Introduction
Cancer and the surrounding stromal cells compose the
tumor microenvironment that provides opportunities for reciprocal
interactions between cancer, fibroblasts, inflammatory cells and
microcapillary vessels. Cytokines, including chemokines, growth
factors, angiogenic factors and prostaglandins, participate in
these interactions. Among these cytokines, transforming growth
factor β1 (TGFβ1) is a multifunctional growth factor produced by
cancer cells, macrophages and fibroblasts, which exerts
wide-ranging activities that affect cancer inhibition and
promotion, epithelial mesenchymal transition (EMT), angiogenesis
and the suppression of regulatory T cell function (1).
In human prostate cancer (PCa) tissues, TGFβ1
expression is increased compared with normal or benign prostate
tissues, and is increased in PCa with lymph node metastasis
compared with cancer without lymph node involvement (2). TGFβ1 suppresses PCa cell growth in a
dose-dependent manner, but this inhibitory effect is lost at later
stages (3,4). Collectively, TGFβ1 is considered to be
a tumor promoter in PCa tissues.
Cancer and inflammation has long been studied in
close connection with carcinogenesis and cancer development.
Cyclooxygenase-2 (COX-2) is the major enzyme that converts
arachidonic acid into prostanoids, which are involved in a number
of pathological events, including inflammation and cancer
progression (5). However, the
mechanistic role of COX-2 in prostate carcinogenesis remains
controversial. One study has shown that benign prostatic disease
expresses higher COX-2 than PCa (6), while another study reported COX-2
overexpression in PCa (7).
Multiple inflammatory cells and mediators are
involved in cancer-related inflammation and compose elements of the
tumor microenvironment (8).
Tumor-associated macrophages (TAM), which are derived from
monocytes, infiltrate tumor tissue, promote the invasive capacity
of cancer cells and in turn, metastasis, which is correlated with a
poor prognosis in patients with prostate and breast cancer
(9–12). The mechanism by which TAM promotes
cancer promotion is considered to involve the production of
angiogenic growth factors, proteases and cytokines, including TGFβ
(13). The reciprocal interactions
between macrophages and the various phenotypes of PCa in the tumor
microenvironment may be diverse. To investigate this issue, the
current study examined the tumor microenvironment model of PCa, and
TGFβ and THP-1 macrophages, where the PCa cells were exposed to
TGFβ for a long period of time. In addition, the cytokine mRNA from
THP-1 macrophages and the regulatory factors from PCa were
analyzed.
Materials and methods
Cell culture and reagents
The human PCa cell line, PC-3, and the subclone, M1
(14), were cultured in RPMI 1640
(R8758; Sigma-Aldrich, St. Louis, MO, USA) with 10% fetal bovine
serum (FBS; Gibco-BRL, Carlsbad, CA, USA), and TGFβ1 (555–83601;
Wako Pure Chemical Industries, Ltd., Osaka, Japan) was added to the
culture medium at a final concentration of 10 ng/ml. The cell lines
were cultured and passaged every seven days. TGFβ1 or vehicle was
added to the culture medium simultaneously on each passage and kept
in the medium until the next passage. The passage was repeated 10
times. TGFβ1 or vehicle long-term exposure for the PC3 and M1 cells
was designated as TbL-PC3 or TbL-M1, and CoL-PC3 or CoL-M1,
respectively. The cell culture of the short-term exposure was
represented by overnight incubation of TGFβ1 or vehicle, and TGFβ1
was removed from the culture medium in subsequent experiments.
Short-term exposure for the PC3 and M1 cells was designated as
TbS-PC3 or TbS-M1, and CoS-PC3 or CoS-M1, respectively. The human
acute monocytic leukemia-derived THP-1 cell line was maintained in
RPMI 1640 medium supplemented with 10% FBS. The antibodies for
western blot analysis and their dilutions were as follows: Rabbit
polyclonal anti-human anti-Smad3 (ab28379; 1:1,000), rabbit
monoclonal anti-human anti-phospho-Smad3 (S423+S425; ab52903,
1:1,000) and rabbit polyclonal anti-rat anti-COX-2 (ab15191;
1:1,000) (All Abcam, Cambridge, UK). The p3TP-Lux plasmid was
kindly provided by Dr Joan Massague (15). The luciferase assay was performed as
follows: The PC-3 cells were transfected with expression and
reporter plasmids together with Lipofectamine (11668019; Invitrogen
Life Technologies, Carlsbad, CA, USA) and harvested 24 h later. The
firefly luciferase activity was counted using a Dual-Luciferase
Reporter Assay System (E1910; Promega Corporation, Madison, WI,
USA). Renilla luciferase activity was also estimated by
cotransfection of the pRL-TK vector (E2241; Promega Corporation) as
an internal control.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated from THP-1 macrophages using
an RNA extraction kit (74104; Qiagen, Hilden, Germany).
