Introduction
The phosphatidylinositol 3-kinase (PI3K) pathway
functions in cell proliferation, migration and survival (1,2).
Mutations of several components of the signaling pathway have been
shown to lead to tumor progression in numerous cancer types,
including glioblastoma (3), breast
(4), ovarian (5), endometrial (6), lung (7) and thyroid (8). PI3Ks, major signaling hubs, are
heterodimeric lipid kinases consisting of the p110 catalytic
subunit and the p85 regulatory subunit, which is encoded by one of
three gened; α, β and γ. p85 has two Src homology 2 (SH2) domains
and an inter-SH2 domain that binds to the p110 catalytic subunit
(1). The interaction between p85
and p110 has effects on the activity of p110, and results in
alterations to downstream signaling.
Previous studies have reported the association
between p85 isoforms and various cancers. Jaiswal et al
(9) indicated that p85α mutants
promote cell survival, Akt activation, anchorage-independent cell
growth and oncogenesis. It was found that mutations in p85α
abrogate its inhibitory effects on p110 from the stabilization
activity, resulting in p110-dependent survival signaling. Sun et
al (10) showed that expression
of mutant p85 protein in chicken embryonic fibroblasts induced
oncogenic transformation and increased proliferation. p85β
expression has additionally been shown to be elevated in breast and
colon carcinomas, and its increased levels correlate with PI3K
pathway activation and tumor progression (11). p85α has been proposed to exert tumor
suppressor properties based on observations in mice with a
liver-specific deletion of the Pik3r1 gene, which encodes
p85 (12). It has also been
demonstrated that inhibition of p85 activity by phosphopeptide 1257
(P-1257) delivery in vivo can significantly inhibit the
proliferation of tumor cells (13).
These studies may suggest that p85 is closely associated with tumor
development, and may therefore be a potential target for
therapeutic approaches. This previous research was preclinical and
focused on cells or animals that did not show an association
between p85 and cancer prognosis.
In the present study, p85 protein expression was
analyzed by immunohistochemistry (IHC) in 126 primary breast tumors
to elucidate the association between p85 expression and the
prognosis of patients.
Materials and methods
Patients
One hundred and twenty six primary invasive breast
carcinoma specimens were obtained from patients admitted between
2002 and 2005 to Beijing Chao-Yang Hospital, affiliated to the
Capital Medical University of China (Beijing, China). The median
age was 53 years (range, 27–84 years). A clinical history,
treatment information and outcomes for each of the patients were
obtained. Disease staging was performed according to the criteria
of the American Joint Committee on Cancer (AJCC) TNM stage
classification, seventh edition (2010) for breast cancer.
Disease-free survival (DFS) was defined as the time from the date
of diagnosis to the appearance of a regional recurrence or distant
metastasis. Overall survival (OS) was defined as the duration from
the date of diagnosis to the death of the patient due to breast
cancer. The study was approved by the ethics committee of Beijing
Chao-Yang Hospital, Capital Medical University (Beijing, China) and
patients provided written informed consent.
IHC and scoring
Immunohistochemical staining was performed by the
immuno-bridge method in formalin-fixed paraffin tissue sections (4
μm). Sections were dewaxed in xylene and rehydrated through a
graded alcohol series. Antigen retrieval was performed by placing
the glass slides in EDTA (pH 9) at 98°C for 10 min under high
pressure. The primary monoclonal rabbit antibody against human p85
protein (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) was incubated on the glass slides overnight at −4°C in a
humidified chamber. The goat polyclonal polyperoxidase
anti-mouse/rabbit immunoglobulin G (Zhongshan Golden Bridge
Biotechnology Co., Ltd.) secondary antibody was then applied for 30
min at 37°C. Diaminobenzidine solution was used as a chromogen and
the sections were counterstained with hematoxylin. Two pathologists
independently assessed the staining results to determine the IHC
score. p85 cytoplasmic staining was scored by multiplying the
staining intensity score (0, 1, 2 and 3) by the percentage of
stained cells (0–100%), to obtain the histochemical score (H-score;
range, 0–300).
