Introduction
Endometrial carcinoma (EC) is the second most common
gynecological malignancy of the female genital tract worldwide
(1). In the United States, with
47,130 new cases and 8,010 mortalities projected in 2012 (2), the majority of women (80–85%) present
with early-stage disease, and surgery in the form of hysterectomy
and bilateral salpingo-oophorectomy is curative. However, a
proportion of the cases present with advanced disease or develop
disease recurrence or metastasis, which are associated with poor
survival (3).
Histologically, endometrial cancer can be divided
into two types (4). The common and
estrogen-dependent type is called type I cancer, and is generally
diagnosed at an early stage and as a result has an improved
prognosis. However, certain cases with advanced stage and high
tumor grade have a poor survival. Type II cancer, which usually has
a papillary serous or clear cell pattern, is likely associated with
p53 mutation. The probability of surviving 5 years with this type
of cancer is considerably lower than that for the type I form, even
with early stage diseases (5).
Therefore, there is an urgent requirement for the identification of
new therapeutic targets.
Enhancer of zeste homolog 2 (EZH2), a critical
component of the polycomb repressive complex 2, has intrinsic
histone methyl transferase activity that mediates gene silencing by
catalyzing trimethylation on lysine 27 of histone H3 (6). EZH2 has been found to be involved in
multiple biological processes, such as tumor proliferation
(7), cell cycle, senescence
(8), metastasis and angiogenesis
(9). EZH2 is overexpressed in
aggressive forms of prostate (10),
breast (11) and bladder (12) cancer.
Nevertheless, EZH2 expression is associated with a
high proliferation rate and aggressive tumor subgroups of
endometrial cancer (13). EZH2
expression has been found to be positively associated with
lipocalin 2 expression, which is associated with aggressive
features of endometrial cancer (14). Inhibition of EZH2 expression is
associated with decreased tumor cell proliferation, migration and
invasion in endometrial cancer cell lines, which is parallel to an
increased expression of Wnt pathway inhibitors, sFRP1 and DKK3, and
a concomitant decrease in β-catenin levels (15).
However, the role of EZH2 in endometrial cancer has
not been fully determined. In the present study, the expression of
EZH2 in endometrial cancer and precancerous lesions was evaluated,
and the potential role of EZH2 in endometrial cancer cell
proliferation was further investigated.
Materials and methods
Cell culture
Human endometrial carcinoma cell lines, Hec-1a and
Ishikawa (provided by Dr Yinhua Yu; Anderson Cancer Center,
Houston, TX, USA), were maintained in McCoy’s 5A medium (Jinuo Co.,
Ltd, Shanghai, China) supplemented with 10% fetal bovine serum
(Gibco-BRL, Rockville, IN, USA). The cells were incubated at 37°C
in 5% CO2.
Tissue samples
A total of 92 endometrial tissues (including 24
normal endometrium, 14 simple hyperplasia, 6 complex hyperplasia,
15 atypical hyperplasia and 33 endometrial cancer samples) were
obtained from patients who underwent surgery between August 2008
and December 2012 at the Obstetrics and Gynecology Hospital, Fudan
University (Shanghai, China). Normal endometrium and simple and
complex hyperplasia samples were from patients who received
dilation and curettage, whereas atypical hyperplasia and cancer
tissues were from patients who received hysterectomy. All specimens
were reviewed by an experienced pathologist. The patients’
demographic profiles and the pathology files were tabulated. For
endometrial cancer patients, the clinicopathological factors, such
as age, tumor grade, depth of myometrial invasion, lymph-vascular
space invasion (LVSI) and nodal metastasis, were analyzed. The
surgical pathology stage was determined by the 1998 International
Federation of Gynecology and Obstetrics (FIGO) guidelines (16). All patients provided written
informed consent permitting the use of their tissue for research at
the time specimens were collected. This study was approved by the
institutional review board of the Obstetrics and Gynecology
Hospital, Fudan University.
