Introduction
The sextant method for performing a prostate biopsy
was introduced by Hodge et al (1) in 1989. Various studies have
demonstrated that extra cores improve the prostate cancer detection
rate (PCDR) (2–7). In previous years, prostate biopsies
were guided by the finger of the operator, however, transrectal
ultrasound (TRUS) is a simple and useful tool that may be used for
detecting and observing prostate tissues. In recent years, the
TRUS-guided biopsy has generally been adopted when a prostate
biopsy is required (1,8,9).
Recently, magnetic resonance imaging (MRI)-guided and
robotic-assisted prostate biopsies have been attempted at various
advanced medical centers (10,11);
however, TRUS-guided prostate biopsy remains the standard regimen
for prostate cancer detection at the majority of medical centers.
The optimal number of biopsy cores and distribution, however,
remains controversial.
In the present study, the 13-year data of finger-
and TRUS-guided biopsies, which were conducted at the Department of
Urology, The First Affiliated Hospital of Nanjing Medical
University (Nanjing, China) were retrospectively analyzed. The
value of entire 13-core biopsy, guided by either TRUS or MRI, with
regard to the PCDR was evaluated; particularly the extra 13th core,
which revealed abnormal TRUS or MRI findings. To the best of our
knowledge, it is the largest and longest single-center study
regarding prostate biopsies in a Han Chinese population.
Patients and methods
Patients
Between July 1999 and June 2012, 2,707 patients from
the Han Chinese population were recruited for a prostate biopsy at
the Department of Urology, The First Affiliated Hospital of Nanjing
Medical University. All patients underwent a digital rectal
examination (DRE), serum prostate-specific antigen (PSA) and free
PSA (fPSA) detection and TRUS to assess the prostate volume (PV)
prior to the biopsy. PSA density (PSAD) was defined as the ratio of
PSA to PV and the f/t ratio was calculated as fPSA divided by PSA.
A finger-guided biopsy was performed on 1,603 patients prior to
July 2009 and 1,104 patients underwent TRUS-guided biopsy after
June 2009. In addition, 60 patients underwent prostate MRI as well
as TRUS after March 2012.
Approval for the study was granted by the ethics
