Introduction
The phosphodiesterases (PDEs) are metallohydrolases
that hydrolyze the secondary messengers, cyclic adenosine
monophosphate (cAMP) or cyclic guanosine monophosphate (cGMP), into
5′-AMP or 5′-GMP, which is considered to be the only pathway to
degrade cAMP and cGMP intracellularly (1). The impairment of cAMP or cGMP
generation by the regulation of PDEs has been indicated in various
cancer pathologies (2,3). The PDE subtype, PDE4D, belongs to the
PDE4 subfamily, which includes the four isoforms, A, B, C and D. It
has been demonstrated that PDE4D functions as a proliferation
promoting factor in prostate cancer through the Sleeping Beauty
transposon system (4). Pullamsetti
et al (5) found that PDE4D
induces human lung cancer proliferation in vitro and in
vivo through the hypoxia-inducible transcription factor
signaling pathway (5). Studies have
also shown that PDE4D is involved in the transforming growth factor
(TGF)-β1-induced epithelial-mesenchymal transition in the human
alveolar epithelial type II A549 cell line. The upregulated PDE4D
expression caused by TGF-β1 may contribute to the invasion and
metastasis of A549 cells (6).
Recently, Lin et al (7)
found that PDE4D protein expression levels were elevated in
multiple types of cancer, including head and neck cancer (HNC).
Nasopharyngeal carcinoma (NPC) is a type of HNC,
however, due to unique clinical, etiological and biological
characteristics, NPCs are distinct from other HNCs. NPC is one of
the most common types of cancer in Southern China, particularly
among those of Cantonese origin (8). Sothern China has the highest incidence
of this disease, peaking at 50 cases per 100,000 individuals per
year (9). Despite NPC being a
generally radiosensitive disease, the presentation of local
recurrence and distant metastasis is observed in certain patients
following radiotherapy as a result of radioresistance (10). The development of therapies
targeting the underlying molecular mechanisms of tumorigenesis has
become a focus of attention, as the use of adjuvant chemotherapy
has shown limited success.
Increasing evidence has shown that the
overexpression of epidermal growth factor receptor (EGFR) is common
in NPC; its signaling may be significant in the pathogenesis of NPC
(11,12). A recent study has proposed EGFR as a
novel target for NPC therapy (13).
Epidermal growth factor (EGF) and TGF-α are natural ligands that
bind to the extracellular domain of EGFR, subsequently activating
EGFR and its downstream signaling proteins. This results in the
modulation or activation of various cellular processes (14). In total, ~200 targets of the EGFR
signaling pathway have been reported (15). One of the most significant signaling
pathways is the phosphoinositide 3-kinase/AKT pathway. EGFR
activates PI3K, which phosphorylates phosphatidylinositol
2-phosphate to phosphatidylinositol 3-phosphate, and subsequently
activates AKT, as well as a number of its downstream effectors.
This induces protein synthesis, cell growth and survival,
proliferation, migration and angiogenesis (16). However, a direct association between
the EGFR/PI3K/AKT axis and the PDE4D-cAMP axis in malignant cells
has not been reported. In the present study, PDE4D was demonstrated
to promote NPC cell proliferation in vitro and in
vivo, which has been associated with the EGFR/PI3K/AKT
signaling pathway.
Patients and methods
Patients and tissue preparation
In total, 40 fresh, poorly-differentiated NPC
tissues and 21 normal nasopharyngeal epithelial tissues were
obtained between January 2009 and December 2012 at the Department
of Otolaryngology, The Second People’s Hospital of Wuxi (Wuxi,
China). All patients had no history of previous malignancies, or
treatment with radiotherapy or chemotherapy. The clinical stage of
the patients was classified according to the Chinese 2008 NPC
staging system (17). All samples
were snap-frozen immediately and stored in liquid nitrogen prior to
protein extraction. The study was approved by the Research Ethics
Committee of the Second People’s Hosiptal of Wuxi (Wuxi, China) and
written informed consent was obtained from all the patients. All
specimens were handled and made anonymous according to the ethical
and legal standards.
Cell culture and lentiviral short hairpin
(sh)RNA infection
The immortalized nasopharyngeal epithelial NP69
cells (Shanghai Bogoo Biological Technology Co., Ltd., Shanghai,
China) were cultured in keratinocyte-SFM (Invitrogen Life
Technologies, Carlsbad, CA, USA) supplemented with bovine pituitary
extract (BD Biosciences, Franklin Lakes, NJ, USA). The human NPC
cell lines, CNE1, CNE2, 5–8F, 6–10B and HNE1 (Shanghai Bogoo
Biological Technology Co., Ltd., Shanghai, China), were cultured in
Roswell Park Memorial Institute 1640 medium (Invitrogen Life
Technologies, Carlsbad, CA, USA). PDE4D-targeted shRNA lentiviral
particles (LV-PDE4D shRNA; sc-44004-v) and control shRNA lentiviral
particles (ctr-shRNA; sc-108080) (both Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) were infected into the CNE2 cells
according to the manufacturer’s instructions. Following ≥14 days of
selection using Puromycin (Invitrogen Life Technologies), the
individual puromycin-resistant colonies were isolated and
expanded.
Cell viability assay and cell cycle
analysis
The cells were plated in 96-well plates at a density
of 2.5×103 cells/well. Cell growth and viability was
detected by MTT assay, as described previously (18). Briefly, the medium was replaced with
fresh medium containing 5 mg/ml MTT reagent (Sigma-Aldrich, St.