Immunohistochemistry
Immunohistochemistry (IHC), antigen retrieval and
antibody dilution were optimized prior to the study onset. All
endometrium specimens were reviewed by experienced pathologists to
confirm the diagnosis. To ensure uniformity, all sections were
processed simultaneously. Four-micrometer paraffin sections
adjacent to the hematoxylin and eosin sections used for
histological assessment were mounted onto Superfrost Plus slides
(Menzel, Braunschweig, Germany). Slides were subjected to
immunoperoxidase staining for EZH2 (5246S; Cell Signaling
Technology, Inc., Danvers, MA, USA). Endogenous peroxidase activity
was blocked using 0.3% hydrogen peroxide. Antigen retrieval was
performed by heating the sections for 30 min in a microwave oven
with 10 mM sodium citrate (pH 6.0). The slides were then incubated
with monoclonal antibodies against EZH2 (1:50; rabbit anti-human,
-rat, -mouse and -monkey; Cell Signaling Technology, Inc.) at 4°C
overnight. Slides were washed and incubated with the biotinylated
secondary antibody (polyclonal goat anti-rabbit; Histostain-Plus
IHC kit; Mingrui Biotech, Shanghai, China) for 45 min at 37°C and
washed with phosphate-buffered saline. Slides were incubated with
avidin-biotin-peroxidase (Histostain-Plus IHC kit; Mingrui Biotech)
for 10 min at room temperature and incubated with diaminobenzidine
(Mingrui Biotech) for 2 min. Finally, slides were counterstained
with hematoxylin and evaluated at a magnification of ×200 using
light microscopy. The intensity of positive cells was graded from 0
to 3 (0, negative; 1, weak; 2, medium; 3, strong). The scores were
determined independently by two observers, and the average of their
scores was used for evaluation. For estrogen receptor (ER) (1:150),
progesterone receptor (PR) (1:160), p53 (1:500) and Ki-67(1:200)
(Dako, Glostrup, Denmark) detection, antigens were unmasked by
treating the slides with Target Retrieval Solution, High pH (Dako)
for 30 min at 95°C. High-grade serous adenocarcinoma of the ovary
was used as positive control for p53 and Ki-67, while ductal
carcinoma of the breast was used as positive control for ER and PR.
Expression of ER and PR was considered as positive when >1% of
the nuclei of cells in the epithelium showed immunoreactivity.
Expression of p53 was considered as positive when
immunohistochemical staining was observed in >5% of the nuclei
of cells in the epithelium. The percentage of the nuclei staining
of Ki-67 was determined independently by two observers.
Western blot analysis
Cells were lysed in RIPA buffer (150 mM NaCl, 50 mM
Tris-base, 5 mM EDTA, 1% NP-40, 0.25% deoxycholate, pH 7.4).
Protein concentrations were measured by the BCA protein assay
(23227; Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts
of protein were resolved by SDS-PAGE, transferred to PVDF membranes
and incubated with appropriate primary antibodies (monoclonal
rabbit anti-human EZH2 antibody, 5246S, 1:1000, Cell Signaling
Technology, Inc.; monoclonal rabbit anti-human GAPDH antibody,
5632–1, 1:5000, Epitomics, Burlingame, CA, USA). Immune complexes
were detected with a goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:5000; SSA005; Sino
Biological Inc., Beijing, China) and enhanced chemiluminescence
reagent (32109; Thermo Fisher Scientific).
EZH2 siRNA transfection
Hec-1a and Ishikawa cells were plated on six-well
plates at a density of 2×105 cells/well and grown
overnight until 30–40% confluency. The cells were transfected with
validated siRNA for EZH2 (sense: 5′-GUGUAUGAGUUUAGAGUCATT-3′) and a
scramble siRNA-FAM (negative control) (synthesized by Jima, Co.,
Ltd, Shanghai, China) at a concentration of 100 nM. using
Lipofectamine 2000 transfection reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. The medium was replaced with standard culture medium
12 h post-transfection. Transfection was repeated 72 h after the
first transfection.
In vitro cell proliferation assay
Cell proliferation was assayed using a cell
proliferation kit, Cell Counting Kit-8 (CCK-8; Dojindo Molecular
technologies, Inc., Kyushu, Japan) according to the manufacturer’s
instructions. Hec-1a and Ishikawa cells were seeded in sextuplicate
onto 96-well tissue culture plates at a density of 2×103
cells/well the day before EZH2 siRNA transfection. Cell growth was
analyzed at a wavelength of 450 nm at 0, 48, 72, 96 and 120 h after
transfection using Multiskan MK3 (Thermo Fisher Scientific).
Experiments were performed in triplicate.
Statistical analysis
The data are presented as the means ± standard
deviation. Statistical analyses were performed using SPSS 15.0
software (SPSS, Inc., Chicago, IL, USA). Two independent samples
non-parametric tests were utilized to analyze the
immunohistochemistry results. Fisher’s exact tests were utilized to
analyze the association between EZH2 methylation levels and
clinicopathological characteristics. Paired-sample t-tests were
utilized to analyze the CCK-8 results of EZH2 knockdown. P<0.05
was considered to indicate a statistically significant
difference.
