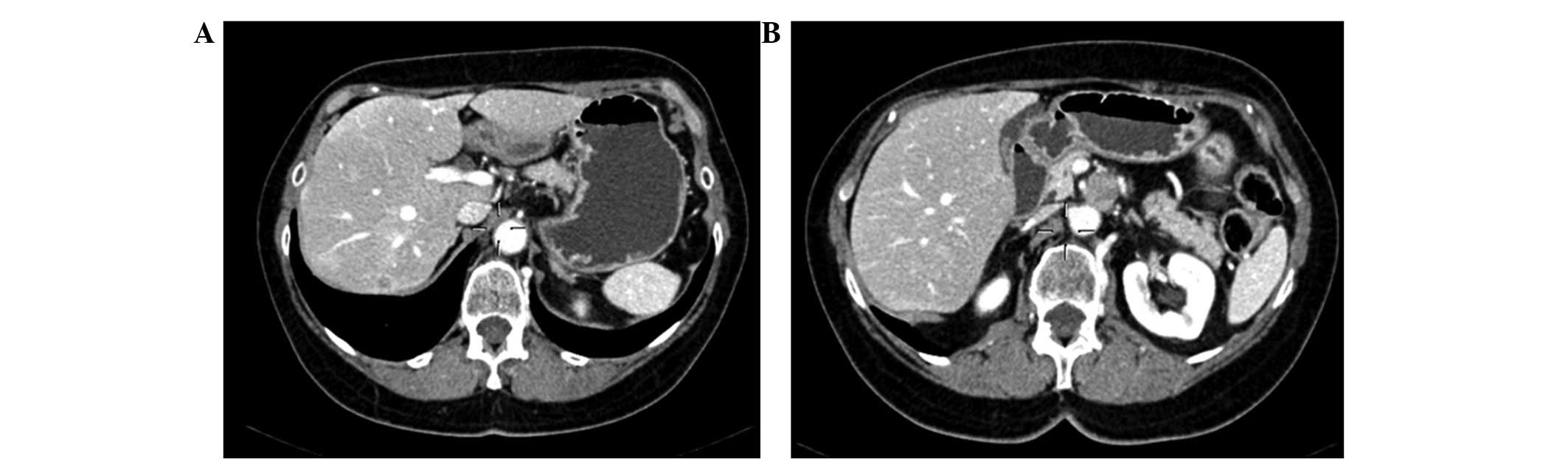
alternative schedule of sunitinib; the CT scans in Fig. 2 demonstrate the stability of the
disease.
Case 3
In December 2003, a 58-year-old male underwent
surgical resection for a GIST in the ileum. The mutational analysis
showed a deletion at exon 11 of the KIT gene (p.V569_Y578 del.). In
January 2005, during follow-up, a CT scan detected three liver
metastases. Treatment with 400 mg imatinib was initiated, however,
the imatinib was administered irregularly as it caused deep
fatigue, mucositis and a skin-rash. In July 2005, the
administration of imatinib was permanently discontinued. Due to a
lack of novel approved drugs, the patient underwent three wedge
resections of the liver, however, in January 2006, a CT scan
revealed six new liver lesions. In July 2006, the patient was
referred to the Ematology and Medical Oncology Unit ‘L&A
Seràgnoli’, S.Orsola-Malpighi Hospital, University of Bologna and
was enrolled in the A6181036 protocol; commencing sunitinib,
initially at a daily dose of 50 mg (4w/2w) and subsequently at the
continuative dose of 37.5 mg/day. The CT scan revealed a clear
response to therapy, with a reduction in size of the primary lesion
and a decrease in tumour density of the remaining lesions. In
September 2008, due to the long-term stability of the disease, the
patient underwent a third surgical intervention (multiple wedge
resections) and surgery was considered to be complete.