Statistical analysis
Determination of the optimal p85 expression level
cut-offs was performed using X-tile bioinformatics software
(version 3.6.1, 2003–2005; Yale University, New Haven, CT, USA)
(14). Statistical analysis was
performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
The association between p85 expression and the clinicopathological
variables of the analyzed breast cancers was analyzed by the
χ2 test. DFS and OS curves were calculated by the
Kaplan-Meier method, and the log-rank test was used to evaluate the
differences. A Cox proportional-hazards model was used to calculate
the hazard ratio for each variable in the multivariate analysis.
For all analyses, P<0.05 was considered to indicate a
statistically significant difference.
Results
p85 protein expression
p85 protein expression in 126 breast cancer tissues
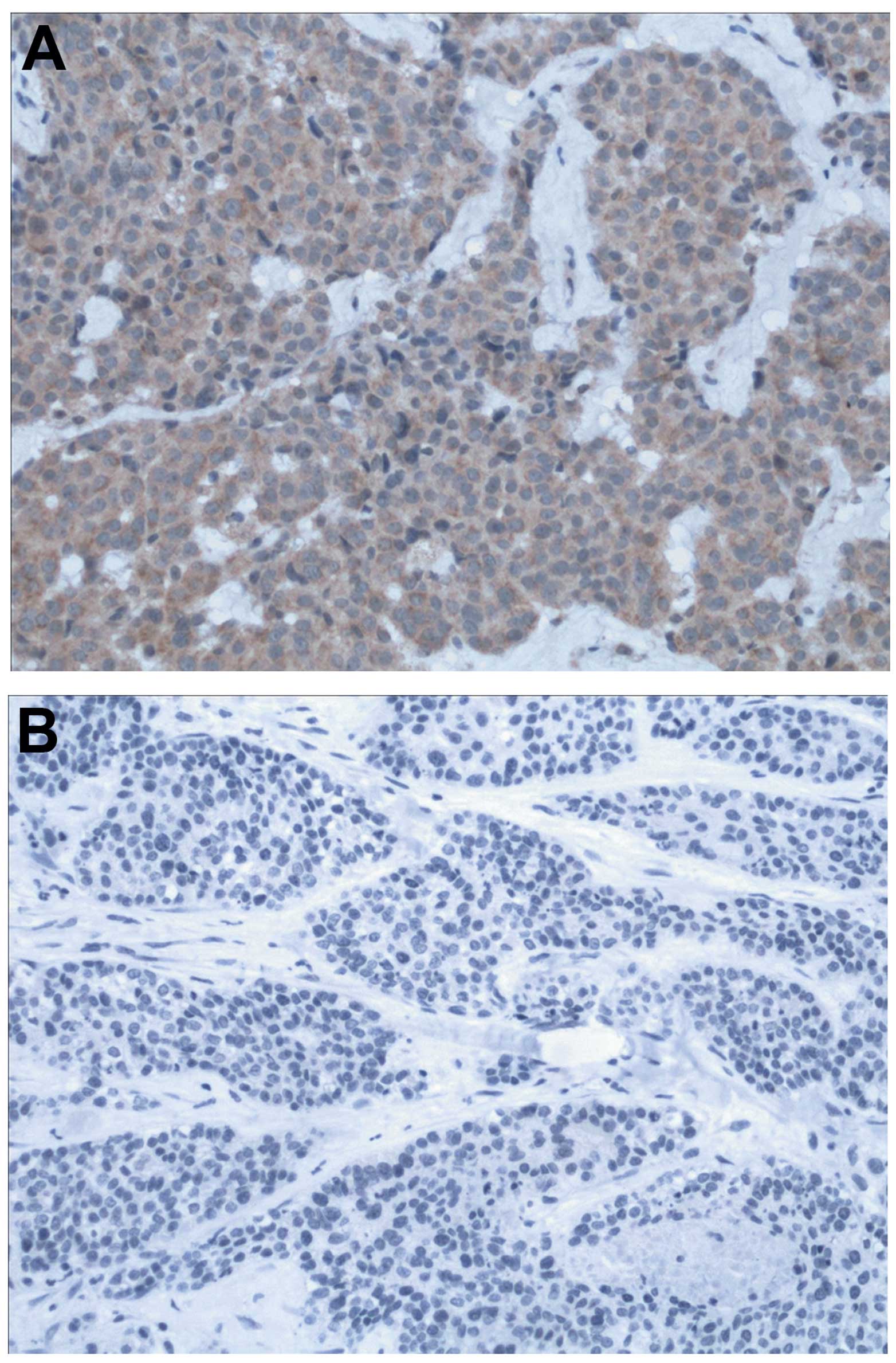
was detected by IHC. The immunohistochemical staining showed that
the expression was detectable in the cytoplasm of tumor cells
(Fig. 1). Estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor 2 (HER2) status, as well as Ki-67 index, tumor size, tumor
grade, lymph node status and vascular invasion status, were
available from postoperative pathological reports.