Results
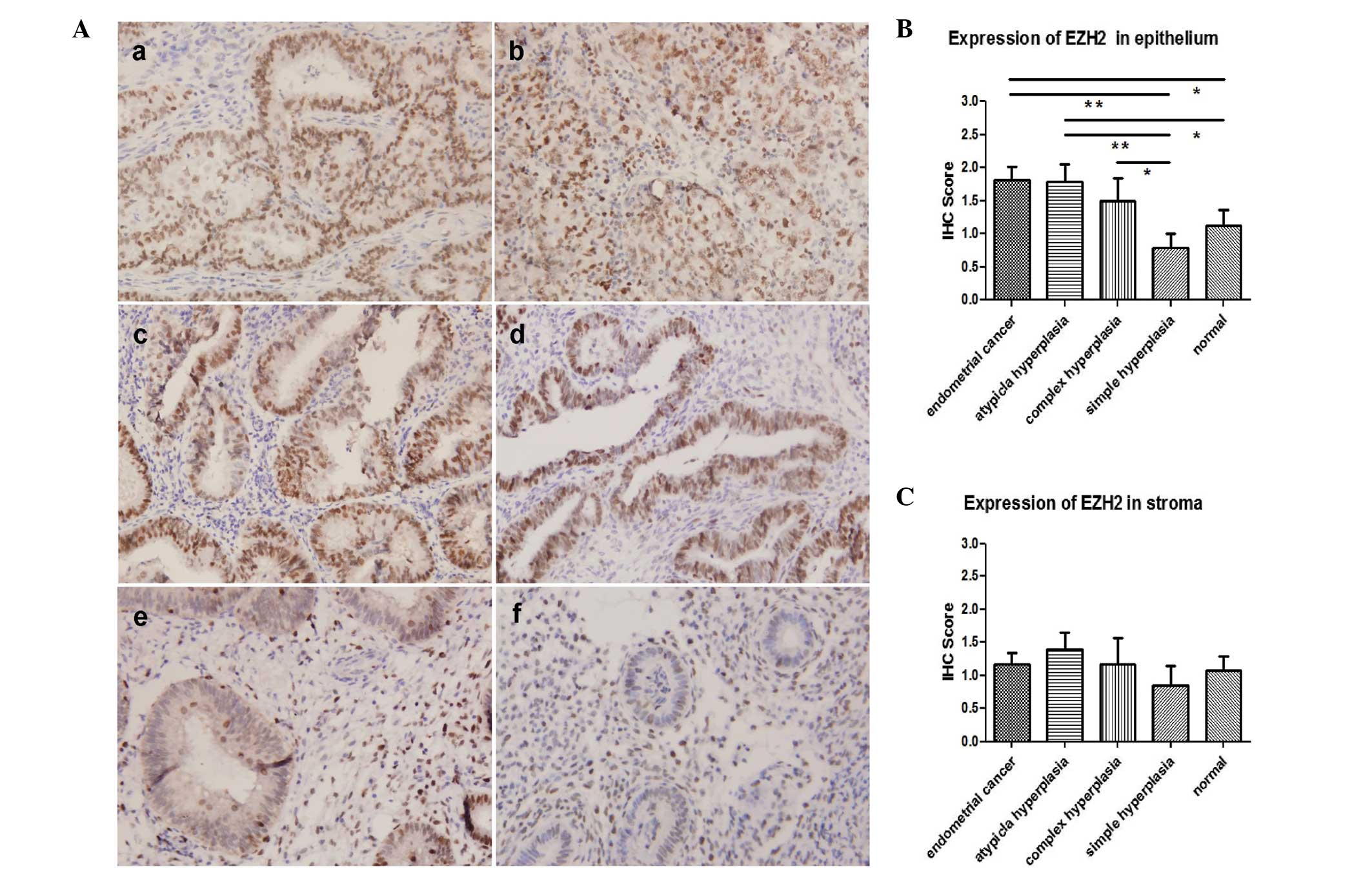
EZH2 is overexpressed in complex
hyperplasia, atypical hyperplasia and endometrial carcinoma
Immunohistochemistry was performed in 92 endometrium
tissues, including 24 normal endometrium (21 proliferative
endometrium, two secretory endometrium and one atrophic
endometrium; age range, 38–66), 14 simple hyperplasia (age range,
30–76), six complex hyperplasia (age range, 44–62), 15 atypical
hyperplasia (age range, 36–64) and 33 endometrial cancers (27 type
I and six type II; age range, 37–78). The age distribution was
similar among groups (P>0.05). Overexpression of EZH2 was
observed in the epithelium of endometrial cancer, atypical
hyperplasia and complex hyperplasia compared with the expression in
simple hyperplasia and normal endometrium. No difference was seen
among endometrial cancer, atypical hyperplasia and complex
hyperplasia, or between simple hyperplasia and normal endometrium,
in terms of epithelial EZH2 expression (Fig. 1A and B). Expression of EZH2 showed
no difference in the stroma among all groups (Fig. 1C).