committee of Nanjing Medical University and written informed
consent was obtained from all patients.
Biopsy procedures
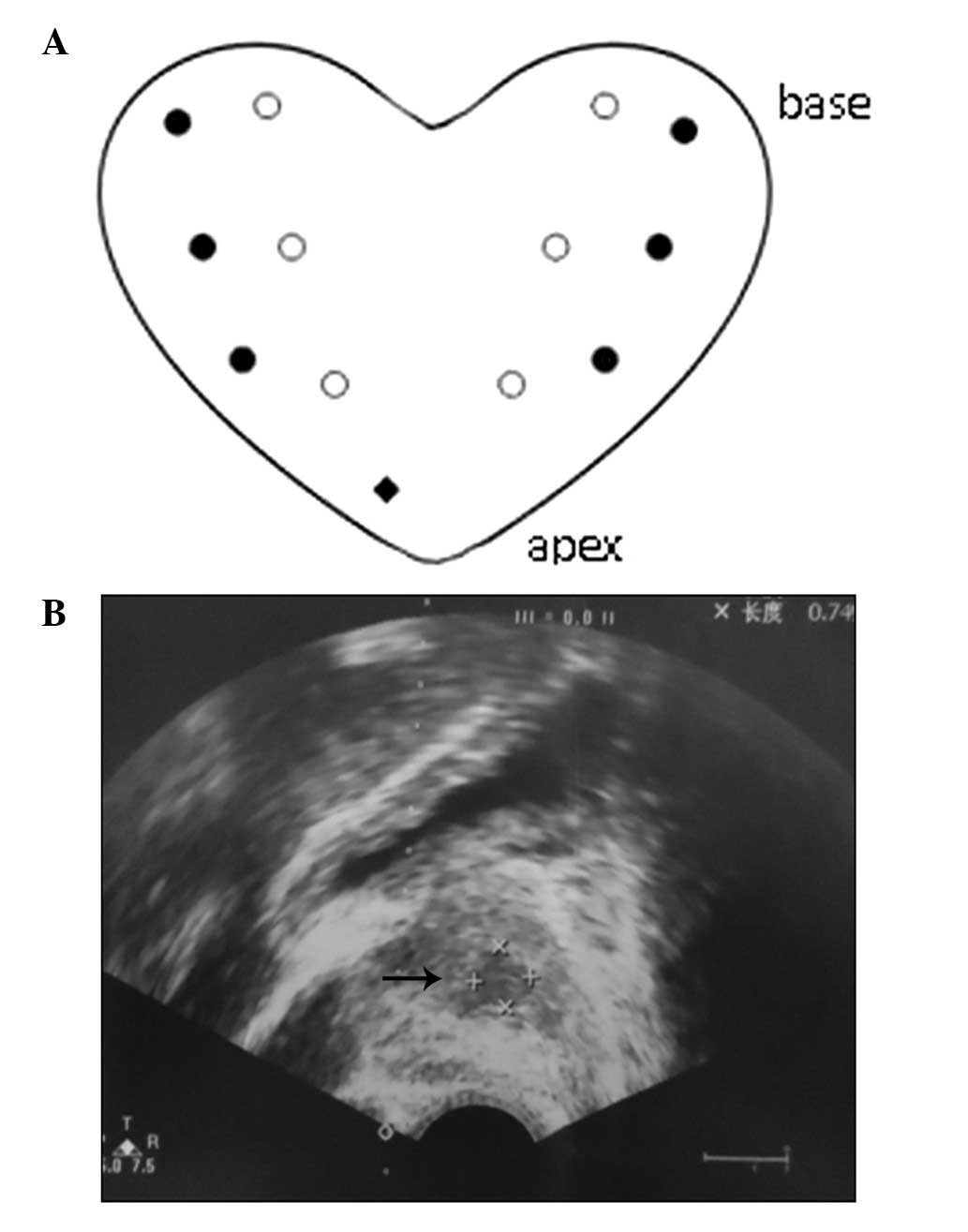
The prostate biopsies were performed as systematic
12-core biopsies and for the TRUS-guided biopsy, an extra 13th core
was added. The 12 cores were evenly distributed around four
vertical planes: Right lateral, right medial, left medial and left
lateral. Three biopsy cores from each plane were respectively
located at the apex, middle and base of the prostate. The extra
13th core was directed towards the hypoechoic lesions on the TRUS
image. In the patients that exhibited an abnormal MRI signal, the
extra 13th core was directed towards the prostate area where the
MRI demonstrated the lesions. For patients with normal TRUS and MRI
images, the extra 13th core was positioned at the apex of the
prostate (Fig. 1). The number and
distribution of the positive cores were recorded. In order to
perform further analyses, the distribution of positive cores were
analyzed retrospectively, and it was assumed that all patients had
undergone four biopsy schemes: Medial 6-core, lateral 6-core,
12-core and entire 13-core (Fig.
1). The data from each of these hypothetical biopsy schemes
were compared. The positive rate of the 13th core was compared with
the mean positive rate of a systematic 12-core biopsy (mean number
of positive cores divided by 12) in patients with confirmed
prostate cancer in order to evaluate the value of the extra 13th
core.
Statistical analysis
Data were expressed as the mean ± standard deviation
and analyzed using SPSS software (version 18.0; SPSS Inc., Chicago,
IL, USA). Differences between the PCa and non-PCa groups were
assessed by the t-test and the χ2 test was used to
compare nonparametric variables. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient demographics and clinical
characteristics
The demographic and clinical characteristics of
2,707 patients are presented in Table
I. 36.2% (979/2,707) of the patients were confirmed with
prostate cancer. The PCDR of the finger- and TRUS-guided biopsies
was 32.5% (521/1,603; data not shown) and 41.5% (458/1,104),
respectively.
 | Table IDemographic and clinical
characteristics of 2,707 patients. |
Table I
Demographic and clinical
characteristics of 2,707 patients.