First-strand cDNA synthesis was performed using the Transcriptor
High Fidelity cDNA Synthesis kit (05081963001; Roche Diagnostics
GmbH, Mannheim, Germany). qPCR was performed with the QuantiTect
SYBR Green PCR kit (204145; Qiagen) according to the manufacturer’s
instructions. The results were analyzed by Rotor-GeneQ software
(9020353; Qiagen) and normalized against GAPDH mRNA levels. The
mRNA expression of 84 genes for human signal transduction molecules
was analyzed by the RT2Profiler PCR Array (PAHS-014A;
Qiagen), with the cDNA synthesis and SYBR Green PCR performed as
aforementioned.
Co-culture assay of the PCa cell line and
THP-1 macrophages
The THP-1 cells were cultured at 5×105
cells/well in 24-well plates and differentiated to THP-1
macrophages with 100 nm phorbol 12-myristate 13-acetate (PMA;
P1585; Sigma-Aldrich) for two days. Following PMA removal from the
culture media, the treated cells were maintained in RPMI 1640 with
10% FBS for an additional two days. The PC-3 cell line (without
TGFβ1) was loaded in a cell culture insert (1.0-μM pore size;
353104; BD Falcon™ Cell Culture Inserts; BD Biosciences, Franklin
Lakes, NJ, USA) at 1×104 cells/300 μl medium and the
inserts were placed in each well of a THP-1 macrophage culture
plate. After two days of co-culture, total RNA was extracted from
the THP-1 macrophages and cDNA was synthesized as
aforementioned.
qPCR primers
The primer sequences used were as follows:
interleukin (IL)-6 forward, 5′-TCAGAACGAATTGACAAACA-3′ and reverse,
5′-TTGAATCCAGATTGGAAGC-3′; TNF-α forward,
5′-GACAAGCCTGTAGCCCATGT-3′ and reverse, 5′-TCTCAGCTCCACGCCATT-3′;
and IL-10 forward, 5′-GCTGGAGGACTTTAAGGGTTACCT-3′ and reverse,
5′-CTTGATGTCTGGGTCTTGGTTCT-3′.
Prostaglandin E2
(PGE2) production and enzyme immunoassay
All the cells were cultured at 5×105
cells/well in triplicates of 24-well plates. The culture medium of
the cells was changed to RPMI 1640 without FBS, but containing 10
μM of arachidonic acid (A3555; Sigma-Aldrich). Following 2 h of
incubation, the media were collected from each well and
PGE2 production was determined by the DetectX
Prostaglandin E2 Enzyme Immunoassay kit (K018-H1; Arbor
Assays, Ann Arbor, MI, USA).
Results
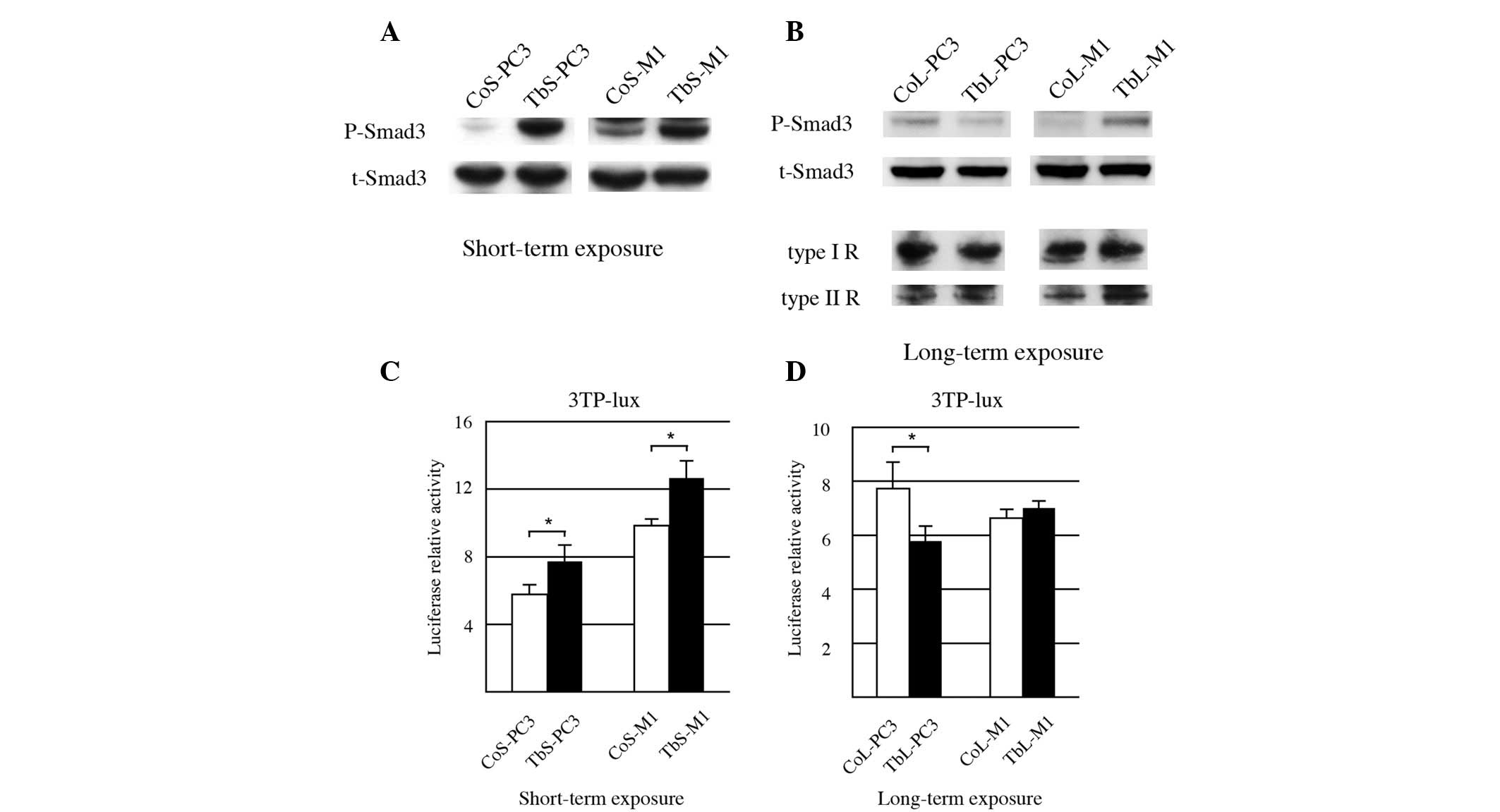
