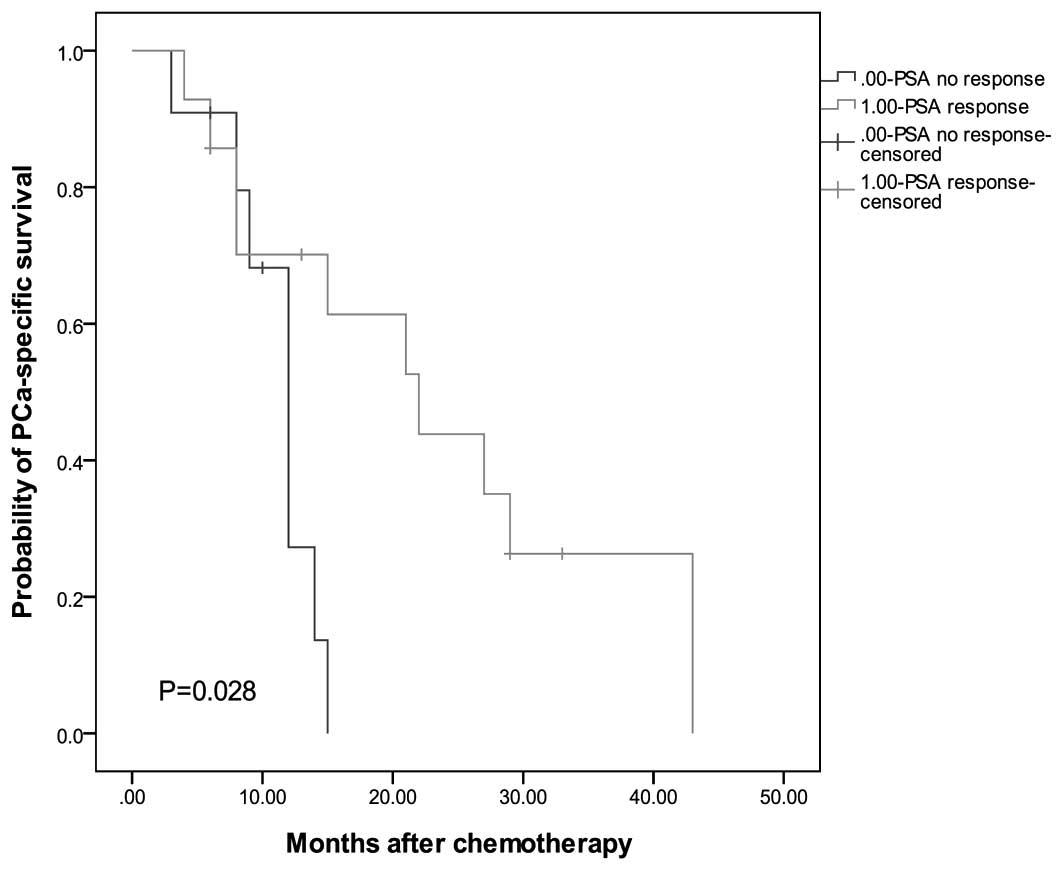
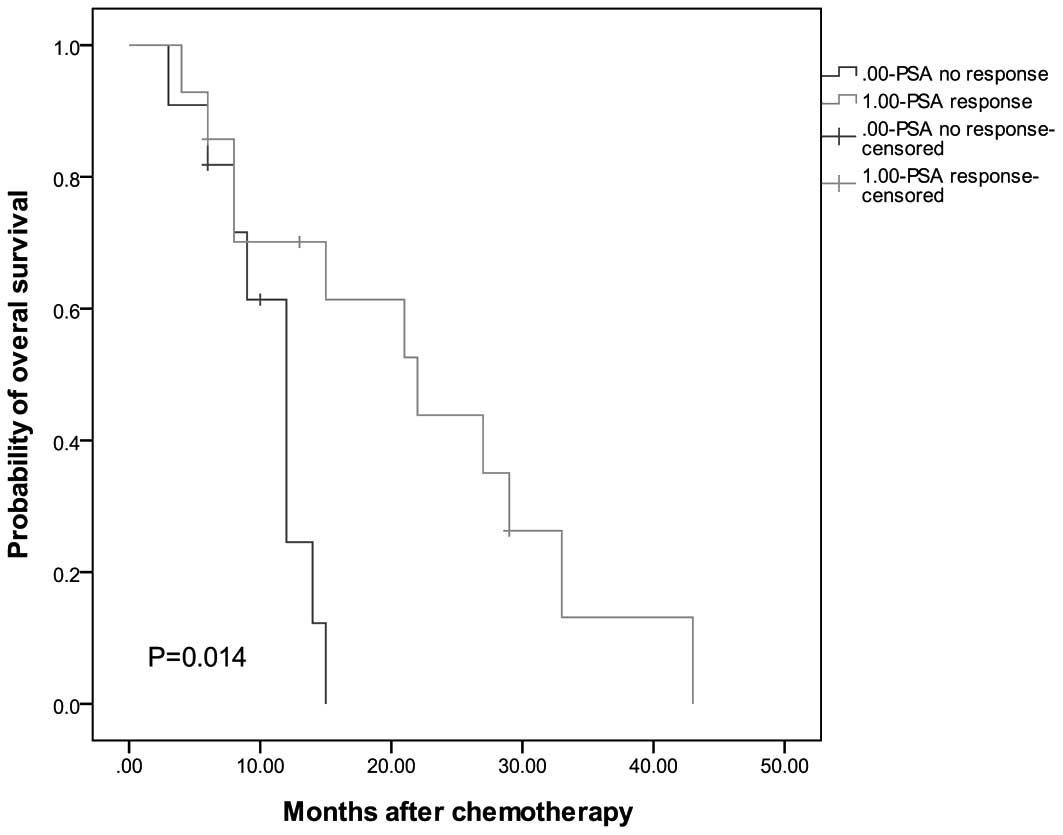
|
1
|
Matsuda T, Marugame T, Kamo K, et al:
Cancer incidence and incidence rates in Japan in 2005: based on
data from 12 population-based cancer registries in the Monitoring
of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol.