| Prostate cancer
detection rate | |
|---|
|
| |
|---|
| Variable | Negative, n (%) | Positive, n (%) | P-value |
|---|
| PSA, ng/ml | | | <0.001 |
| 0–4 | 140 (87.0) | 21 (13.0) | |
| 4.01–10 | 664 (80.4) | 162 (19.6) | |
| 10.01–20 | 602 (72.8) | 225 (27.2) | |
| 20.01–30 | 182 (58.3) | 130 (41.7) | |
| >30 | 140 (24.1) | 441 (75.9) | |
| Age, years | 68.3±8.12 | 71.1±7.12 | 0.008 |
| fPSA, ng/ml | 2.3±3.35 | 8.5±36.40 | <0.001 |
| PV,
cm3 | 52.28±29.25 | 41.27±22.85 | <0.001 |
| f/t ratio | 0.17±0.098 | 0.12±0.072 | <0.001 |
| PSAD,
ng/ml/cm3 | 0.32±0.42 | 2.04±9.36 | <0.001 |
| DRE finding | | | <0.001 |
| Negative | 1525 (76.9) | 457 (23.1) | |
| Positive | 203 (28.0) | 522 (72.0) | |
| Echo level | | | <0.001 |
| Regular | 730 (72.0) | 284 (28.0) | |
| Irregular | 998 (58.9) | 695 (41.1) | |
| Hypoechoic | | | <0.001 |
| Negative | 1342 (77.3) | 393 (22.7) | |
| Positive | 386 (39.7) | 586 (60.3) | |
|
Microcalcification | | | <0.001 |
| Negative | 1282 (71.4) | 513 (28.6) | |
| Positive | 446 (48.9) | 466 (51.1) | |
PCDR of the finger- and TRUS-guided
biopsies
The PCDR of the finger- and TRUS-guided biopsies in
different PSA and PV subgroups was further analyzed (Table II). In the patients with PSA ≤30
ng/ml or PV >46 cm3, the PCDR of the TRUS-guided
biopsy was found to be significantly higher than that of the
finger-guided biopsy (30.0% vs. 22.2%, P<0.001 and 31.7% vs.
18.1%, respectively). There was no statistical difference
identified in the PCDR at PSA >30 ng/ml (79.1% vs. 73.4%,
P=0.111) or PV ≤46 cm3 (46.8% vs. 44.3%, P=0.336).
 | Table IIProstate cancer detection rate of
finger- and TRUS-guided biopsy stratified by PSA values and the
PVs. |
Table II
Prostate cancer detection rate of
finger- and TRUS-guided biopsy stratified by PSA values and the
PVs.
| Prostate cancer
detection rate | |
|---|
|
| |
|---|
| Variable | Negative, n (%) | Positive, n (%) | P-value |
|---|
| PSA, ng/ml |
| 0–30 | | | <0.001 |
| Finger-guided | 996 (77.8) | 284 (22.2) | |
| TRUS-guided | 592 (70.0) | 254 (30.0) | |
| >30 | | | 0.111 |
| Finger-guided | 86 (26.6) | 237 (73.4) | |
| TRUS-guided | 54 (20.9) | 204 (79.1) | |
| PV,
cm3 |
| 0–46 | | | 0.336 |
| Finger-guided | 490 (55.7) | 390 (44.3) | |
| TRUS-guided | 379 (53.2) | 334 (46.8) | |
| >46 | | | <0.001 |
| Finger-guided | 592 (81.9) | 131 (18.1) | |
| TRUS-guided | 267 (68.3) | 124 (31.7) | |
PCDR of various TRUS-guided biopsies
Table III shows
the PCDR of the hypothetical medial 6-core, lateral 6-core, 12-core
and entire 13-core TRUS-guided biopsies. The PCDR of the medial
6-core biopsy was identified to be significantly inferior to the
lateral 6-core and 12-core biopsies (32.2% vs. 37.0%, P=0.020 and
32.2% vs. 40.7%, p<0.001, respectively). However, there was no
obvious difference in the PCDR between the lateral 6-core and
12-core biopsies (37.0% vs. 40.7%, P=0.081). The PCDR of the entire
13-core biopsy was found to be significantly higher than the
lateral 6-core biopsy (41.5% vs. 37.0%, P=0.033), although there
was no obvious difference when compared with the 12-core biopsy
(41.5% vs. 40.7%, P=0.729).