Immunohistochemistry determined the diagnosis of a GIST, which was
characterized by a good histological response to therapy. Treatment
with sunitinib was continued following surgery and periodic CT
scans were performed until September 2010, when a liver relapse of
37 mm was detected in segment VIII. Since it was the only site of
relapse and there was no alternative approved therapy at that time,
a loco-regional treatment approach with radiofrequency (RFA) and/or
percutaneous ethanol injection (PEI) was adopted. Thus, in December
2010, considering the site and size of the lesion, an RFA + PEI
treatment was performed and the post-procedure CT scan revealed a
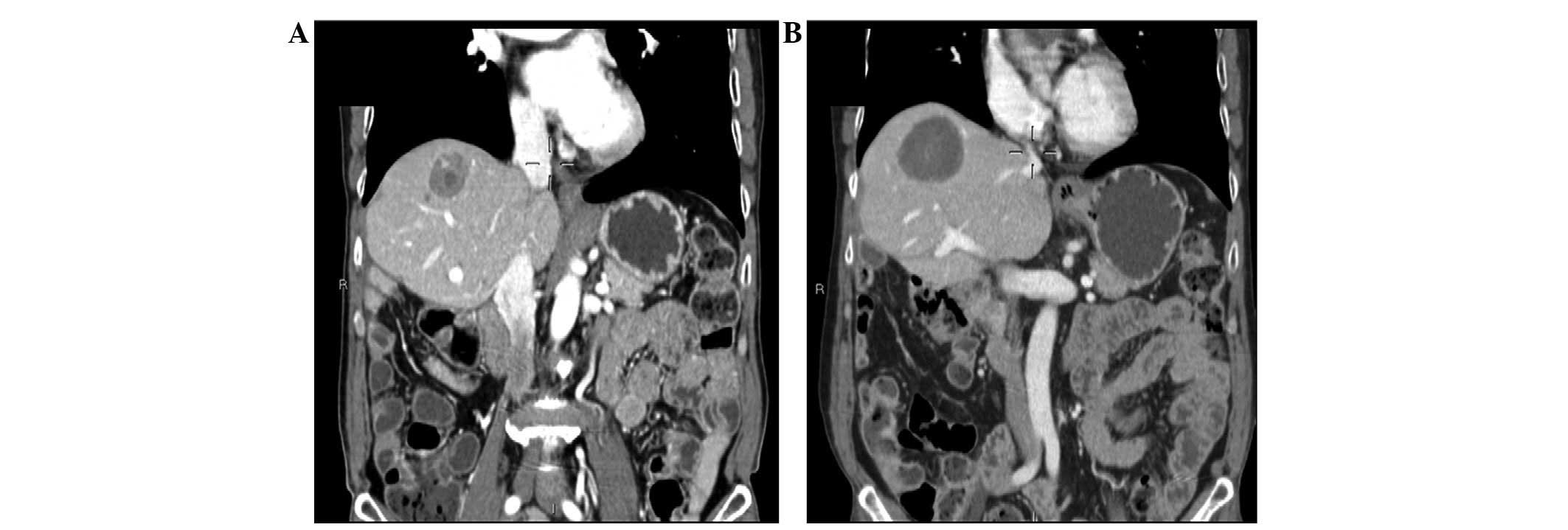
necrotic area without any sign of active disease (Fig. 3). This result has been maintained
over time and the sunitinib treatment has been continued.
Discussion
Sunitinib is the only approved second-line treatment
option for patients with advanced GIST, who are resistant or
intolerant to imatinib. It provides clinical benefit following the
failure of imatinib treatment and it has been shown to be more
active than first-line treatments in patients with wild-type GIST
and KIT exon 9 mutations (all of which are relatively resistant to
imatinib), as well as those with KIT exon 11 mutations.
Sunitinib-associated activity was also observed in patients with
secondary KIT mutations in exons 13 and 14 (23).
Sunitinib-associated AEs are generally mild to
moderate, however, in clinical practice, intolerance caused by
toxicities frequently leads to dose reductions and/or breaks in
treatment. Therefore, effective therapy management is key to avoid
inadequate dosages and loss of treatment efficacy.
With the advent of regorafenib, a newly approved
therapeutic agent for GISTs, the likelihood of treating this type
of tumour is improved. However, regorafenib is currently not
globally available and it is associated with considerable side
effects. In the GRID trial, 98% of patients presented
treatment-associated AEs of all grades and 61% presented serious
AEs, including hand-foot syndrome, skin reactions, diarrhoea,
abdominal pain and fever (21).