Smad3 phosphorylation status of PCa cells
in response to short- and long-term TGFβ1 stimulation
To determine whether long-term TGFβ1 exposure can
modify PCa cell signaling events, the PCa cell line, PC-3, and
subclone, M1 (14), were used.
Since IL-8 expression, which is regulated by TGFβ, is slightly
different in PC-3 and M1 cells, we hypothesized that these cell
lines may respond differently to TGFβ.
A growth inhibitory effect was observed when the
PC-3 cells were exposed to TGFβ1 (4); PC-3 cells express TGFβ1 target genes
(16), therefore, alterations in
signaling caused by TGFβ1 stimuli should be observable.
When the PC-3 cells were incubated with TGFβ1, Smad2
C-terminal phosphorylation (Ser465/467) was induced, but was not
robust (data not shown). In the majority of the commercially
available antibodies, the C-terminal phosphorylated form of Smad2
and Smad3 is not distinguishable. The anti-phospho-Smad3 antibody,
described in the Materials and methods section, does not
cross-react with phospho-Smad2. Therefore, TGFβ1 signaling was
evaluated using the phosphorylated status of Smad3.
As predicted, robust Smad3 phosphorylation was
observed in the PC-3 and M1 cell lines tested following short-term
TGFβ1 exposure (Fig. 1A). To
further confirm Smad3 activity, a luciferase assay was conducted
using the promoter for PAI-1, a Smad3 target gene. As shown in
Fig. 1C, when the PC-3 and M1 cells
were exposed to TGFβ1 in the short-term, the PAI-1 promoter
activity was unregulated.
Following long-term exposure to TGFβ1, Smad3
phosphorylation in the TbL-PC3 and TbL-M1 cells was highly
diminished compared with that in the vehicle-exposed cells (CoL-PC3
and CoL-M1), while TGFβ receptor expression was compatible between
vehicle and TGFβ1 exposure (Fig.
1B). In contrast to the short-term exposure, PAI-1 promoter
activity of the PC3 cells with long-term exposure was diminished
compared with the cells exposed to the control treatment (Fig. 1D). PAI-1 promoter activity of the M1
cells with long-term exposure was at a similar level to that of the
cells exposed to the control treatment (Fig. 1D). These results indicated that Smad
signaling is attenuated in the PC-3 and M1 PCa cell lines exposed
to long-term TGFβ1 treatment.