The cut-off points were set using the X-tile
bioinformatics software to divide the specimens into negative,
moderately positive and strongly positive expression level
subgroups. The optimal H-score cut-off points were 120 and 180, and
the intervals of the three subgroups were 0–120, 121–180 and
181–300. The number of patients in each subgroup was 76 (60.3%), 28
(22.2%), and 22 (17.5%), respectively.
Association between p85 protein
expression levels and clinicopathological characteristics
The association between p85 protein expression
levels and clinicopathological parameters is summarized in Table I. The expression levels of p85
protein were not correlated with patient age, menopausal status,
clinical stage, tumor size, lymph node status or Ki-67 index. p85
protein expression levels were significantly higher in patients
with a higher tumor grade, vascular invasion and recurrence and/or
metastasis (P<0.05).
 | Table Ip85 expression and clinicopathological
characteristics. |
Table I
p85 expression and clinicopathological
characteristics.
| PI3K p85 expression,
n (%) | |
|---|
|
| |
|---|
| Clinicopathological
parameter | Negative (n=76) | Moderately positive
(n=28) | Strongly positive
(n=22) | P-valuea |
|---|
| Age, years | | | | 0.458b |
| <60 | 52 (60.5) | 17 (19.8) | 17 (19.8) | |
| ≥60 | 24 (60.0) | 11 (27.5) | 5 (12.5) | |
| Menopausal
status | | | | 0.730b |
| Premenopausal | 15 (62.5) | 4 (16.7) | 5 (20.8) | |
| Postmenopausal | 61 (59.8) | 24 (23.5) | 17 (16.7) | |
| Clinical stage | | | | 0.195b |
| I | 25 (78.1) | 4 (12.5) | 3 (9.4) | |
| II | 35 (56.5) | 15 (24.2) | 12 (19.4) | |
| III | 16 (50.0) | 9 (28.1) | 7 (21.9) | |
| Tumor size, cm | | | | 0.334b |
| ≤2 | 39 (67.2) | 11 (19.0) | 8 (13.8) | |
| >2 | 37 (54.4) | 17 (25.0) | 14 (20.6) | |
| Tumor gradec | | | | 0.004b |
| 1 | 29 (82.9) | 4 (11.4) | 2 (5.7) | |
| 2 | 35 (60.3) | 12 (20.7) | 11 (19.0) | |
| 3 | 12 (36.4) | 12 (36.4) | 9 (27.3) | |
| Lymph node
status | | | | 0.182b |
| Negative | 41 (67.2) | 13 (21.3) | 7 (11.5) | |
| Positive | 35 (53.8) | 15 (23.1) | 15 (23.1) | |
| Vascular
invasion | | | | <0.001b |
| No | 69 (81.2) | 14 (16.5) | 2 (2.4) | |
| Yes | 7 (17.1) | 14 (34.1) | 20 (48.8) | |
| Ki-67, n (%) | | | | 0.109b |
| <14 | 24 (70.6) | 8 (23.5) | 2 (5.9) | |
| ≥14 | 52 (56.5) | 20 (21.7) | 20 (21.7) | |
|
Recurrence/Metastasis | | | | <0.001b |
| No | 65 (75.6) | 14 (16.3) | 7 (8.2) | |
| Yes | 11 (27.5) | 14 (35.0) | 15 (37.5) | |
Association between p85 protein
expression levels and subtypes of breast cancer
The patients were classified into three subtypes
according to the receptor status: ER- and/or PR-positive,
HER2-positive and triple-negative (ER-, PR- and HER2-negative). The
number of patients in each of these groups was 69 (54.8%), 25
(19.8%), and 32 (25.4%), respectively. As shown in Table II, p85 protein expression levels
were significantly associated with breast cancer subtype
(χ2=13.791; P=0.008). The proportions of moderately and
strongly positive expression among the HER2-positive and
triple-negative subtypes were higher as compared with the
ER/PR-positive subtype.