Hence, in current clinical practice, the most common
therapeutic alternatives upon sunitinib failure are as follows:
Rechallenge of imatinib; nilotinib; sorafenib; or the best
supportive care (24). Overall, the
clinical benefit rate (CBR), median progression free survival
(mPFS) and mOS that were obtained with those treatment options,
ranged between 11 and 42%, from 2.1 to 4.9 months, and from 2.4 to
11.8 months, respectively. These results, together with those
obtained with regorafenib, are summarized in Table II.
 | Table IIPatterns of the most common third-line
therapies and associated CBRs, mPFS and mOS. |
Table II
Patterns of the most common third-line
therapies and associated CBRs, mPFS and mOS.
| Treatment type, no.
of patients (ref) | CBR (%) | mPFS (months) | 95% CI | mOS (months) | 95% CI |
|---|
| Rechallenge with
imatinib, 40 (23) | 25 | 2.9 | 2.2–3.5 | 7.5 | 4.0–10.9 |
| Nilotinib, 67
(23) | 35 | 4.1 | 2.8–5.3 | 11.8 | 7.2–16.3 |
| Sorafenib, 55
(23) | 42 | 4.9 | 2.2–7.6 | 10.7 | 7.1–14.2 |
| Regorafenib, 133
(21) | 53 | 4.8 | 1.4–9.2 | - | - |
| Best supportive care,
18 (23) | 11 | 2.1 | 1.3–2.8 | 2.4 | 1.8–2.9 |
Three clinical cases have been presented in which
therapy, using sunitinib, was continued via the adoption of
alternative reduced schedules or an additional loco-regional
treatment, in order to manage toxicities or overcome PD. These
decisions were predominantly driven by the absence of alternative
approved therapeutic agents at the time of progression or due to an
intolerance to sunitinib. Furthermore, the selection of alternative
treatments was reinforced by the long response that was previously
obtained from administering sunitinib to these patients.
In case 1, an elderly women affected by a metastatic
GIST at diagnosis was treated with sunitinib for 46 months in
total. Following the first cycle, a dose reduction, initially to 25
mg/day, then to 25 mg/day 1d/1d was prescribed (due to grade 3
hematologic AEs) and improved the bone marrow toxicity profile,
whilst maintaining disease control until the present.
Although evaluation of sunitinib blood levels was
not conducted in the patients in the current study, it is likel
that adequate sunitinib levels to prevent PD have been maintained,
despite does reduction, due to: i) Old age (88-years-old at the
time of switching to an alternative schedule) with consequent
age-associated decline in sunitinib metabolism, and changes in the
therapeutic agent and the availability of metabolites (25); and ii) low body mass index (BMI) of
17.98.
Concerning BMI, certain available results
demonstrate the importance of adapting the dosage of cytotoxic
chemotherapy to weight, or derivatives of weight, such as BMI,
whilst a small quantity of data are available regarding BMI and
targeted therapies (26,27). Thus, sunitinib is applied at a
constant dose of 50 mg/day, 4w/2w or 37.5 mg/day continuously, not
accounting for several sources of interindividual variance, such as
body size, which are often considerable, explaining differences in
therapeutic agent concentration, metabolism and ultimately
tolerance. This is an interesting and novel area of research,
however, further studies are required to obtain definitive
results.
In the next case reported, a 70-year-old female
showed rapid PD during imatinib treatment (13 months). Conversely,
the patient obtained good disease control when administered with
sunitinib. However, due to diarrhoea and HTN, the patient required
treatment adjustment to 25 mg/day. Again, the patient’s tolerance
improved and disease control was preserved.
The clinical history of this patient during
sunitinib treatment was marked by severe HTN. This AE is
characterised in the most recent literature as a significant
biomarker of sunitinib efficacy (28,29).
In addition to hypothyroidism and hand-foot syndrome, it is defined
as a mechanism-based toxicity as it is caused by the mechanism of
action of sunitinib (30,31).
HTN affects 11–28% of GIST patients and generally
begins at the end of the first or second treatment cycle (10,12).
Hypotheses have been proposed concerning the occurrence of this
event, according to which the administration of sunitinib may lead
to an increase in vascular resistance by reducing the production of
nitric oxide. Furthermore, inhibition of VEGFR by sunitinib may
result in a density decrease of the small arterioles and
capillaries (vascular rarefaction) (32).