 | Table IIIProstate cancer detection rate of
hypothetical medial 6-core, lateral 6-core, 12-core and entire
13-core biopsies guided by transrectal ultrasound. |
Table III
Prostate cancer detection rate of
hypothetical medial 6-core, lateral 6-core, 12-core and entire
13-core biopsies guided by transrectal ultrasound.
| Prostate cancer
detection rate | |
|---|
|
| |
|---|
| Variable | Negative, n (%) | Positive, n (%) | P-value |
|---|
| Medial 6-core vs.
lateral 6-core | | | 0.020 |
| Medial 6-core | 749 (67.8) | 355 (32.2) | |
| Lateral 6-core | 696 (63.0) | 408 (37.0) | |
| Medial 6-core vs.
12-core | | | <0.001 |
| Medial 6-core | 749 (67.8) | 355 (32.2) | |
| 12-core | 655 (59.3) | 449 (40.7) | |
| Lateral 6-core vs.
12-core | | | 0.081 |
| Lateral 6-core | 696 (63.0) | 408 (37.0) | |
| 12-core | 655 (59.3) | 449 (40.7) | |
| Lateral 6-core vs.
entire 13-core | | | 0.033 |
| Lateral 6-core | 696 (63.0) | 408 (37.0) | |
| Entire 13-core | 646 (58.5) | 458 (41.5) | |
| 12-core vs. entire
13-core | | | 0.729 |
| 12-core | 655 (59.3) | 449 (40.7) | |
| Entire 13-core | 646 (58.5) | 458 (41.5) | |
Positive rate of the 13th core in TRUS-
or MRI-guided biopsy
Table IV
demonstrates the positive rate of the extra 13th core and the mean
positive rate of the systematic 12-core in patients with confirmed
prostate cancer, guided by TRUS or MRI. In 151 patients with
hypoechoic lesions identified on the TRUS image, the positive rate
of the extra 13th core was 70.9%. The 32 patients out of the total
60 who also underwent MRI exhibited abnormal signals, in which the
positive rate of the extra 13th core was 81.2%. The mean number of
positive cores in each patient undergoing TRUS-guided biopsy was
6.8, thus, the mean positive rate of the systematic 12-core biopsy
was 56.7% (6.8/12). The positive rate of the extra 13th core, which
was directed towards the abnormal TRUS or MRI findings, was found
to be significantly higher than the mean positive rate of the
systematic 12-core biopsy (70.9% vs. 56.6%, P<0.001 and 81.2%
vs. 56.6%, P=0.006, respectively). Although the MRI-guided biopsy
was associated with a higher PCDR than the TRUS-guided biopsy, the
difference was not identified to be significant (81.2% vs. 70.9%,
P=0.280).
 | Table IVPositive rate of the 13th core that
was directed towards abnormal TRUS or MRI findings and the mean
positive rate of systematic 12-core biopsy in patients with
confirmed prostate cancer. |
Table IV
Positive rate of the 13th core that
was directed towards abnormal TRUS or MRI findings and the mean
positive rate of systematic 12-core biopsy in patients with
confirmed prostate cancer.