 | Table IIp85 expression and breast cancer
subtypes. |
Table II
p85 expression and breast cancer
subtypes.
| PI3K expression | |
|---|
|
| |
|---|
| Parameter | Negtive, (n=76) | Moderately positive
(n=28) | Strongly positive
(n=22) | P-valuea |
|---|
| ER/PR-positive, n
(%) | 50 (72.5) | 13 (18.8) | 6 (8.7) | 0.008b |
| HER2-positive, n
(%) | 14 (56.0) | 6 (24.0) | 5 (20.0) | |
| Triple-negative, n
(%) | 12 (37.5) | 9 (28.1) | 11 (34.4) | |
Association between p85 protein
expression levels and survival
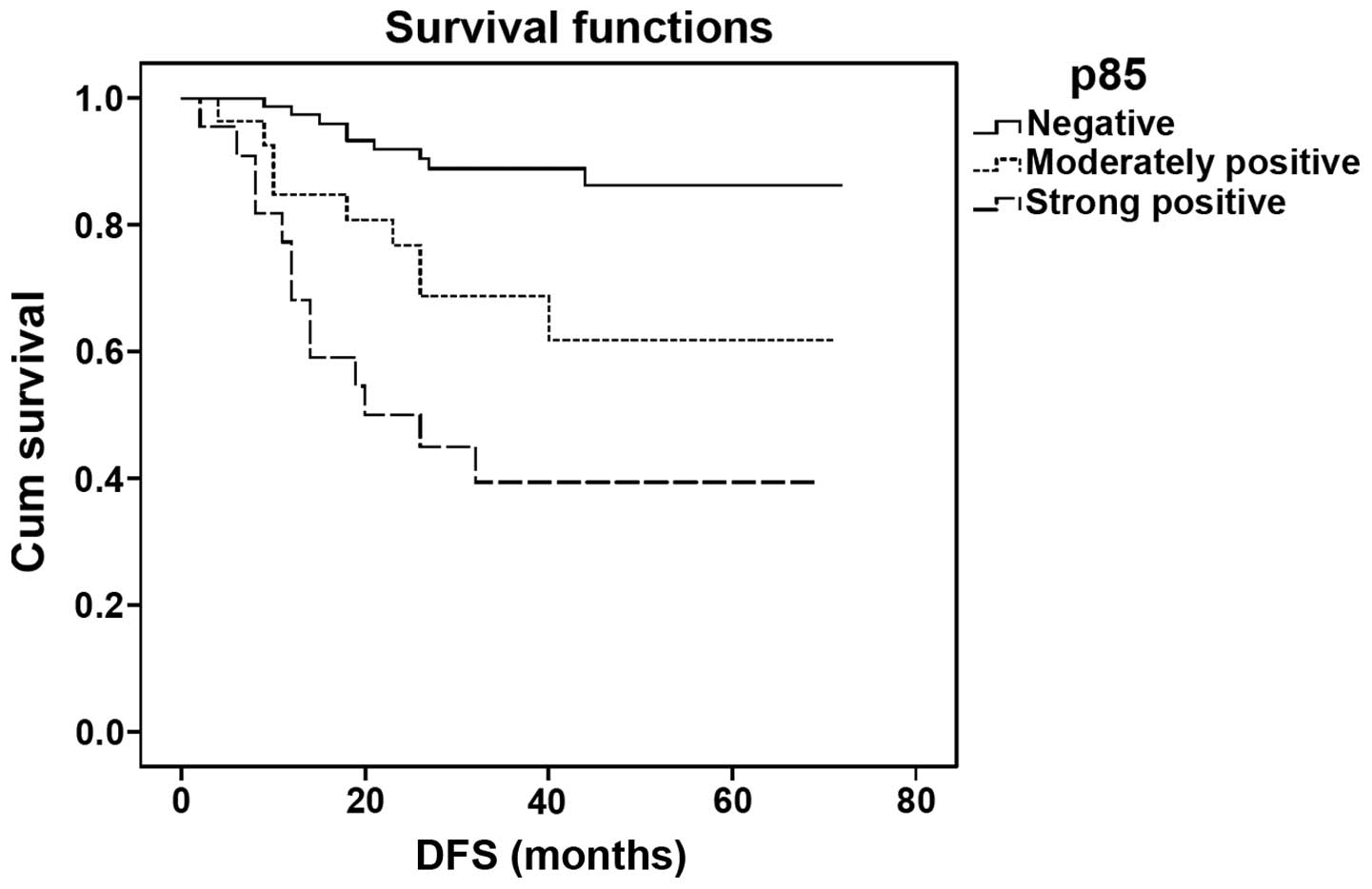
The median DFS time of the patients in this study
was 34.5 months (range 2–72 months) and the median OS time was 40
months (range 5–72 months). Patients with higher p85 protein
expression levels showed a shorter DFS as compared with those with
lower expression levels (log-rank=28.078; P<0.001; Fig. 2). The DFS time of patients who were
negative for p85 expression was significantly different from that
of patients with moderately and strongly positive expression
(P=0.006 and P<0.001, respectively). However, there was no
significant difference between the groups with moderately and
strongly positive expression (P=0.058).