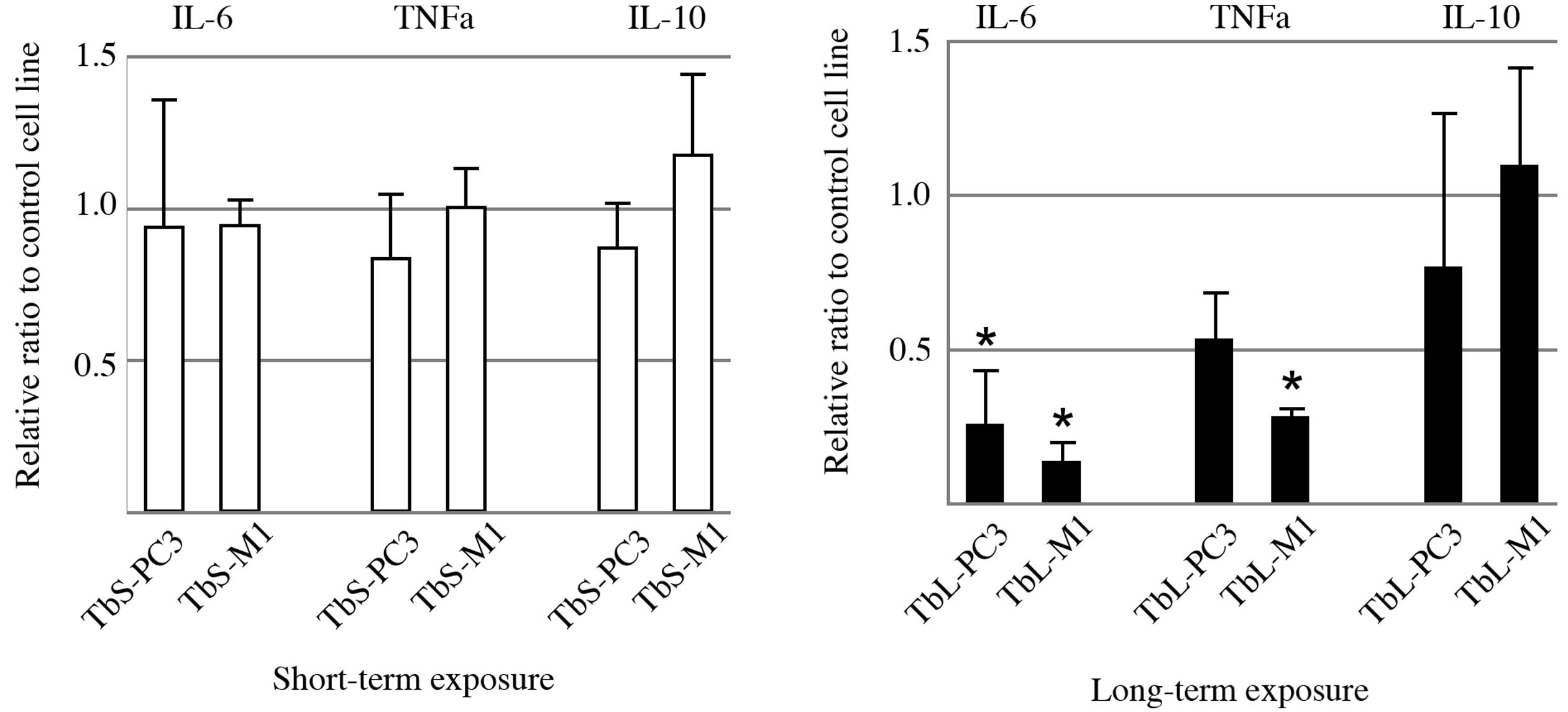
Long-term TGFβ1 exposure of PCa cell
suppresses cytokine production by THP-1 differentiated
macrophages
To mimic the tumor microenvironment, the reciprocal
interactions between TGFβ1-exposed PCa cells and inflammatory
cells, in this case, macrophages, was examined. The activated
macrophages were characterized with respect to the cytokines and
receptors they produced and were designated as polarized
macrophages (17). Since primary
tissue macrophages are not easily obtainable, the human monocytic
leukemia THP-1 cell line has been utilized in a number of studies
(18–20). PMA treatment of THP-1 cells induces
their differentiation into macrophage-like cells (THP-1
macrophages) that mimic the characteristics of monocyte-derived
macrophages (21). As described in
the Materials and methods section, cytokine production from THP-1
macrophages following reciprocal interactions with PCa cells was
assessed by a chamber assay, where THP-1 macrophages and PCa cells
were separated and could not make direct contact. When the THP-1
macrophages were co-cultured with the PC-3 cell line without any
treatment, all cytokine production was increased, as previously
described (22) (data not
shown).