Expression of EZH2 is associated with
myometrial invasion of endometrial cancer
The association between expression of EZH2 and
clinicopathological characteristics of 33 endometrial cancer
tissues was analyzed. The samples were grouped based on whether
they had high (IHC score, 2 or 3) or low (IHC score, 0 or 1)
expression of EZH2 (Table I). Tumor
grade; FIGO stage; depth of myometrial invasion; LVSI; nodal
metastasis status; ER, PR and p53 expression and Ki-67 labeling
index of the tumor were determined, and patients ranged in age from
37 to 78 years (medium age, 55 years). High expression of EZH2 was
observed in 64% of these cases. As shown in Table I, high EZH2 expression was
associated with deep myometrial invasion. In samples of low EZH2
expression, two cases (17%) were limited to the endometrium, 10
(83%) cases occupied less than half of the myometrium and none
occupied more than half of the myometrium. However, in the high
EZH2 expression group, 14 cases (67%) were found to occupy less
than half of the myometrium, while seven (33%) cases demonstrated
deep (≥1/2) myometrium infiltration (P=0.013). Cases with deeper
myometrial invasion were more likely to be EZH2-overexpressing.
 | Table IThe relationship between expression of
EZH2 and clinicopathological characteristics in endometrial
cancer. |
Table I
The relationship between expression of
EZH2 and clinicopathological characteristics in endometrial
cancer.
| EZH2 expression,
n | |
|---|
|
| |
|---|
| Score=0/1 | Score=2/3 | P-value |
|---|
| Case no. | 12 | 21 | |
| Age, years |
| <60 | 10 | 11 | 0.133 |
| ≥60 | 2 | 10 | |
| Tumor grade |
| G1 | 5 | 10 | 0.694 |
| G2 | 3 | 2 | |
| G3 | 2 | 5 | |
| Type 2 | 2 | 4 | |
| FIGO stage |
| I | 11 | 19 | 1.000 |
| II, III and IV | 1 | 2 | |
| Depth of myometrial
invasion |
| Limited to
endometrium | 2 | 0 | 0.013 |
| <1/2 | 10 | 14 | |
| ≥1/2 | 0 | 7 | |
| LVSI |
| No | 12 | 15 | 0.065 |
| Yes | 0 | 6 | |
| Nodal metastasis |
| Negative | 10 | 17 | 0.133 |
| Positive | 2 | 4 | |
| Estrogen
receptor |
| Negative | 2 | 3 | 1.000 |
| Positive | 10 | 18 | |
| Progesterone
receptor |
| Negative | 1 | 3 | 1.000 |
| Positive | 11 | 18 | |
| P53 |
| Negative | 9 | 15 | 1.000 |
| Positive | 3 | 6 | |
| Ki-67 |
| <10%
positive | 0 | 3 | 0.573 |
| 10–39% positive | 4 | 6 | |
| ≥40% positive | 8 | 12 | |
In addition, high EZH2 expression appeared to be
associated with the presence of LVSI. Although the P-value (0.065)
was not significant, this may have been due to the small sample
size. No correlation was noted between EZH2 expression and other
clinicopathological characteristics.