HTN has recently been shown to correlate with the
clinical outcome in mRCC (28).
Subsequently, a retrospective analysis of three phase I–III trials
(319 patients) was conducted to examine the correlations between
sunitinib-associated HTN and antitumour efficacy in GIST (29). The results of the study showed that
HTN correlates with an improved overall response rate [16% in
patients with HTN vs. 3% in patients without HTN (P=0.004)], PFS
[34 weeks in patients with HTN vs. 16 weeks in patients without HTN
(P<0.0001)] and OS [87 weeks in patients with HTN vs. 53 weeks
in patients with no HTN (P=0.0003)].
In the last case, a 67-year-old male who benefited
from sunitinib for a particularly long duration (50 months) was
described; complete liver surgery was planned 26 months following
the initiation of sunitinib. No relevant AE arose other than
moderate fatigue. Thus, with the onset of a single liver
metastasis, a current treatment was integrated using a
loco-regional approach, rather than considering a rechallenge of
imatinib or searching for novel experimental therapeutic
agents.
Liver surgery combined with systemic therapy is an
established technique to improve the outcome of patients affected
by metastases from multiple tumours. The same approach has been
investigated in GISTs using surgery/TKI therapy integration, which
showed favourable results, especially in imatinib-responder
patients (33,34). A small number of cases of
sunitinib-responders have also been reported (35).
RFA appears to be an interesting option for the
treatment of small-size liver GIST metastases. It has shown
encouraging results in primary and metastatic liver tumours
measuring ≤3 cm, obtaining a rate of local control that is
equivalent to that of surgery resection, with reduced morbidity and
mortality rates. A retrospective study was conducted to assess the
role of RFA in the multimodality management of GIST, demonstrating
that RFA is a feasible, safe and useful option in patients with
liver metastasis of GIST (36).
This is the case, particularly when performed upon achievement of
the optimum clinical response to TKIs and in combination with
post-RFA resumption of the therapeutic agent. In the present case,
RFA was used against local progression under sunitinib therapy,
with the aim of ablating individual lesions, which developed a
resistance to sunitinib prior to spreading, thus allowing the
continuation and prolonging the efficacy of the second-line
systemic therapy. As a result of this, the patient was able to
continue sunitinib to date, maintaining disease control for an
additional 30 months following thermoablation.
In conclusion, sunitinib represents an effective
therapeutic treatment against GISTs, exhibiting a direct antitumour
and antiangiogenic effect. This is particularly true in a subgroup
of patients whose boundaries have not yet been precisely
identified. However, sunitinib treatment is characterized by
multiple, varying AEs. The present report of clinical cases and the
observations regarding dose adjustment and the dose/efficacy
correlation may facilitate the management of patients who are
affected by GISTs. Notably, the patients in the current report are
representative of the general population receiving sunitinib.
The decision to continue administering sunitinib
(despite PD) by using alternative schedules to overcome AEs was due
to the following: i) A lack of approved third-line therapies; and
ii) the poor likelihood of restoring the efficacy of an imatinib
rechallenge considering the patient’s mutational status, and
previous response and tolerability to first-line therapies.
These observations remain relevant, when the
increasing knowledge on this rare type of tumour drives the
development and evaluation of TKIs. Furthermore, physicians may
consider an increasingly wide spectrum of treatment options, basing
their decision on the specific characteristics and clinical history
of each patient, with the aim of maximizing the duration of each
therapeutic method and, ultimately, the overall sequential
treatment strategy.
References
|
1
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors - definition, clinical, histological,
immunohistochemical, and molecular genetic features and
differential diagnosis. Virchows Arch. 438:1–12. 2001.
|
|
2
|
Hirota S, Isozaki K, Moriyama Y, et al:
Gain-of-function mutations of c-kit in human gastrointestinal
stromal tumors. Science. 279:577–580. 1998.
|
|
3
|
Heinrich MC, Corless CL, Duensing A, et
al: PDGFRA activating mutations in gastrointestinal stromal tumors.