Louis, MO, USA) following various durations of culture (at 24, 36,
48 and 96 h). The cells were cultured for an additional 4 h at
37°C, then 100 μl dimethyl sulfoxide was added to each well to
solubilize the colored crystals produced within the living cells.
The absorbance was measured at a wavelength of 490 nm using a
BioTek microplate reader (Bio-Rad, Hercules, CA, USA). In addition,
cell cycle analysis was performed by flow cytometric analysis, as
described previously (18).
Briefly, the transfected cells were seeded onto a six well plate at
a density of 105 cells/well in RPMI 1640 medium for 48 h. Next, the
cells were harvested and fixed in ice cold 70% (v/v) ethanol for 15
min. The cells were then treated with RNase A (Sigma-Aldrich) and
stained with 50 μg/ml propidium iodide (Abcam, Cambridge, UK),
followed by incubation at 37°C for 30 min in the dark. Samples were
analyzed using a FACScan flow cytometer (Becton Dickinson, Franklin
Lakes, NJ, USA), according to the manufacturer’s instructions. In
addition, the DNA content in the G1, S and G/M phases was analyzed
using BD FACSDivaTM software (Becton Dickinson).
Western blot analysis
Western blot analyses were performed as described
previously (19). Briefly, a total
of 50 μg protein from each sample was added to gel loading buffer
(pH, 6.8; 125 mM 2× Tris-HCl; 4% SDS; 20% glycerol; 0.1%
bromophenol blue; 2.5% β-mercaptoethanol), boiled for 5 min,
separated by 10% SDS-polyacrylamide gel and then transferred onto
polyvinylidene difluoride membranes. The blotted membranes were
then incubated with primary polyclonal anti-PDE4D (sc-25814;
1:500), monoclonal anti-EGFR (sc-373746; 1:1,000), monoclonal anti
-phosphorylated (p)-EGFR (sc-57543), monoclonal anti-AKT (sc-5298;
1:1,000) and polyclonal anti-pAKT (sc-33437; 1:300) antibodies,
which were purchased from Santa Cruz Biotechnology, Inc., at 4°C
overnight. After being washed with Tris-buffered saline with Tween
20 (SunShine Biotechnology Co., Ltd., Nanjing, China), the
membranes were incubated with monoclonal horseradish
peroxidase-conjuagted anti-rabbit or anti-mouse secondary
antibodies for 1 h at room temperature. Protein bands were
visualized using BeyoECL Plus Detection System (Beyotime Beyotime
Institute of Biotechnology, Jiangsu, China). Protein expression
levels were quantified using FluorChem FC2 and presented as the
densitometric ratio of the targeted protein to β-actin. Cell
protein lysate assays were performed in triplicate.
Colony-forming assay
The cells were plated on six-well plates at
3×102 cells per well and grown for 14 days. Next, the
cells were washed twice with phosphate-buffered saline (SunShine
Biotechnology Co., Ltd.), fixed with methanol/acetic acid (3:1;
v/v: SunShine Biotechnology Co., Ltd.) and stained with 0.5%
crystal violet (C3886; Sigma-Aldrich). The number of colonies was
counted under a microscope (TE2000, Nikon Corp., Tokyo, Japan).
EGF stimulation assay
CNE2 cells infected with ctr-shRNA or LV-PDE4D shRNA
were treated with 100 ng/ml recombinant human EGF (AF-100-15;
PerproTech, Rocky Hill, NJ, USA) for 2 h. Western blot analysis was
used to detect protein levels at various treatment times (0, 10, 60
min and 6 h). The cell viability assay, cell cycle analysis and
colony-forming assay were applied to detect the cell proliferation
following EGF stimulation.
Nude mice experiment
Five-week-old male BALB/c nude mice were purchased
from the Institute of Laboratory Animal Sciences (Beijing, China).
The CNE2 cells infected with LV-PDE4D shRNA and control (referred
to as the LV-PDE4D shRNA and ctr-shRNA groups, respectively) were
isolated. A total of 5×105 cells of each type were
injected subcutaneously into the dorsal flanks of two groups of
nude mice, with each group containing five mice. Tumor size was
measured every two days and the tumor volume was calculated as
follows: Tumor volume (mm3) = (length ×
width2) × 0.5. Two weeks later, the mice were sacrificed
using cervical dislocation and the tumors were dissected. The
experiment was repeated three times. The use of animals in the
present study complies with the Guide for the Care and Use of
Laboratory Animals (National Research Council) (20). The study was approved by the
Institutional Animal Care and Use Committee, Wuxi, Jiangsu,
China.
Immunohistochemistry
Immunohistochemistry was performed as described
previously (19). Formalin-fixed,
paraffin-embedded tissues of the transplanted tumors were sectioned
to a 4-mm thickness and analyzed for anti-p-EGFR and anti-pAKT
expression. Visualization was achieved using the EnVision
peroxidase system (Dako, Carpinteria, CA, USA). The degree of
immunostaining of the formalin-fixed, paraffin-embedded sections
was reviewed and scored by two independent pathologists in a
blinded manner. The percentage of positively-stained nuclear tumor
cells was recorded. Scoring was determined according to the
percentage of positive cells as follows: 1, ≤5% of cells; 2, 6–35%
of cells; 3, 36–70% of cells; and 4, ≥71% of cells. An additional
score was determined according to the intensity of staining as
follows: 1, negative staining; 2, weak staining (light yellow); 3,
moderate staining (yellowish brown); and 4, strong staining (brown)
(20).