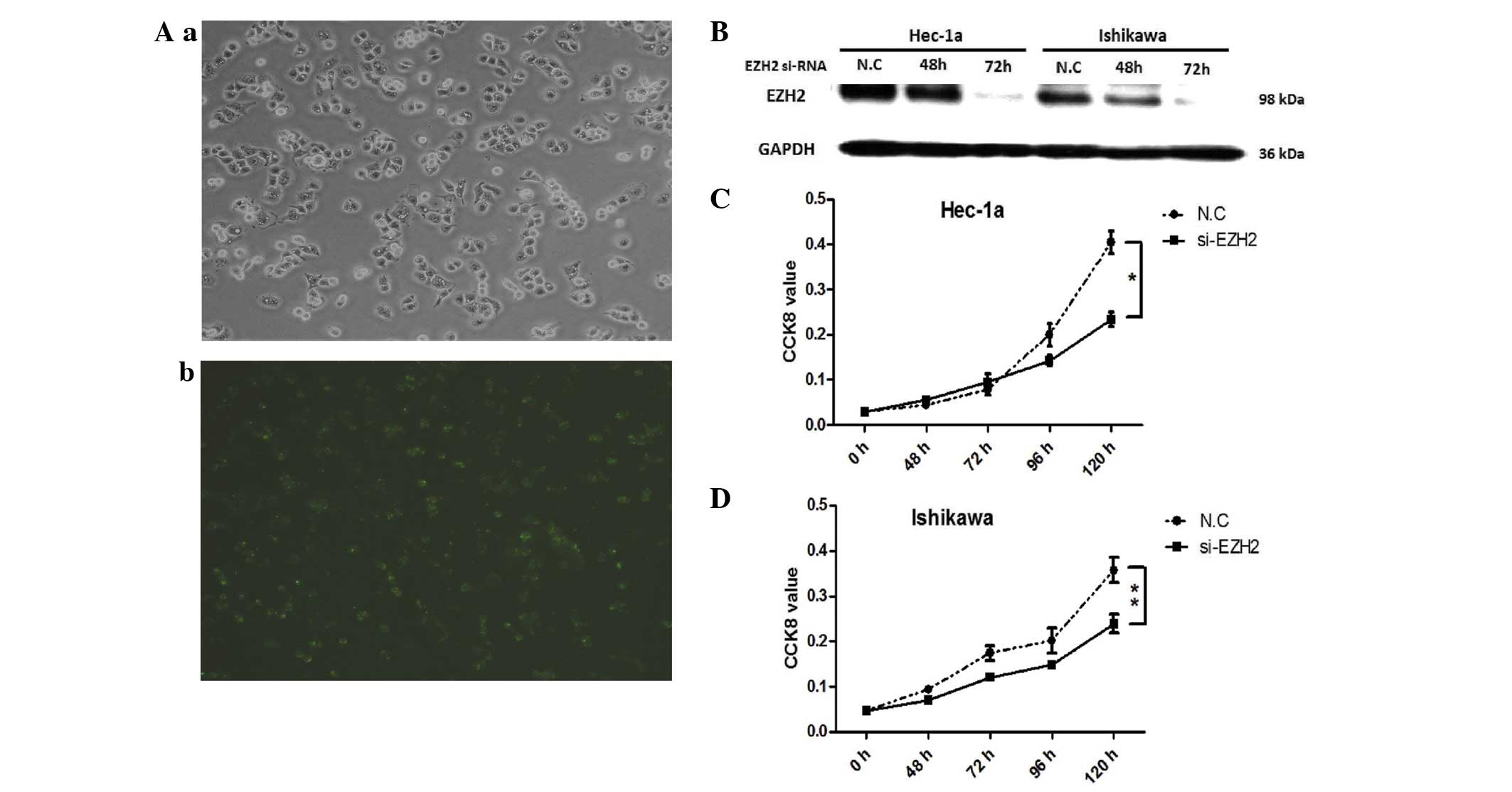
EZH2 is involved in cell proliferation of
endometrial carcinoma
To evaluate the effect of EZH2 on cell
proliferation, knockdown of EZH2 was performed in endometrial
cancer cells, Hec-1a and Ishikawa, by siRNA. Subsequently, cell
viability was analyzed using the CCK-8 assay. Scrambled siRNA,
labeled by FAM, was transfected at the same time to observe the
transfection efficiency (Fig. 2A),
and the knockdown effect on EZH2 was validated by western blotting
(Fig. 2B). The inhibition effect
started 48 to 96 h after cell transfection and was significantly
inhibited in Hec-1a and Ishikawa cells at 120h (Fig. 2C and D). Cell growth was inhibited
after EZH2 knockdown in endometrial cancer cells.
Discussion
The widely used World Health Organization system
classifies endometrial hyperplasia into four levels according to
glandular crowing and nuclear appearance: Simple, complex, simple
atypical and complex atypical hyperplasia (17). Simple hyperplasia refers to diffuse
and variably sized glands with a normal ratio of glands to stroma;
complex hyperplasia consists of architecturally irregular glands
and an increased gland-to-stroma ratio. When there is nuclear
enlargement with chromatin evenly dispersed or clumped, it is
called simple or complex atypical hyperplasia. In the present
study, normal endometrium and simple hyperplasia did not show
significantly elevated expression of EZH2, while complex
hyperplasia displayed significantly increased expression of EZH2.