| Prostate cancer
detection rate | |
|---|
|
| |
|---|
| Variable | Negative, n (%) | Positive, n (%) | P-value |
|---|
| TRUS-guided | | | <0.001 |
| Mean positive
rate | 2387 (43.4) | 3109 (56.6) | |
| Extra 13th core | 44 (29.1) | 107 (70.9) | |
| MRI-guided | | | 0.006 |
| Mean positive
rate | 2387 (43.4) | 3109 (56.6) | |
| Extra 13th core | 6 (18.8) | 26 (81.2) | |
| TRUS vs. MRI | | | 0.280 |
| TRUS | 44 (29.1) | 107 (70.9) | |
| MRI | 6 (18.8) | 26 (81.2) | |
Discussion
The present study summarizes the 13-year experience
of prostate biopsies on a large, Han Chinese population at the
Department of Urology, The First Affiliated Hospital of Nanjing
Medical University. TRUS-guided biopsies have been widely adopted
in advanced medical centers in China; however, finger-guided biopsy
continues to be performed at certain primary hospitals. The present
data demonstrates that TRUS-guided biopsy is superior when compared
with finger-guided biopsy with regard to PCDR, particularly in
patients with PSA ≤30 ng/ml or PV >46 cm3. Due to
improvements in economic and health conditions, routine PSA
screening and TRUS examinations have been introduced in elder
males. Therefore, an increasing number of PCa patients have been
detected in the early stage, and the overall PSA level has
decreased. As a result of this, the use of TRUS-guided biopsy
should be encouraged in developing countries, such as China. In
certain advanced medical centers, MRI or other tools, such as
elastography and contrast-enhanced TRUS, are also considered for
assisting with biopsies.
Although novel biopsy tools and methods have been
approved quickly, the optimal number of cores and distribution for
conducting prostate biopsies remain controversial. Numerous studies
proposed that the PCDR increases as the number of biopsy cores
increases. Elabbady et al (12) reported that the 12-core biopsy
increased the PCDR from 25.8% to 36.4% during a comparison with
6-core biopsy. Similarly, the PCDR was improved from 7.7% to 13.8%
in the studies of Kojima et al (13) and Matsumoto et al (14). Certain studies showed different
conclusions. In a randomized trial conducted by Naughton et
al (8) no significant
difference in PCDR between 6-core and 12-core biopsies was found.
However, in the study by Kim et al (15), the PCDR of 12-core biopsy was
identified to be lower than that of the 6-core biopsy (14.4% vs.
17.2%). In the current study, the lateral 6-core and 12-core
biopsies were associated with a higher PCDR when compared with that
of the medial 6-core biopsy. This may have been due to the prostate
cancer predominantly occurring in the prostatic peripheral zone.
The distribution of the cores in the present study were directed by
TRUS and the results demonstrate that the distribution of biopsy
cores is important when detecting the lesions. This may explain why
certain studies found a significant improvement in the PCDR in
cases where more biopsy cores were used, while other studies showed
negative results. Notably, the 12-core biopsy did not demonstrate
obvious superiority when compared with lateral 6-core biopsy,
although the 12-core biopsy did exhibit a higher PCDR. This may be
due to the positive rate of the medial 6-core biopsy, which reduced
the difference in positive detection rates between the 12-core and
lateral 6-core biopsies. Thus, the results identified the critical
value of lateral biopsy cores in prostate cancer detection.
The PCDR of the entire 13-core biopsy was comparable
with the 12-core biopsy, which appeared to demonstrate that the
extra 13th core was insignificant. However, the entire 13-core
biopsy was significantly superior with regard to PCDR, when
compared with the lateral 6-core biopsy. During the biopsy
procedures, particularly the 12-core biopsy, one or two cores were
located in the areas that exhibited abnormal TRUS or MRI findings.
This may explain the similar PCDR between the entire 13-core biopsy
and the 12-core biopsy. Further analysis demonstrated that the
positive rate of the extra 13th core, which was directed towards
the hypoechoic lesions on the TRUS image or the abnormal MRI
signal, was significantly higher than the mean positive rate of the
systematic 12-core biopsy. This verified that the areas with
abnormal TRUS or MRI findings exhibited a higher positive rate of
cancer than other areas. Therefore, the systematic 12-core biopsy
plus the extra 13th core biopsy was beneficial for improving the
PCDR.
In conclusion, the positive rate of the extra 13th
core, which was directed towards the abnormal MRI signal showed an
insignificant superiority when compared with the hypoechoic lesions
that were observed on the TRUS image. MRI has an obvious advantage
over TRUS when detecting and observing prostate tissues. However,
MRI was not used directly to guide the biopsy in the current study,
which is a limitation of the study. Another limitation was the
restricted number of cases included in the present study. Only 32
patients were included for the analysis, thus, an increased number
of cases and the performance of genuine MRI-guided biopsies are
required in future studies to evaluate the value of MRI in
determining PCDR.