Science. 299:708–710. 2003.
|
|
4
|
Dematteo RP, Heinrich MC, El-Rifai WM and
Demetri G: Clinical management of gastrointestinal stromal tumors:
before and after STI-571. Hum Pathol. 33:466–477. 2002.
|
|
5
|
Edmonson JH, Marks RS, Buckner JC and
Mahoney MR: Contrast of response to dacarbazine, mitomycin,
doxorubicin, and cisplatin (DMAP) plus GM-CSF between patients with
advanced malignant gastrointestinal stromal tumors and patients
with other advanced leiomyosarcomas. Cancer Invest. 20:605–612.
2002.
|
|
6
|
Demetri GD, von Mehren M, Blanke CD, et
al: Efficacy and safety of imatinib mesylate in advanced
gastrointestinal stromal tumors. N Engl J Med. 347:472–480.
2002.
|
|
7
|
DeMatteo RP, Lewis JJ, Leung D, et al: Two
hundred gastrointestinal stromal tumors: recurrence patterns and
prognostic factors for survival. Ann Surg. 231:51–58. 2000.
|
|
8
|
Antonescu CR, Besmer P, Guo T, et al:
Acquired resistance to imatinib in gastrointestinal stromal tumor
occurs through secondary gene mutation. Clin Cancer Res.
11:4182–4190. 2005.
|
|
9
|
Mendel DB, Laird AD, Xin X, et al: In
vivo antitumor activity of SU11248, a novel tyrosine kinase
inhibitor targeting vascular endothelial growth factor and
platelet-derived growth factor receptors: determination of a
pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res.
9:327–337. 2003.
|
|
10
|
Demetri GD, van Oosterom AT, Garrett CR,
et al: Efficacy and safety of sunitinib in patients with advanced
gastrointestinal stromal tumor after failure of imatinib: a
randomised controlled trial. Lancet. 368:1329–1338. 2006.
|
|
11
|
Demetri GD, Huang X, Garrett CR, et al:
Novel statistical analysis of long-term survival to account for
crossover in a phase III trial of sunitinib (SU) vs. placebo (PL)
in advanced GIST after imatinib (IM) failure. J Clin Oncol.
26:105242008.
|
|
12
|
George S, Blay JY, Casali PG, et al:
Clinical evaluation of continuous daily dosing of sunitinib malate
in patients with advanced gastrointestinal stromal tumour after
imatinib failure. Eur J Cancer. 45:1959–1968. 2009.
|
|
13
|
Reichardt P, Kang Y, Ruka W, et al:
Detailed analysis of survival and safety with sunitinib (SU) in a
worldwide treatment-use trial of patients with advanced GIST. J
Clin Oncol. 26(Suppl 15): 105482008.
|
|
14
|
Houk BE, Bello CL, Poland B, et al:
Relationship between exposure to sunitinib and efficacy and
tolerability endpoints in patients with cancer: results of a
pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother
Pharmacol. 66:357–371. 2010.
|
|
15
|
Saponara M, Pantaleo MA, Nannini M and
Biasco G: Chronic therapy in gastrointestinal stromal tumors
(GISTs): the big gap between theory and practice. Target Oncol.
7:243–246. 2012.
|
|
16
|
Joensuu H, Trent JC and Reichardt P:
Practical management of tyrosine kinase inhibitor-associated side
effects in GIST. Cancer Treat Rev. 37:75–88. 2011.
|
|
17
|
Najjar YG, Elson P, Wood LS, et al:
Association of a 2-weeks-on and 1-week-off schedule of sunitinib
with decreased toxicity in metastatic renal cell carcinoma. J Clin
Oncol. 31(Suppl 6): 4062013.
|
|
18
|
Atkinson BJ, Kalra S, Wang X, Tannir NM
and Jonasch E: A single-center retrospective review of outcomes
associated with sunitinib alternative schedule compared to
traditional schedule. J Clin Oncol. 31(Suppl 6): 3812013.
|
|
19
|
Britten CD, Kabbinavar F, Hecht JR, et al:
A phase I and pharmacokinetic study of sunitinib administered daily
for 2 weeks, followed by a 1-week off period. Cancer Chemother
Pharmacol. 61:515–524. 2008.
|
|
20
|
Reichardt P, Kang YK, Rutkowski P, et al:
Continued sunitinib treatment after progressive disease (PD) in a
worldwide treatment-use trial of patients (pts) with
gastrointestinal stromal tumor (GIST). In: Poster presented at the
37th ESMO congress; Vienna, Austria. September 28–October 2, 2012;
(abstr 1490P).