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation. The statistical significance of the differences
between the two groups was analyzed using a two-sided, unpaired
Student’s t-test for equal variances or Welch’s corrected t-test
for unequal variances. Statistical analysis was performed using
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
PDE4D is upregulated in clinical
specimens and human NPC cell lines
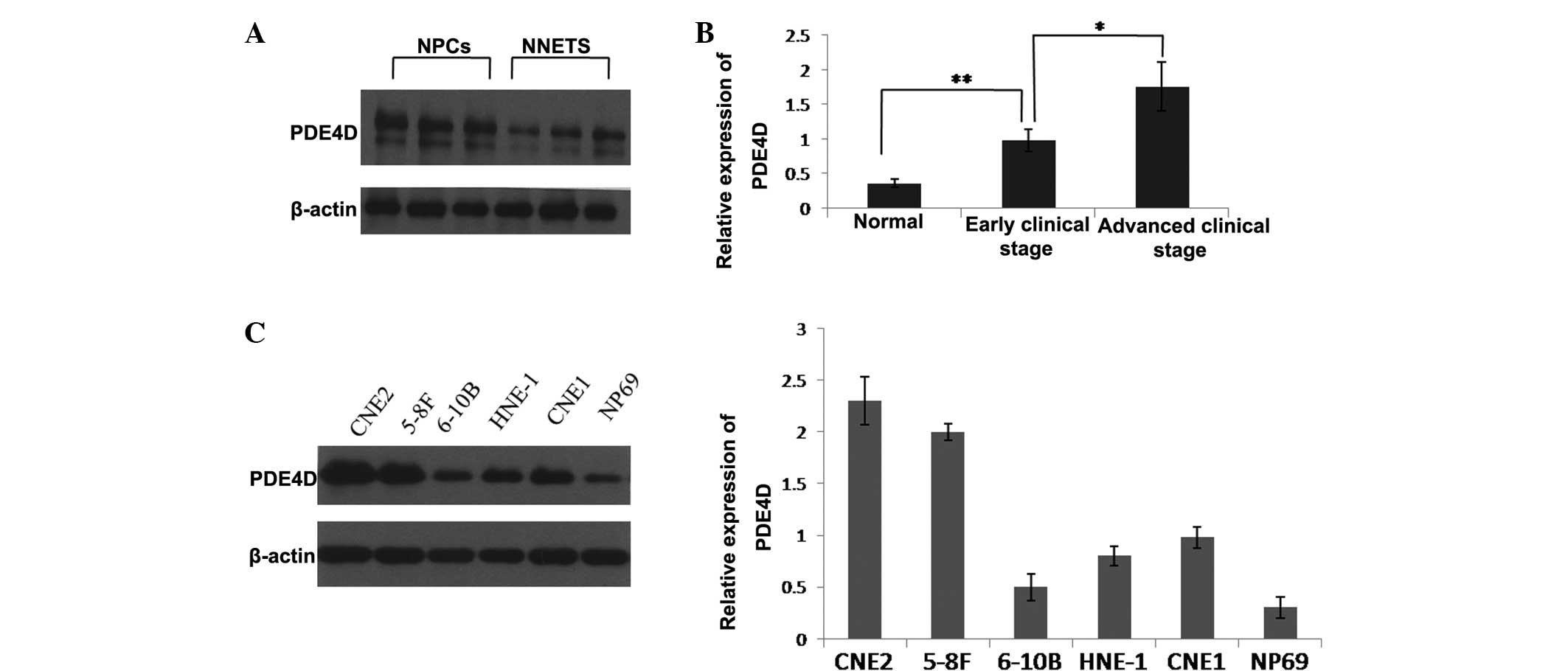
The clinical significance of PDE4D was investigated
in 40 human NPC samples. The results showed that PDE4D was
significantly upregulated in the NPC samples compared with the
normal nasopharynx epithelial samples (Fig. 1A). The upregulation of PDE4D
expression was found to correlate with an advanced clinical stage
of NPC (Fig. 1B). The expression of
PDE4D was further examined in human NPC cell lines and the
immortalized non-tumorigenic NP69 cell line. Western blot analysis
showed that PDE4D was increased in all five NPC cell lines compared
with the NP69 cell line. Among the five NPC cell lines, CNE2 and
5–8F showed relatively higher PDE4D expression levels, while 6–10B
showed a relatively lower PDE4D expression level (Fig. 1C).
Knockdown of PDE4D inhibits the growth of
NPC cells
CNE2 and 5–8F cells were selected for further study
due to their relatively high PDE4D expression level. The results
revealed that the infection efficiency of the CNE2 and 5–8F cells
was 89.68±1.7 and 87.32±1.54%, respectively. In addition, the
western blot analysis showed that PDE4D was successfully inhibited
in the CNE2 and 5–8F cells (80.5±1.6 and 83.23±2.4%, respectively;
P<0.01; Fig. 2A). To explore the
effect of PDE4D on NPC cell growth, the MTT assay was applied to
detect proliferation. It was demonstrated that CNE2 cell growth was
inhibited by 36.3% (P<0.05), while 5–8F cell growth was
inhibited by 29.7% (P<0.05) (Fig.