IL-6 expression from the THP-1 macrophages was
significantly decreased upon co-culturing with the PC-3 and M1
cells exposed to TGFβ1 for a long-term period (Fig. 2). TNF-α expression from the THP-1
macrophages was also markedly suppressed in the TbL-PC3 and TbL-M1
cells compared with the control cells. IL-10 expression was not
altered significantly. However, IL-6 expression was increased,
rather than decreased, by the addition of TGFβ1 to the THP-1
macrophage culture (data not shown).
Since IL-6 is a key regulator in PCa progression
(23,24), the mechanisms of IL-6 downregulation
in the THP-1 macrophages were investigated. Several growth factors,
cytokines and prostanoids, such as hepatocyte growth factor (HGF),
IL-1β, IL-4 and PGE2, have been found to regulate IL-6
production from macrophages or peripheral blood monocytes (25–27).
Therefore, we hypothesized that TGFβ1 suppresses or stimulates
pleiotropic factor secretion from PCa cells and consequently
downregulates IL-6 production by THP-1 macrophages. Several of
these potential factors were assessed at an mRNA level by qPCR
(data not shown), which showed that HGF mRNA was unchanged in the
CoL-PC3, TbL-PC3, CoL-M1 and TbL-M1 cells regardless of TGFβ1
exposure (data not shown). Although HGF was reported to
downregulate IL-6 production from monocytic cell lines (25), this appears to have less relevance
for IL-6 reduction in THP-1 macrophages.
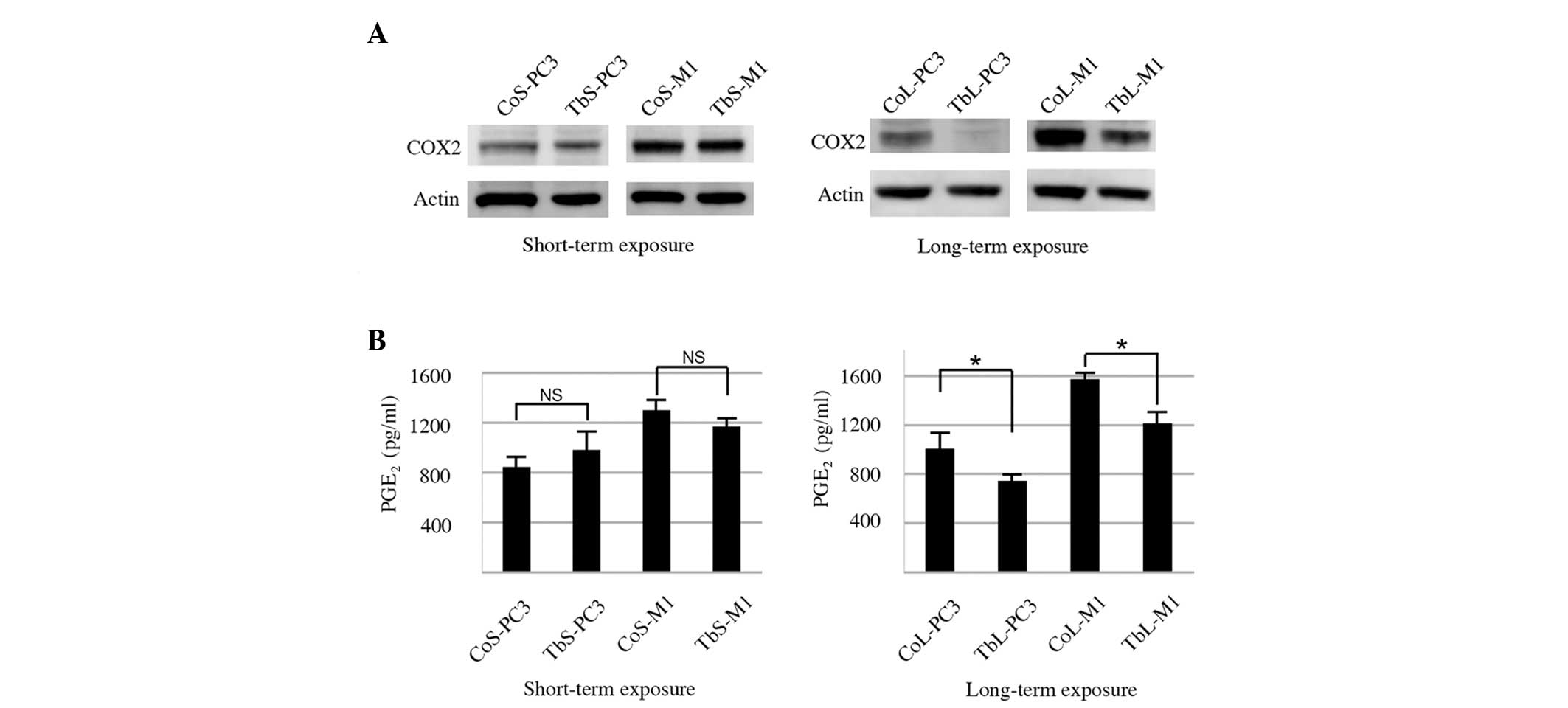
Next, the possibility of non-canonical signal
activity was explored in the cell lines with long-term TGFβ1
exposure through use of qPCR-based array analysis, which profiled
the expression of key genes that are representative of various
signal transduction pathways. Overall, the PCR array analysis
showed that the TGFβ-related genes exhibited no marked changes in
gene expression, with all changes observed being less than two-fold
(Table I). Meanwhile, for the genes
involved in the phospholipase C pathways, the expression of the
COX-2 gene was downregulated in response to long-term TGFβ1
exposure. COX-2 protein expression was reduced by long-term TGFβ1
exposure in the TbL-PC3 and TbL-M1 cells (Fig. 3A).