Acknowledgements
The present study was supported by grants from The
Program for Development of Innovative Research Team of the First
Affiliated Hospital of Nanjing Medical University (Nanjing, China),
the Provincial Initiative Program for Excellency Disciplines
(Jiangsu, China), the National Natural Science Foundation of China
(grant nos. 81201571 and 81201998) and by a project funded by the
Priority Academic Program Development of Jiangsu Higher Education
Institutions (grant no. JX10231801).
References
|
1
|
Hodge KK, McNeal JE and Stamey TA:
Ultrasound guided transrectal core biopsies of the palpably
abnormal prostate. J Urol. 142:66–70. 1989.
|
|
2
|
Terris MK, Wallen EM and Stamey TA:
Comparison of mid-lobe versus lateral systematic sextant biopsies
in the detection of prostate cancer. Urol Int. 59:239–242.
1997.
|
|
3
|
Eskew LA, Bare RL and McCullough DL:
Systematic 5 region prostate biopsy is superior to sextant method
for diagnosing carcinoma of the prostate. J Urol. 157:199–203.
1997.
|
|
4
|
Chang JJ, Shinohara K, Bhargava V and
Presti JC Jr: Prospective evaluation of lateral biopsies of the
peripheral zone for prostate cancer detection. J Urol.
160:2111–2114. 1998.
|
|
5
|
Babaian RJ, Toi A, Kamoi K, Troncoso P,
Sweet J, Evans R, Johnston D and Chen M: A comparative analysis of
sextant and an extended 11-core multisite directed biopsy strategy.
J Urol. 163:152–157. 2000.
|
|
6
|
Stamatiou K, Alevizos A, Karanasiou V,
Mariolis A, Mihas C, Papathanasiou M, Bovis K and Sofras F: Impact
of additional sampling in the TRUS-guided biopsy for the diagnosis
of prostate cancer. Urol Int. 78:313–317. 2007.
|
|
7
|
Inahara M, Suzuki H, Kojima S, Komiya A,
Fukasawa S, Imamoto T, Naya Y and Ichikawa T: Improved prostate
cancer detection using systematic 14-core biopsy for large prostate
glands with normal digital rectal examination findings. Urology.
68:815–819. 2006.
|
|
8
|
Naughton CK, Smith DS, Humphrey PA,
Catalona WJ and Keetch DW: Clinical and pathologic tumor
characteristics of prostate cancer as a function of the number of
biopsy cores: a retrospective study. Urology. 52:808–813. 1998.
|
|
9
|
Levine MA, Ittman M, Melamed J and Lepor
H: Two consecutive sets of transrectal ultrasound guided sextant
biopsies of the prostate for the detection of prostate cancer. J
Urol. 159:471–476. 1998.
|
|
10
|
Ehdaie B and Shariat SF: Magnetic
resonance imaging-targeted prostate biopsy: back to the future. Eur
Urol. 63:141–144. 2013.
|
|
11
|
Ho H, Yuen JS, Mohan P, Lim EW and Cheng
CW: Robotic transperineal prostate biopsy: pilot clinical study.
Urology. 78:1203–1208. 2011.
|
|
12
|
Elabbady AA and Khedr MM: Extended 12-core
prostate biopsy increases both the detection of prostate cancer and
the accuracy of Gleason score. Eur Urol. 49:49–53. 2006.
|
|
13
|
Kojima M, Hayakawa T, Saito T, Mitsuya H
and Hayase Y: Transperineal 12-core systematic biopsy in the
detection of prostate cancer. Int J Urol. 8:301–307. 2001.
|
|
14
|
Matsumoto K, Satoh T, Egawa S, Shimura S,
Kuwao S and Baba S: Efficacy and morbidity of transrectal
ultrasound-guided 12-core biopsy for detection of prostate cancer
in Japanese men. Int J Urol. 12:353–360. 2005.
|
|
15
|
Kim JW, Lee HY, Hong SJ and Chung BH: Can
a 12 core prostate biopsy increase the detection rate of prostate
cancer versus 6 core? : a prospective randomized study in Korea.
Yonsei Med J. 45:671–675. 2004.
|