|
|
21
|
Demetri GD, Reichardt P, Kang YK, et al:
GRID study investigators: Efficacy and safety of regorafenib for
advanced gastrointestinal stromal tumours after failure of imatinib
and sunitinib (GRID): an international, multicentre, randomised,
placebo-controlled, phase 3 trial. Lancet. 381:295–302. 2013.
|
|
22
|
National Cancer Institute. National Cancer
Institute common terminology criteria for adverse events version
4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Accessed June 14, 2010
|
|
23
|
Maleddu A, Pantaleo MA, Nannini M, et al:
Mechanisms of secondary resistance to tyrosine kinase inhibitors in
gastrointestinal stromal tumours (Review). Oncol Rep. 21:1359–1366.
2009.
|
|
24
|
Italiano A, Cioffi A, Coco P, et al:
Patterns of care, prognosis, and survival in patients with
metastatic gastrointestinal stromal tumors (GIST) refractory to
first-line imatinib and second-line sunitinib. Ann Surg Oncol.
19:1551–1559. 2012.
|
|
25
|
Kinirons MT and O’Mahony MS: Drug
metabolism and ageing. Br J Clin Pharmacol. 57:540–544. 2004.
|
|
26
|
Antoun S, Baracos VE, Birdsell L, Escudier
B and Sawyer MB: Low body mass index and sarcopenia associated with
dose-limiting toxicity of sorafenib in patients with renal cell
carcinoma. Ann Oncol. 21:1594–1598. 2010.
|
|
27
|
Telli ML, Witteles RM, Fisher GA and
Srinivas S: Cardiotoxicity associated with the cancer therapeutic
agent sunitinib malate. Ann Oncol. 19:1613–1618. 2008.
|
|
28
|
Rini BI, Cohen DP, Lu DR, et al:
Hypertension as a biomarker of efficacy in patients with metastatic
renal cell carcinoma treated with sunitinib. J Natl Cancer Inst.
103:763–773. 2011.
|
|
29
|
George S, Reichardt P, Lechner T, et al:
Hypertension as a potential biomarker of efficacy in patients with
gastrointestinal stromal tumor treated with sunitinib. Ann Oncol.
23:3180–3187. 2012.
|
|
30
|
Schmidinger M, Vogl UM, Bojic M, et al:
Hypothyroidism in patients with renal cell carcinoma: blessing or
curse? Cancer. 117:534–544. 2011.
|
|
31
|
Puzanov I, Michaelson MD, Cohen DP, et al:
Evaluation of hand-foot syndrome (HFS) as a potential biomarker of
sunitinib (SU) efficacy in patients (pts) with metastatic renal
cell carcinoma (mRCC) and gastrointestinal stromal tumor (GIST). J
Clin Oncol. 29(Suppl): 211132011.
|
|
32
|
Eskens FA and Verweij J: The clinical
toxicity profile of vascular endothelial growth factor (VEGF) and
vascular endothelial growth factor receptor (VEGFR) targeting
angiogenesis inhibitors; a review. Eur J Cancer. 42:3127–3139.
2006.
|
|
33
|
DeMatteo RP, Maki RG, Singer S, et al:
Results of tyrosine kinase inhibitor therapy followed by surgical
resection for metastatic gastrointestinal stromal tumor. Ann Surg.
245:347–352. 2007.
|
|
34
|
Gronchi A, Fiore M, Miselli F, et al:
Surgery of residual disease following molecular-targeted therapy
with imatinib mesylate in advanced/metastatic GIST. Ann Surg.
245:341–346. 2007.
|
|
35
|
Pantaleo MA, Di Battista M, Catena F, et
al: Surgical debulking of gastrointestinal stromal tumors: is it a
reasonable option after second-line treatment with sunitinib? J
Cancer Res Clin Oncol. 134:625–630. 2008.
|
|
36
|
Hakimé A, Le Cesne A, Deschamps F, et al:
A role for adjuvant RFA in managing hepatic metastases from
gastrointestinal stromal tumors (GIST) after treatment with
targeted systemic therapy using kinase inhibitors. Cardiovasc
Intervent Radiol. 37:132–139. 2014.
|