 | Table IqPCR analysis of human signal
transduction molecules. |
Table I
qPCR analysis of human signal
transduction molecules.
| Pathway | Gene | Fold-change of
TbL-PC3 to CoL-PC3a |
|---|
| TGFβ | CDKN1A (p21) | 1.670 |
| CDKN1B (p27) | 0.835 |
| CDKN2A (p16) | 1.558 |
| CDKN2B (p15) | 0.727 |
| Phospholipase C | FOS | 0.363 |
| ICAM1 | 0.959 |
| NOS2A | 0.389 |
| COX2 | 0.257 |
PGE2 is known to induce IL-6 production
from macrophages and is regulated by COX-2 activity (27), one of the rate-limiting enzymes for
prostanoid biosynthesis (5).
The ability of the cells to produce PGE2
was also examined in the present study. The PGE2 level
was not significantly different following short-term TGFβ1
exposure. However, following long-term TGFβ1 exposure,
PGE2 production in the TbL-PC3 and TbL-M1 cells was
reduced compared with that in the control cells (Fig. 3B).
Thus, these results indicated that COX-2 attenuation
may be responsible for the reduction in PGE2 caused by
long-term TGFβ1 exposure in TbL-PC3 and TbL-M1 cells, which
consequently reduces IL-6 production in THP-1 macrophages.
Discussion
In the current study, Smad signaling was shown to be
diminished in the PCa cells following long-term TGFβ1 exposure.
Cytokine production from THP-1 macrophages, particularly IL-6, was
downregulated upon co-culture with PCa cells, producing lower
levels of COX-2 and PGE2 by long-term TGFβ1
exposure.
The dynamic function of TGFβ1 allows it to be
involved in a variety of intracellular signal transduction pathways
(28). Several lines of evidence
support the fact that a number of TGFβ1 signal transductions are
independent from Smad canonical activation (29). The underlying mechanism for the
reductions in phospho-Smad3 expression following long-term TGFβ1
exposure has not yet been fully elucidated. The turnover of
phospho-Smad is mediated by the specific phosphatases PPM1A, PDP
and SCP1, 2 and 3, or proteasomal degradation with the ubiquitin E3
ligase, NEDD4L (30–33). Neither the PPM1A transcript nor the
NEDD4L protein expression were altered following exposure of the
PCa cells to TGFβ1 or vehicle (data not shown). Other unidentified
phosphatases or ubiquitin ligases may therefore be involved in the
suppression of phospho-Smad3.
In the tumor microenvironment, TGFβ1 is produced by
a variety of cells and acts as an intercellular signaling molecule
that induces the expression of cytokines and angiogenic factors,
which consequently promote tumor growth, invasion and metastasis
(1). Long-term reciprocal
interactions between cancer cells and fibroblasts, which are a
source of TGFβ1 production (34),
give rise to an altered cancer cell phenotype that may affect
stromal components. In the present study, the long-term exposure of
the PCa cells to TGFβ1 was found to suppress THP-1 macrophage
activation in a co-culture system. This result concurs with a colon
cancer study in which a COX-2-degrading enzyme was upregulated by
TGFβ1 (35), suggesting that the
long-term exposure of PCa cells to TGFβ1 may have a similar COX-2
suppression mechanism. The current in vitro results may have
relevance for physiological cancer tissues, wherein certain
populations of cancer cells may control inflammatory cell function
and gain survival advantages. A study revealed that when NF-κB
signaling was repressed in TAMs, those TAMs showed cytotoxicity
against tumor cells (36). In
addition, normal mammary epithelial cells (MECs) exposed to TGFβ1
underwent EMT and acquired features of stromal cells. These
immortalized and transformed MECs with EMT-regulated gene
expression also showed increased mammosphere formation, a surrogate
measure of stemness (37). Taken
together, these results suggested that the long-term exposure of
PCa cells to TGFβ1 may also promote a stem cell-like character.