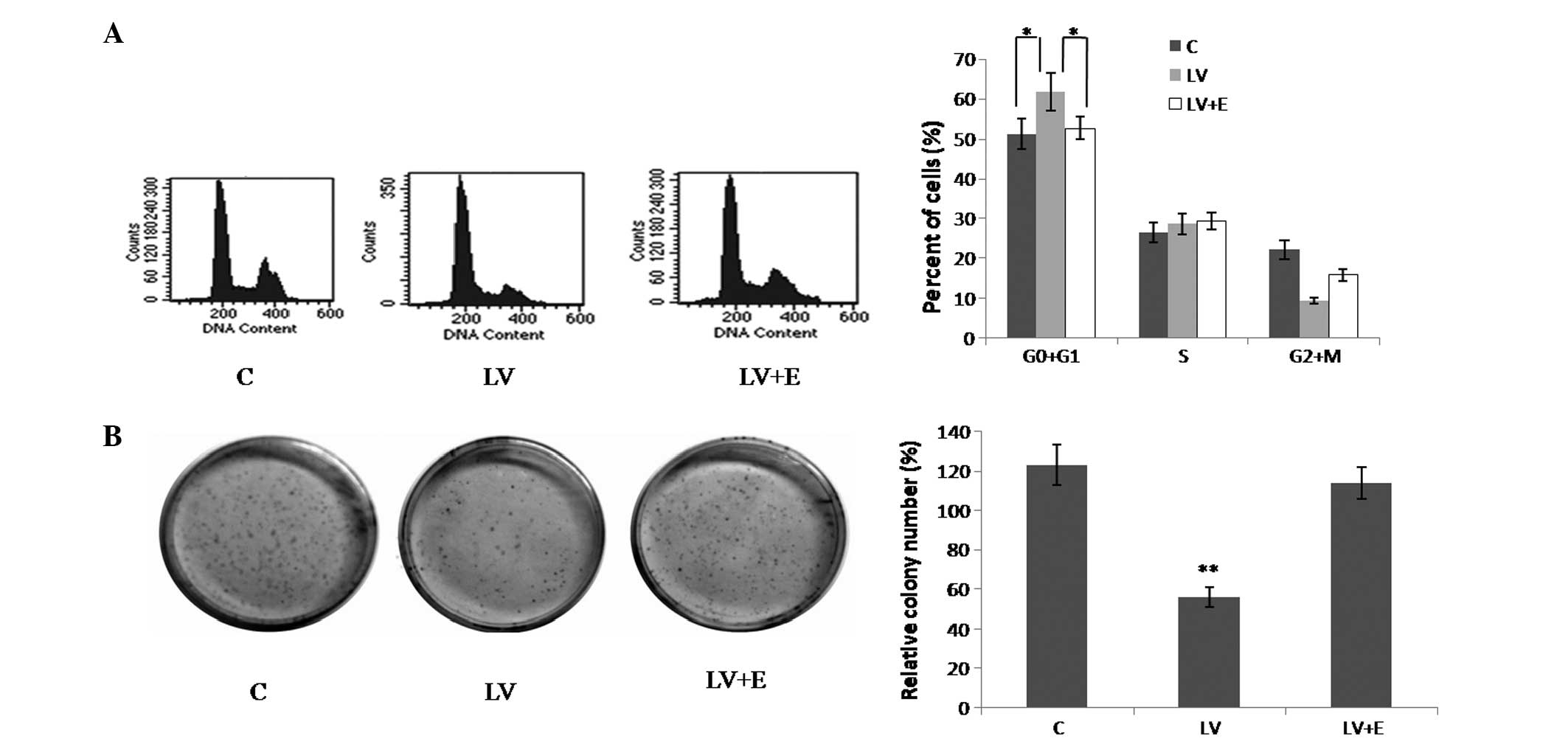
2B). As revealed in the colony formation assay, CNE2 cells
infected with LV-PDE4D shRNA exhibited much smaller colonies
compared with the control cells (Fig.
3B).
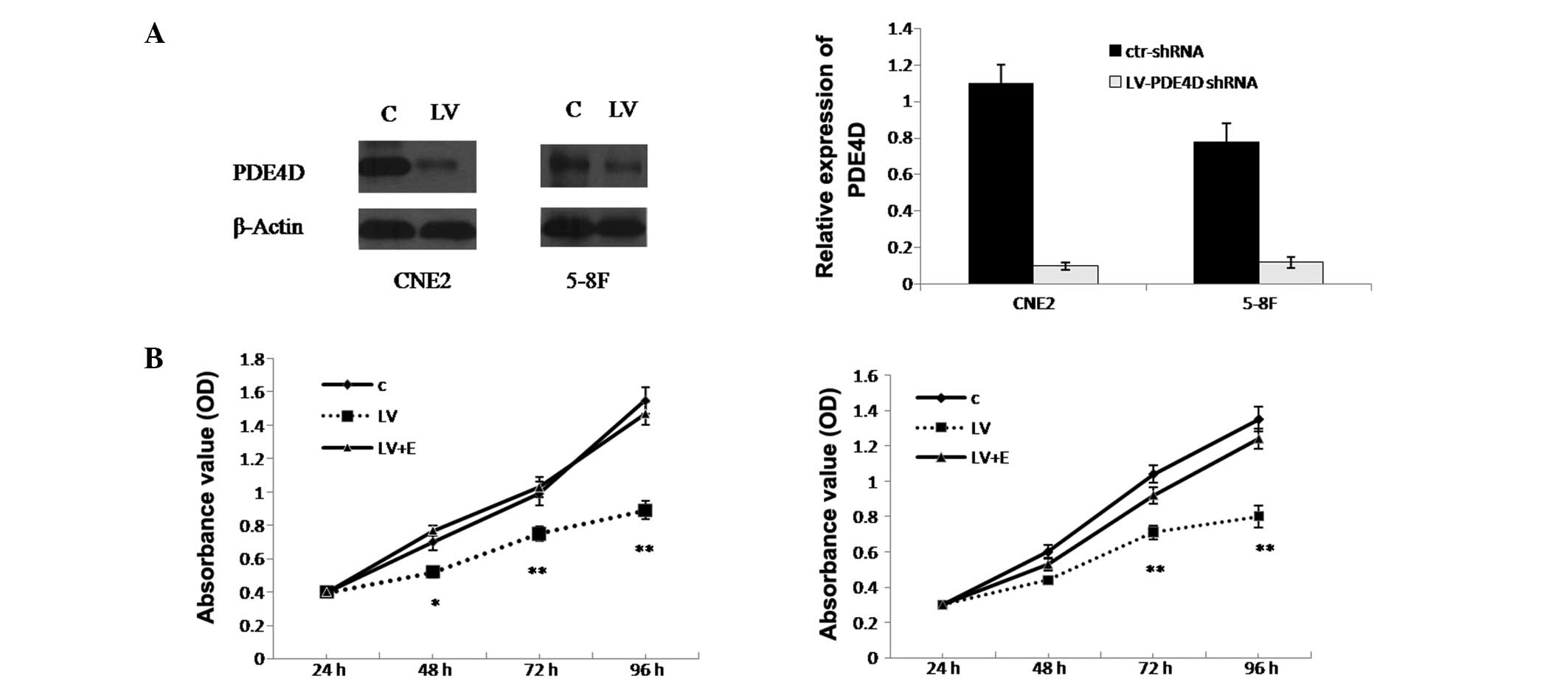 | Figure 2Knockdown of PDE4D inhibits the growth
of NPC cells, which is reversed by EGF stimulation. (A) Western
blot analysis results and graph presenting the infection efficiency
of PDE4D-targeted shRNA lentiviral particles in CNE2 and 5–8F
cells. Following infection, PDE4D expression was significantly
inhibited in the two cell lines. (B) Effect of PDE4D-targeted shRNA
lentiviral particles on cell proliferation measured by MTT assay
following infection in CNE2 and 5–8F cells. The effect of EGF
stimulation on the proliferation of the NPC cells, which were
infected with PDE4D-targeted shRNA lentiviral particles, is also
shown. *P<0.05 and **P<0.01 vs. C and
LV+E. PDE4D, phosphodiesterase 4D; NPC, nasopharyngeal carcinoma;
EGF, epidermal growth factor; shRNA, short hairpin RNA; OD, optical
density; C, cells infected with control shRNA lentiviral particles;
LV, cells infected with PDE4D-targeted shRNA lentiviral particles;
LV+E, cells infected with PDE4D-targeted shRNA lentiviral particles
followed by 100 ng/ml EGF treatment; ctr-shRNA, control shRNA
lentiviral particles. |
Knockdown of PDE4D induces cell cycle
arrest in the G0/G1 phase in CNE2 cells
Flow cytometry was performed to examine the CNE2
cell cycle. The results showed that compared with the control, the
CNE2 cells infected with LV-PDE4D shRNA exhibited an increased
percentage of cells in the G0/G1 phase and
fewer cells in the G2/M phase (Fig. 3A; P<0.05).
Knockdown of PDE4D inhibits the
phosphorylation of EGFR and AKT
The expression of EGFR is common in NPC tissues and
cell lines, and the EGFR/PI3K/AKT signaling pathway is important in
the pathogenesis of NPC (20).
However, the effect of PDE4D on the EGFR/PI3K/AKT signaling pathway