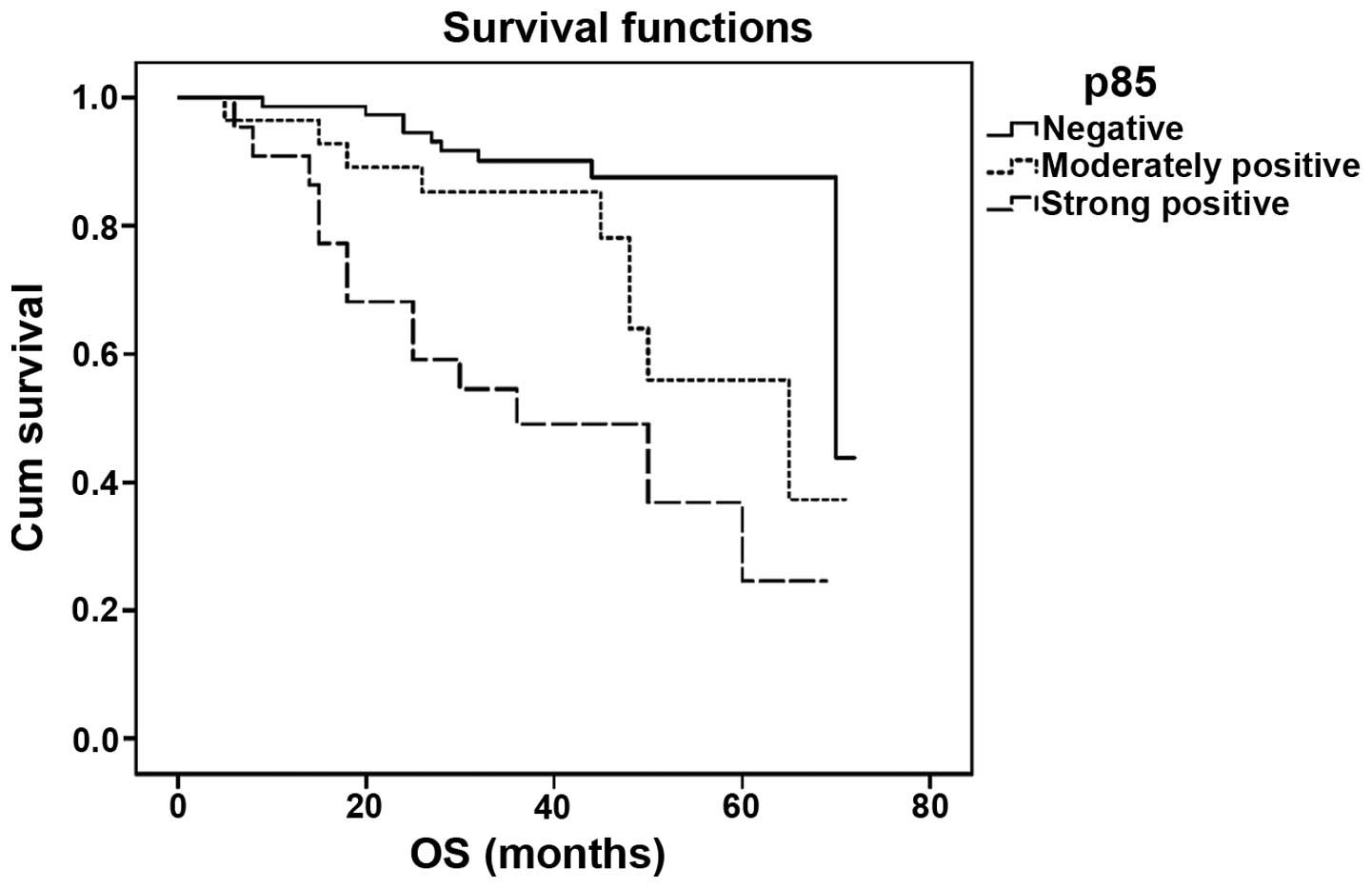
The OS time of patients with higher p85 protein
expression levels was shorter than that of patients with lower
levels (log-rank=26.043; P<0.001; Fig. 3), and the difference between each
group was significant (P=0.023 for negative versus moderately
positive; P<0.001 for negative versus strongly positive; and
P=0.037 for moderately versus strongly positive). Cox
proportional-hazards analysis, however, showed that p85 expression
was not an independent prognostic factor in this model. The only
variable correlated with survival was recurrence/metastasis
(P<0.001).
Discussion
Deregulation of the PI3K signaling pathway has been
previously identified in breast cancer. Mutations to genes of the
PI3K signaling pathway occur in >70% of breast cancers (15). The hyperactivation of the PI3K
signaling pathway has been considered to promote resistance to
current breast cancer therapies (15). A mutated form of the p85 regulatory
subunit of PI3K has additionally been considered to be associated
with hyperactivation of PI3K the pathway (10). In the present study, by using the
X-tile bioinformatics software, p85 protein expression levels and
the association with clinicopathological characteristics in breast
carcinoma subtypes and the prognosis of patients, was
investigated.
According to the H-scores of p85, patients in this
study were divided into three subgroups: Negative, moderate, and
strong positive expression level subgroups. The correlation between
the PI3K p85 protein expression levels and the clinicopathological
parameters were analyzed. The results indicated that the p85
expression levels were significantly higher in patients with a
higher tumor grade, vascular invasion, and recurrence and/or
metastasis. In a lung cancer study, the overexpression of p85 was
demonstrated to correlate with the poor differentiation of primary
lung cancer, and only weak or no expression was observed in the
bronchial epithelial cells with phenotypic signs of metaplasia