However, whether PCa with stem cell-like characteristics can
suppress macrophage activity in tumors has not been fully
investigated. The current in vitro results indicated the
possibility of a macrophage inhibitory mechanism in the tumor
microenvironment.
Acknowledgements
This study was supported by Grants-in Aid from the
Ministry to Education for Science and Culture of Japan (grant no.
22591765).
Abbreviations:
|
TGFβ1
|
transforming growth factor β1
|
|
COX-2
|
cyclooxygenase-2
|
|
EMT
|
epithelial mesenchymal transition
|
|
PGE2
|
prostaglandin E2
|
|
TAM
|
tumor-associated macrophage
|
References
|
1
|
Yingling JM, Blanchard KL and Sawyer JS:
Development of TGF-beta signalling inhibitors for cancer therapy.
Nat Rev Drug Discov. 3:1011–1022. 2004.
|
|
2
|
Eastham JA, Truong LD, Rogers E, et al:
Transforming growth factor-beta 1: comparative immunohistochemical
localization in human primary and metastatic prostate cancer. Lab
Invest. 73:628–635. 1995.
|
|
3
|
Kim IY, Ahn HJ, Zelner DJ, et al: Genetic
change in transforming growth factor beta (TGF-beta) receptor type
I gene correlates with insensitivity to TGF-beta 1 in human
prostate cancer cells. Cancer Res. 56:44–48. 1996.
|
|
4
|
Wilding G, Zugmeier G, Knabbe C, Flanders
K and Gelmann E: Differential effects of transforming growth factor
beta on human prostate cancer cells in vitro. Mol Cell
Endocrinol. 62:79–87. 1989.
|
|
5
|
Fosslien E: Molecular pathology of
cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 30:3–21. 2000.
|
|
6
|
Zha S, Gage WR, Sauvageot J, et al:
Cyclooxygenase-2 is up-regulated in proliferative inflammatory
atrophy of the prostate, but not in prostate carcinoma. Cancer Res.
61:8617–8623. 2001.
|
|
7
|
Gupta S, Srivastava M, Ahmad N, Bostwick
DG and Mukhtar H: Over-expression of cyclooxygenase-2 in human
prostate adenocarcinoma. Prostate. 42:73–78. 2000.
|
|
8
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444.
2008.
|
|
9
|
Hagemann T, Wilson J, Kulbe H, et al:
Macrophages induce invasiveness of epithelial cancer cells via
NF-kappa B and JNK. J Immunol. 175:1197–1205. 2005.
|
|
10
|
Qian B, Deng Y, Im JH, et al: A distinct
macrophage population mediates metastatic breast cancer cell
extravasation, establishment and growth. PLoS One. 4:e65622009.
|
|
11
|
Lissbrant IF, Stattin P, Wikstrom P, et
al: Tumor associated macrophages in human prostate cancer: relation
to clinicopathological variables and survival. Int J Oncol.
17:445–451. 2000.
|
|
12
|
Denardo DG, Brennan DJ, Rexhepaj E, et al:
Leukocyte complexity predicts breast cancer survival and
functionally regulates response to chemotherapy. Cancer Discov.
1:54–67. 2011.
|
|
13
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010.
|
|
14
|
Hirokawa YS, Takagi A, Uchida K, et al:
High level expression of STAG1/PMEPA1 in an androgen-independent
prostate cancer PC3 subclone. Cell Mol Biol Lett. 12:370–377.
2007.
|
|
15
|
Wrana JL, Attisano L, Cárcamo J, et al:
TGF beta signals through a heteromeric protein kinase receptor
complex. Cell. 71:1003–1014. 1992.
|
|
16
|
Park BJ, Park JI, Byun DS, Park JH and Chi
SG: Mitogenic conversion of transforming growth factor-beta1 effect
by oncogenic Ha-Ras-induced activation of the mitogen-activated
protein kinase signaling pathway in human prostate cancer. Cancer
Res. 60:3031–3038. 2000.
|
|
17
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002.
|
|
18
|
El Fiky A, Perreault R, McGinnis GJ and
Rabin RL: Attenuated expression of interferon-β and interferon-λ1
by human alternatively activated macrophages. Hum Immunol.