remains unknown. The present study investigated whether the
EGFR/PI3K/AKT axis is associated with the PDE4D-cAMP axis in CNE2
cells. p-EGFR and p-AKT were found to be expressed in the control
CNE2 cells. Following the stable knockdown of PDE4D, p-EGFR and
p-AKT levels were significantly decreased (Fig. 4A). Furthermore, following treatment
by 100 ng/ml recombinant human EGF for 2 h, the CNE2 cells infected
with LV-PDE4D shRNA showed increased phosphorylation of EGFR and
AKT (Fig. 4A).
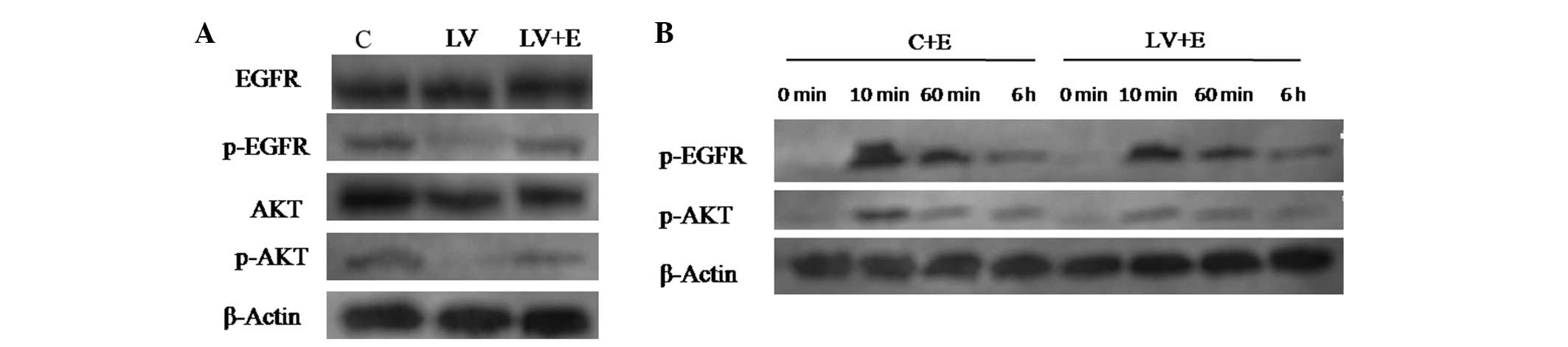 | Figure 4Western blot analysis of EGFR
signaling pathway proteins of the CNE2 cells following infection
with LV-PDE4D shRNA and EGF stimulation. (A) Knockdown of PDE4D
inactivates EGFR and AKT in CNE2 cells, and EGF stimulation
reverses the efficiency of LV-PDE4D shRNA. (B) Various time points
following stimulation with 100 ng/ml of EGF. The phosphorylation of
EGFR and AKT for C+E compared with LV+E cells. After 10 min, EGF
was found to promote the phosphorylation of of EGFR and AKT,
however, following PDE4D knockdown, EGF stimulation decreased. Each
experiment was performed in triplicate and the mean value was
calculated. PDE4D, phosphodiesterase 4D; NPC, nasopharyngeal
carcinoma; EGFR, epidermal growth factor receptor; shRNA, short
hairpin RNA; C, cells infected with control shRNA lentiviral
particles; LV, cells infected with PDE4D-targeted shRNA lentiviral
particles; LV+E, cells infected with PDE4D-targeted shRNA
lentiviral particles followed by 100 ng/ml EGF treatment; C+E,
control cells stimulated by 100 ng/ml EGF; p-EGFR, phosphorylated
EGFR; p-AKT, phosphorylated AKT. |
A further 100 ng/ml EGF was added to the CNE2 cells
infected with ctr-shRNA and LV-PDE4D shRNA. At ~10 min, EGF began
to promote the phosphorylation of EGFR and AKT. However, as time
went by (0 min–6 h), the extent of EGFR and AKT phosphorylation was
gradually weakened. The LV-PDE4D shRNA-infected CNE2 cells showed