(17).
Breast cancer is a heterogeneous group of tumors and
can be classified into subtypes according to ER, PR and HER2
status. Patients who are ER- and/or PR-positive are often
considered to have a favorable prognosis, while patients with
HER2-positive and triple-negative breast cancers (TNBCs) have a
relatively poor outcome (18,19).
In the present study, it was demonstrated that p85 expression
levels were significantly associated with breast cancer subtype.
Patients with the HER2-positive and TNBC subtypes of breast cancer
displayed higher levels of expression of p85 than those with the
ER/PR-positive subtype. The results suggested that the expression
of p85 was different among the three subtypes of breast cancer.
Furthermore, it was demonstrated that patients with
higher p85 protein expression levels had shorter DFS and OS times
as compared with those with lower levels of p85 expression. This
indicated that p85 may be a prognostic factor for patients with
breast cancer. These findings were consistent with a previous
observation in non-small cell lung cancer specimens, which
suggested that high p85 expression was associated with poor
survival (20). Patients with a
strongly and moderately positive expression of p85 had a higher
risk of mortality risk as compared with those with negative
expression. However, there was no significant difference among the
three groups.
In a previous study, the P-1257 inhibitor of p85 was
administered to breast cancer cells in vitro and in
vivo, and was found to possess strong potential to inhibit the
PI3K pathway (13). This indicates
that p85 may be a valid target for therapeutic intervention and can
be utilized for the development of novel drugs.
In conclusion, p85 is a marker protein correlated
with prognostic characteristics. p85 may serve as a predictive
factor for patients with breast cancer, the inhibition of which may
present as a useful therapeutic approach. However, further
evaluation of the p85 inhibitor in breast cancer is warranted.