74:1524–1530. 2013.
|
|
19
|
Wu TH, Li YY, Wu TL, Chang JW, Chou WC,
Hsieh LL, Chen JR and Yeh KY: Culture supernatants of different
colon cancer cell lines induce specific phenotype switching and
functional alteration of THP-1 cells. Cell Immunol. 290:107–115.
2014.
|
|
20
|
Danielsen PH, Møller P, Jensen KA, Sharma
AK, Wallin H, Bossi R, Autrup H, Mølhave L, Ravanat JL, Briedé JJ,
et al: Oxidative stress, DNA damage, and inflammation induced by
ambient air and wood smoke particulate matter in human A549 and
THP-1 cell lines. Chem Res Toxicol. 24:168–184. 2011.
|
|
21
|
Daigneault M, Preston JA, Marriott HM,
Whyte MK and Dockrell DH: The identification of markers of
macrophage differentiation in PMA-stimulated THP-1 cells and
monocyte-derived macrophages. PLoS One. 5:e86682010.
|
|
22
|
Tsagozis P, Eriksson F and Pisa P:
Zoledronic acid modulates antitumoral responses of prostate
cancer-tumor associated macrophages. Cancer Immunol Immunother.
57:1451–1459. 2008.
|
|
23
|
Spiotto MT and Chung TD: STAT3 mediates
IL-6-induced growth inhibition in the human prostate cancer cell
line LNCaP. Prostate. 42:88–98. 2000.
|
|
24
|
Smith PC, Hobisch A, Lin DL, Culig Z and
Keller ET: Interleukin-6 and prostate cancer progression. Cytokine
Growth Factor Rev. 12:33–40. 2001.
|
|
25
|
Kamimoto M, Mizuno S and Nakamura T:
Reciprocal regulation of IL-6 and IL-10 balance by HGF via
recruitment of heme oxygenase-1 in macrophages for attenuation of
liver injury in a mouse model of endotoxemia. Int J Mol Med.
24:161–170. 2009.
|
|
26
|
Donnelly RP, Crofford LJ, Freeman SL, et
al: Tissue-specific regulation of IL-6 production by IL-4.
Differential effects of IL-4 on nuclear factor-kappa B activity in
monocytes and fibroblasts. J Immunol. 151:5603–5612. 1993.
|
|
27
|
Williams JA, Pontzer CH and Shacter E:
Regulation of macrophage interleukin-6 (IL-6) and IL-10 expression
by prostaglandin E2: the role of p38 mitogen-activated
protein kinase. J Interferon Cytokine Res. 20:291–298. 2000.
|
|
28
|
Meulmeester E and Ten Dijke P: The dynamic
roles of TGF-β1 in cancer. J Pathol. 223:205–218. 2011.
|
|
29
|
Moustakas A and Heldin CH: Non-Smad
TGF-beta signals. J Cell Sci. 118:3573–3584. 2005.
|
|
30
|
Lin X, Duan X, Liang YY, et al: PPM1A
functions as a Smad phosphatase to terminate TGFbeta signaling.
Cell. 125:915–928. 2006.
|
|
31
|
Chen HB, Shen J, Ip YT and Xu L:
Identification of phosphatases for Smad in the BMP/DPP pathway.
Genes Dev. 20:648–653. 2006.
|
|
32
|
Sapkota G, Knockaert M, Alarcón C, et al:
Dephosphorylation of the linker regions of Smad1 and Smad2/3 by
small C-terminal domain phosphatases has distinct outcomes for bone
morphogenetic protein and transforming growth factor-beta pathways.
J Biol Chem. 281:40412–40419. 2006.
|
|
33
|
Gao S, Alarcón C, Sapkota G, et al:
Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta
signaling. Mol Cell. 36:457–468. 2009.
|
|
34
|
Bissell MJ and Radisky D: Putting tumours
in context. Nat Rev Cancer. 1:46–54. 2001.
|
|
35
|
Yan M, Rerko RM, Platzer P, et al:
15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist,
is a TGF-beta-induced suppressor of human gastrointestinal cancers.
Proc Natl Acad Sci USA. 101:17468–17473. 2004.
|
|
36
|
Hagemann T, Lawrence T, McNeish I, et al:
‘Re-educating’ tumor-associated macrophages by targeting NF-kappaB.
J Exp Med. 205:1261–1268. 2008.
|
|
37
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008.
|