inferior phosphorylation of the two proteins compared with the
control following EGF stimulation (Fig.
4B).
EGF stimulation reverses the effect of
LV-PDE4D shRNA on NPC cells
As demonstrated by MTT assay (Fig 2B), following treatment with 100 ng/ml
recombinant human EGF, the LV-PDE4D shRNA-infected CNE2 and 5–8F
cells exhibited increased cell proliferation compared with the
untreated cells, which reversed the effect of LV-PDE4D shRNA on the
NPC cells. Similar results were detected in the colony formation
assay of the CNE2 cells (Fig.
3B).
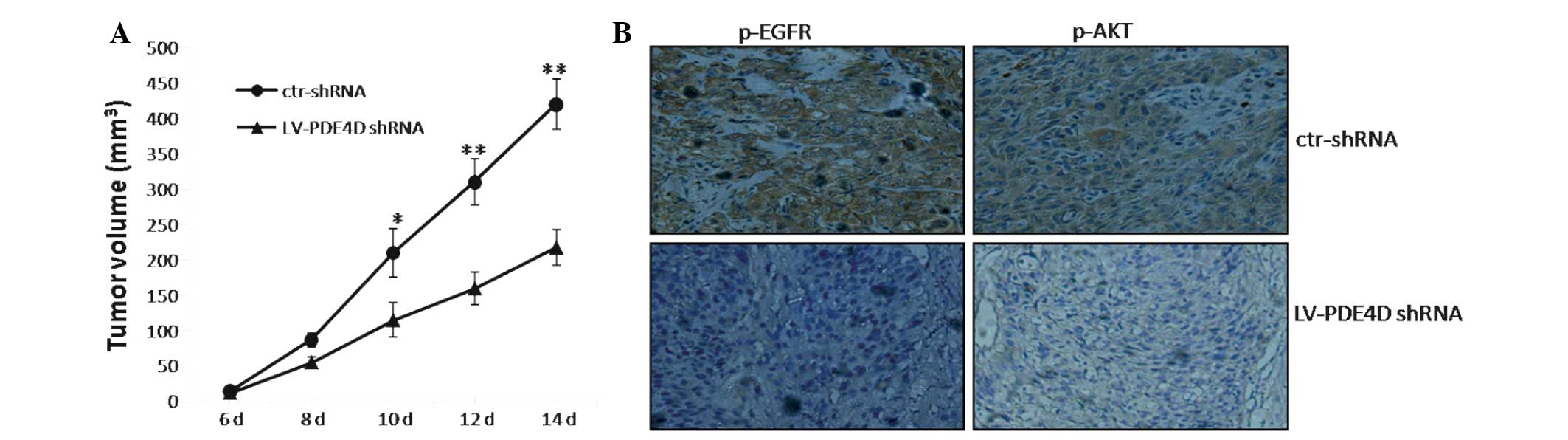
Knockdown of PDE4D suppresses the tumor
growth of NPC cells in nude mice
The CNE2 cells that were infected with LV-PDE4D
shRNA were injected subcutaneously into the dorsal flank of the
nude mice. The tumor became palpable between six and seven days
post-inoculation. All the mice developed tumors at the end of the
experiment. At ~10 days post-implantation, a statistically
significant difference was identified between the volume of the
transplanted tumors in the two groups (P<0.05; Fig. 5A). At two weeks post-implantation,
the mice injected with LV-PDE4D shRNA carried smaller burdens
compared with the controls. The average tumor volume and weight of
the LV-PDE4D shRNA-treated group were markedly reduced compared
with the control group (P=0.023 and P=0.034, respectively). In
addition, the staining intensity of p-EGFR and p-AKT in the tumor
cells was significantly decreased compared with the control
(P<0.05; Fig. 5B).
Discussion
It has been reported that PDE4D functions as a
proliferation-promoting factor in certain cancer types, including
HNC (4–7). From genomic investigation, Lin et
al (7) showed that the PDE4D
gene was highly expressed in HNC. Analysis of the survival data
from the Cancer Genome Atlas projects showed that high PDE4D mRNA
expression levels are associated with a poor prognosis in HNC.