Acknowledgements
The authors would like to thank Ms. HongYing Zhao
and Ms. FeiFei Liu for their technical assistance.
References
|
1
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase-AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002.
|
|
2
|
Bader AG, Kang S, Zhao L and Vogt PK:
Oncogenic PI3K deregulates transcriptionand translation. Nat Rev
Cancer. 5:921–929. 2005.
|
|
3
|
Wang SI, Puc J, Li J, et al: Somatic
mutations of PTEN in glioblastoma multiforme. Cancer Res.
57:4183–4186. 1997.
|
|
4
|
Sun M, Paciga JE, Feldman RI, et al:
Phosphatidylinositol-3-OH-Kinase (PI3K)/AKT2, activated in breast
cancer, regulates and is induced by estrogen receptor alpha
(ERalpha) via interaction between ERalpha and PI3K. Cancer Res.
61:5985–5991. 2001.
|
|
5
|
Shayesteh L, Lu Y, Kuo WL, et al: PIK3CA
is implicated as an oncogene in ovarian cancer. Nat Genet.
21:99–102. 1999.
|
|
6
|
Yokoyama Y, Wan X, Shinohara A, et al:
Expression of PTEN and PTEN pseudogene in endometrial carcinoma.
Int J Mol Med. 6:47–50. 2000.
|
|
7
|
Forgacs E, Biesterveld EJ, Sekido Y, et
al: Mutation analysis of the PTEN/MMAC1 gene in lung cancer.
Oncogene. 17:1557–1565. 1998.
|
|
8
|
Dahia PL, Marsh DJ, Zheng Z, et al:
Somatic deletions and mutations in the Cowden disease gene, PTEN,
in sporadic thyroid tumors. Cancer Res. 57:4710–4713. 1997.
|
|
9
|
Jaiswal BS, Janakiraman V, Kljavin NM, et
al: Somatic mutations in p85alpha promote tumorigenesis through
class IA PI3K activation. Cancer Cell. 16:463–474. 2009.
|
|
10
|
Sun M, Hillmann P, Hofmann BT, Hart JR and
Vogt PK: Cancer-derived mutations in the regulatory subunit
p85alpha of phosphoinositide 3-kinase function through the
catalytic subunit p110alpha. Proc Natl Acad Sci USA.
107:15547–15552. 2010.
|
|
11
|
Cortés I, Sánchez-Ruíz J, Zuluaga S, et
al: p85β phosphoinositide 3-kinase subunit regulates tumor
progression. Proc Natl Acad Sci USA. 109:11318–11823. 2012.
|
|
12
|
Taniguchi CM, Winnay J, Kondo T, et al:
The phosphoinositide 3-kinase regulatory subunit p85alpha can exert
tumor suppressor properties through negative regulation of growth
factor signaling. Cancer Res. 70:5305–5315. 2010.
|
|
13
|
Folgiero V, Di Carlo SE, Bon G, et al:
Inhibition of p85, the non-catalytic subunit of
phosphatidylinositol 3-kinase, exerts potent antitumor activity in
human breast cancer cells. Cell Death Dis. 3:e4402012.
|
|
14
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: a new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004.
|
|
15
|
Miller TW, Rexer BN, Garrett JT and
Arteaga CL: Mutations in the phosphatidylinositol 3-kinase pathway:
role in tumor progression and therapeutic implications in breast
cancer. Breast Cancer Res. 13:2242011.
|
|
16
|
NCCN clinical practice guidelines in
breast cancer. 2013, version 2 http://www.nccn.org/.
Accessed May 1 2013
|
|
17
|
Lin X, Böhle AS, Dohrmann P, et al:
Overexpression of phosphatidylinositol 3-kinase in human lung
cancer. Langenbecks Arch Surg. 386:293–301. 2001.
|
|
18
|
Sorlie T, Tibshirani R, Parker J, et al:
Repeated observation of breast tumor subtypes in independent gene
expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003.
|
|
19
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948.
2010.
|
|
20
|
Zito CR, Jilaveanu LB, Anagnostou V, et
al: Multi-level targeting of the phosphatidylinositol-3-kinase
pathway in non-small cell lung cancer cells. PLoS One.
7:e313312012.
|