41:139–147. 2011.
|
|
2
|
Chen CH, Yao HH, Huang SW, et al: Using
age-referenced prostate-specific antigen percentile to predict
survival outcomes in screened Taiwanese men. Int J Cancer.
132:1927–1932. 2013.
|
|
3
|
Tannock IF, de Wit R, Berry WR, et al; TAX
327 Investigators. Docetaxel plus prednisone or mitoxantrone plus
prednisone for advanced prostate cancer. N Engl J Med.
351:1502–1512. 2004.
|
|
4
|
Kuramoto T, Inagaki T, Fujii R, et al:
Docetaxel in combination with estramustine and prednisolone for
castration-resistant prostate cancer. Int J Clin Oncol. 18:890–897.
2013.
|
|
5
|
Petrylak DP, Tangen CM, Hussain MH, et al:
Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004.
|
|
6
|
Mottet N, Bellmunt J, Bolla M, et al: EAU
guidelines on prostate cancer. Part II: Treatment of advanced,
relapsing, and castration-resistant prostate cancer. Eur Urol.
59:572–583. 2011.
|
|
7
|
Miura N, Numata K, Kusuhara Y, et al:
Docetaxel-prednisolone combination therapy for Japanese patients
with hormone-refractory prostate cancer: a single institution
experience. Jpn J Clin Oncol. 40:1092–1098. 2010.
|
|
8
|
National Cancer Institute. National Cancer
Institute common terminology criteria for adverse events version
4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Accessed April 20, 2013
|
|
9
|
Wilson C, Scullin P, Worthington J, et al:
Dexamethasone potentiates the antiangiogenic activity of docetaxel
in castration-resistant prostate cancer. Br J Cancer. 99:2054–2064.
2008.
|
|
10
|
Miyake H, Sakai I, Terakawa T, Harada K
and Fujisawa M: Oncological outcome of docetaxel-based chemotherapy
for Japanese men with metastatic castration-resistant prostate
cancer. Urol Oncol. 31:733–738. 2013.
|
|
11
|
Naito S, Tsukamoto T, Koga H, et al:
Docetaxel plus prednisolone for the treatment of metastatic
hormone-refractory prostate cancer: a multicenter Phase II trial in
Japan. Jpn J Clin Oncol. 38:365–372. 2008.
|
|
12
|
Lee JL, Kim JE, Ahn JH, et al: Efficacy
and safety of docetaxel plus prednisolone chemotherapy for
metastatic hormone-refractory prostate adenocarcinoma: single
institutional study in Korea. Cancer Res Treat. 42:12–17. 2010.
|
|
13
|
Nelius T, Klatte T, de Riese W and Filleur
S: Impact of PSA flare-up in patients with hormone-refractory
prostate cancer undergoing chemotherapy. Int Urol Nephrol.
40:97–104. 2008.
|
|
14
|
Thuret R, Massard C, Gross-Goupil M, et
al: The postchemotherapy PSA surge syndrome. Ann Oncol.
19:1308–1311. 2008.
|
|
15
|
Olbert PJ, Hegele A, Kraeuter P, et al:
Clinical significance of a prostate-specific antigen flare
phenomenon in patients with hormone-refractory prostate cancer
receiving docetaxel. Anticancer Drugs. 17:993–996. 2006.
|
|
16
|
Ide H, Kikuchi E, Kono H, et al: Docetaxel
in combination with prednisolone for hormone refractory prostate
cancer. Jpn J Clin Oncol. 40:79–84. 2010.
|
|
17
|
Pond GR, Armstrong AJ, Wood BA, et al:
Evaluating the value of number of cycles of docetaxel and
prednisone in men with metastatic castration-resistant prostate
cancer. Eur Urol. 61:363–369. 2012.
|
|
18
|
Masago T, Watanabe T, Nemoto R and Motoda
K: Interstitial pneumonitis induced by bicalutamide given for
prostate cancer. Int J Clin Oncol. 16:763–765. 2011.
|
|
19
|
Matsumoto A, Inoue A, Yokoi S, et al:
Evaluation of docetaxel plus estramustine in the treatment of
patients with hormone-refractory prostate cancer. Int J Urol.
16:687–691. 2009.
|
|
20
|
Qi WX, Shen Z and Yao Y: Docetaxel-based
therapy with or without estramustine as first-line chemotherapy for
castration-resistant prostate cancer: a meta-analysis of four
randomized controlled trials. J Cancer Res Clin Oncol.
137:1785–1790. 2011.
|
|
21
|
Huang SP, Bao BY, Wu MT, et al: Impact of
prostate-specific antigen (PSA) nadir and time to PSA nadir on
disease progression in prostate cancer treated with
androgen-deprivation therapy. Prostate. 71:1189–1197. 2011.
|
|
22
|
Qu YY, Dai B, Kong YY, Ye DW, Yao XD,
Zhang SL, et al: Prognostic factors in Chinese patients with
metastatic castration-resistant prostate cancer treated with
docetaxel-based chemotherapy. Asian J Androl. 15:110–115. 2013.
|