However, there has been no further investigation on PDE4D in HNC.
PDE4D was of great interest in the present study of NPC, as NPC is
a unique type of HNC that has a high incidence in Southern China
(8).
The present study first examined PDE4D in human NPC
tissues and cells. The results revealed that PDE4D was
significantly upregulated in the NPC samples and cells, which was
found to correlate with an advanced clinical stage of NPC. These
results are consistent with the study by Lin et al (7), which used immunohistochemical staining
to show that PDE4D proteins are overexpressed in ovarian and
endometrial tumors and melanomas, compared with corresponding
non-transformed tissues (7). In the
present study, for the expression of PDE4D in the NPC cells, the
poorly-differentiated CNE2 cell line and the 5–8 F cell line with
high metastatic potential exhibited relatively higher expression
levels. While the highly-differentiated CNE1 cell line and the 6–10
cell line with poor metastatic potential exhibited relatively lower
expression levels, which suggested that PDE4D may correlate with
the differentiation and metastatic potential of the NPC cells.
Subsequently, the CNE2 and 5–8 F cell lines were selected for
further in vitro experiments.
To examine the role of PDE4D in the growth of NPC
cells, LV-PDE4D-shRNA infection was used to knockdown PDE4D
expression in the CNE2 and 5–8F cells. The results showed that the
knockdown of PDE4D significantly inhibited the cell growth of the
two cell lines. The cells were then treated with 100 ng/ml EGF,
which reversed the cell proliferation inhibition induced by
LV-PDE4D-shRNA. In addition, the colony formation assay showed a
similar result in the CNE2 cells. The cell cycle assay further
demonstrated that LV-PDE4D-shRNA inhibits CNE2 cell proliferation
by inducing a G0/G1 arrest, which was also
reversed by EGF stimulation. These results suggested that PDE4D may
function by affecting the EGFR signal pathway in the growth of NPC
cells.
The EGFR is commonly expressed in NPC cells and is
one of the identified molecular targets for cancer treatment
(23). In various NPC cell lines,
the downregulation of EGFR by monoclonal antibodies and the use of
specific pharmacological inhibitors have been shown to inhibit the
EGFR signaling pathway and subsequently induce cell cycle arrest
and cell proliferation suppression (24–26).
However, the association between the EGFR signaling pathway and
PDE4D has not been reported in cancer cells. In the present study,
to further demonstrate the potential crosstalk existing between the
PDE4D and EGFR/AKT axis, the EGFR signaling pathway was detected in
the CNE2 cells by western blot analysis. It was demonstrated that
following the knockdown of PDE4D in the CNE2 cells, the
phosphorylation of EGFR and AKT were significantly inhibited.
Following treatment with 100 ng/ml EGF, this inhibition was
reversed. EGF-stimulated LV-PDE4D shRNA CNE2 cells showed inferior
phosphorylation of EGFR and AKT compared with the control following
EGF stimulation. Overall, the results suggested that the knockdown
of PDE4D may suppress NPC cell growth through inhibition of the
EGFR/AKT signaling pathway.
In vivo experiments further demonstrated that
the knockdown of PDE4D suppressed tumorigenesis in a NPC murine
xenograft model, suggesting that PDE4D may be a tumor-promoting
factor in NPC. These results are consistent with the findings in
melanoma, and breast and ovarian cancer (7). In order to demonstrate the crosstalk
between PDE4D and the EGFR/PI3K/AKT axis, p-EGFR and p-AKT were
examined in a nude mouse xenograft. The xenograft, which was
injected with LV-PDE4D-shRNA-infected CNE2 cells, showed lower
p-EGFR and p-AKT expression levels compared with the control
xenograft. This suggested that PDE4D affects the EGFR/PI3K/AKT
signaling pathway not only in vitro, but also in
vivo, which is not affected by the internal environment.
Taken together, the present study demonstrated that
the knockdown of PDE4D inhibits cell growth in NPC, partly by
affecting the EGFR signal pathway, by suppressing the
phosphorylation of EGFR and AKT. This suggests that PDE4D may
contribute to the proliferation of NPC cells, and therefore, PDE4D
is predicted to be a potential therapeutic target for the treatment
of NPC.
Acknowledgements
This study was supported by grants from the
Foundation of Nanjing Medical University (no. 2011NJMU112) and the
key program of Medical Science and Technology Development Fund of
the Medical Control Center in Wuxi City (no. YGZ1111).
References
|
1
|
Houslay MD: Adaptation in cyclic AMP
signalling processes: a central role for cyclic AMP
phosphodiesterases. Semin Cell Dev Biol. 9:161–167. 1998.
|
|
2
|
Zhang L, Murray F, Zahno A, et al: Cyclic
nucleotide phosphodiesterase profiling reveals increased expression
of phosphodiesterase 7B in chronic lymphocytic leukemia. Proc Natl
Acad Sci USA. 105:19532–19537. 2008.
|
|
3
|
Marko D, Romanakis K, Zankl H,
Furstenberger G, Steinbauer B and Eisenbrand G: Induction of
apoptosis by an inhibitor of cAMP-specific PDE in malignant murine
carcinoma cells overexpressing PDE activity in comparison to their
nonmalignant counterparts. Cell Biochem Biophys. 28:75–101.
1998.
|
|
4
|
Rahrmann EP, Collier LS, Knutson TP, et
al: Identification of PDE4D as a proliferation promoting factor in
prostate cancer using a Sleeping Beauty transposon-based somatic
mutagenesis screen. Cancer Res. 69:4388–4397. 2009.
|
|
5
|
Pullamsetti SS, Banat GA, Schmall A, et
al: Phosphodiesterase-4 promotes proliferation and angiogenesis of
lung cancer by crosstalk with HIF. Oncogene. 32:1121–1134.
2012.
|
|
6
|
Kolosionek E, Savai R, Ghofrani HA, et al:
Expression and activity of phosphodiesterase isoforms during
epithelial mesenchymal transition: the role of phosphodiesterase 4.
Mol Biol Cell. 20:4751–4765. 2009.
|
|
7
|
Lin DC, Xu L, Ding LW, et al: Genomic and
functional characterizations of phosphodiesterase subtype 4D in
human cancers. Proc Natl Acad Sci USA. 9:6109–6114. 2013.
|
|
8
|
Lo KW, Chung GT and To KF: Deciphering the
molecular genetic basis of NPC through molecular, cytogenetic, and
epigenetic approaches. Semin Cancer Biol. 22:79–86. 2012.
|
|
9
|
Spano JP, Busson P, Atlan D, Bourhis J,
Pignon JP, Esteban C and Armand JP: Nasopharyngeal carcinomas: an
update. Eur J Cancer. 39:2121–2135. 2003.
|
|
10
|
Lee AW, Poon YF, Foo W, et al:
Retrospective analysis of 5037 patients with nasopharyngeal
carcinoma treated during 1976–1985: overall survival and patterns
of failure. Int J Radiat Oncol Biol Phys. 23:261–270. 1992.
|
|
11
|
Chua DT, Nicholls JM, Sham JS and Au GK:
Prognostic value of epidermal growth factor receptor expression in
patients with advanced stage nasopharyngeal carcinoma treated with
induction chemotherapy and radiotherapy. Int J Radiat Oncol Biol
Phys. 59:11–20. 2004.
|
|
12
|
Leong JL, Loh KS, Putti TC, Goh BC and Tan
LK: Epidermal growth factor receptor in undifferentiated carcinoma
of the nasopharynx. Laryngoscope. 114:153–157. 2004.
|
|
13
|
Ruan L, Li XH, Wan XX, et al: Analysis of
EGFR signaling pathway in nasopharyngeal carcinoma cells by
quantitative phosphoproteomics. Proteome Sci. 9:352011.
|
|
14
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signaling network: receptor heterodimerization in
development and cancer. EMBO J. 19:3159–3167. 2000.
|
|
15
|
Blagoev B, Ong SE, Kratchmarova I and Mann
M: Temporal analysis of phosphotyrosine-dependent signaling
networks by quantitative proteomics. Nat Biotechnol. 22:1339–1345.
2004.
|
|
16
|
Berg M and Soreide K: EGFR and downstream
genetic alterations in KRAS/BRAF and PI3K/AKT pathways in
colorectal cancer: implications for targeted therapy. Discov Med.
14:207–214. 2012.
|
|
17
|
Chinese Committee for Staging of
Nasopharyngeal Carcinoma. Report on revision of the Chinese 1992
staging system for nasopharyngeal carcinoma. J Radiat Oncol.
2:233–240. 2013.
|
|
18
|
Xu T, Xiao D and Zhang X: ECRG4 inhibits
growth and invasiveness of squamous cell carcinoma of the head and
neck in vitro and in vivo. Oncol Lett. 5:1921–1926.
2013.
|
|
19
|
Xu T, Yu CY, Sun JJ, et al: Bone
morphogenetic protein-4-induced epithelial-mesenchymal transition
and invasiveness through Smad1-mediated signal pathway in squamous
cell carcinoma of the head and neck. Arch Med Res. 42:128–137.
2011.
|
|
20
|
National Research Council. Guide for the
Care and Use of Laboratory Animals. 8th edition. The National
Academies Press; Washington, DC: 2011
|
|
21
|
Efferson CL, Winkelmann CT, Ware C, et al:
Downregulation of Notch pathway by a gamma-secretase inhibitor
attenuates AKT/mammalian target of rapamycin signaling and glucose
uptake in an ERBB2 transgenic breast cancer model. Cancer Res.
70:2476–2484. 2010.
|
|
22
|
Yip WK, Leong VC, Abdullah MA, Yusoff S
and Seow HF: Overexpression of phospho-Akt correlates with
phosphorylation of EGF receptor, FKHR and BAD in nasopharyngeal
carcinoma. Oncol Rep. 19:319–328. 2008.
|
|
23
|
Baselga J: The EGFR as a target for
anticancer therapy - focus on cetuximab. Eur J Cancer. 37(Suppl 4):
S16–S22. 2001.
|
|
24
|
Sung FL, Pang RT, Ma BB, Lee MM, Chow SM,
Poon TC and Chan AT: Pharmacoproteomics study of cetuximab in
nasopharyngeal carcinoma. J Proteome Res. 5:3260–3267. 2006.
|
|
25
|
Zhu XF, Liu ZC, Xie BF, Li ZM, Feng GK,
Yang D and Zeng YX: EGFR tyrosine kinase inhibitor AG1478 inhibits
cell proliferation and arrests cell cycle in nasopharyngeal
carcinoma cells. Cancer Lett. 169:27–32. 2001.
|
|
26
|
Huang WC, Hsu RM, Chi LM, Leu YL, Chang YS
and Yu JS: Selective downregulation of EGF receptor and downstream
MAPK pathway in human cancer cell lines by active components
partially purified from the seeds of Livistona chinensis R.
Brown. Cancer Lett. 248:137–146. 2007.
|