Furthermore, high EZH2 expression also presented in the majority of
atypical hyperplasia and endometrial cancer samples. EZH2 starts to
become expressed in the precursor lesions of endometrial cancer,
which indicates that high EZH2 expression is an early event of
endometrial cancer carcinogenesis.
The identification of biological markers in normal
and hyperplastic endometrium that reliably predict an increased
risk of progression to endometrial cancer would provide important
clinical benefits. The long-term risk among women with simple or
complex hyperplasia is <5%, but the risk among women with
atypical hyperplasia is ~30% (18).
The results of the present study showed that there was a
significant difference in endometrial EZH2 levels between normal
subjects and patients with complex hyperplasia. This implicates
that endometrial EZH2 expression may be used as a screening
approach to identifying high-risk subpopulation with a potential to
progress to carcinoma. Current WHO classification of endometrial
hyperplasia is problematic due to poor diagnostic reproducibility.
The significant differences in epithelial expression of EZH2 may
provide clues to identify simple or complex hyperplasia.
Moreover, the present study demonstrated that EZH2
overexpression was associated with myometrial invasion in
endometrial cancer. The high level expression of EZH2 (IHC score, 2
or 3) was observed in 100% of cases with deep (≥1/2) myometrial
infiltration, while no patients with low EZH2 expression exhibited
deep myometrial infiltration. This indicates that endometrial
cancers with high expression levels of EZH2 tend to be more
invasive.
EZH2 has been reported to be involved in the
regulation of invasion-related factors, including E-cadherin,
β-catenin and MMP9 (19). By
silencing EZH2, the mRNA expression levels of E-cadherin and
Keratin 18 increased by 177 and 158%, respectively; while
mesenchymal markers, β-catenin and N-cadherin, decreased by 18.04
and 41.18%, respectively, in nasopharyngeal carcinoma cells
(20). However, overexpression of
EZH2 was correlated with reduced expression of E-cadherin, which
led to reduced cell migration and invasion (21). Moreover, knocking down EZH2
expression suppresses the cell invasion by downregulating E2F1 and
MMP9 in endometrial (22) and in
colorectal (23) cancer. In the
present study, the finding that EZH2 was associated with deep
myometrial invasion supports the hypothesis that EZH2 may be an
important factor in the invasion of endometrial cancer cells by
regulating E-cadherin, β-catenin and MMP9.
The depth of myometrial invasion is an important
factor in prognosis, determination of clinical stage or surgical
procedure selection. The differential expression of EZH2 in stage
IA, IB and IC endometrial cancer was not significantly different
and indicated that it is more important in the discrimination of
early-stage endometrial cancer than advanced endometrial cancer
(stage II, III and IV).
The biological function of EZH2 in endometrial
cancer was further evaluated. Reduced cell proliferation was
identified after EZH2 was silenced by siRNA, indicating that EZH2
was involved in the cell proliferation of endometrial cancer. It
has been reported that EZH2 expression is positively correlated
with expression of Ki-67 (24).
Ki-67, a marker of cell proliferation, is involved in cell mitosis
and is positively correlated with the number of cells that are
about to enter mitotic phase. Overexpression of Ki-67 is often
observed in malignant tumors and can be a reliable marker of
enhanced proliferation. Although no correlation was identified
between the expression of EZH2 and Ki-67 in the present study, this
may have been due to the insufficient sample size.
In the present study, high EZH2 expression appeared
to be associated with LVSI. High expression of EZH2 was seen in 56%
(15/27) of cases without LVSI, while it was observed in all (6/6)
cases with LVSI. However, a significant positive correlation
between the overexpression of EZH2, focal adhesion kinase (FAK) and
phosphorylated FAK, as wells as angiolymphatic invasion and lymph
node metastasis in endometrial cancer were identified by Zhou et
al (25), suggesting that EZH2
may regulate endometrial cancer migration along with FAK through
modulating E-cadherin.
In conclusion, overexpression of EZH2 correlates
with deep myometrial invasion, LVSI and enhanced cell proliferation
of endometrial cancer cells. It may predict a more aggressive
biological behavior in endometrial carcinoma and may serve as a
potential therapeutic target for the treatment of endometrial
cancer. Future studies are required to further evaluate the
biological function of EZH2 in endometrial tissue and prospectively
assess its potential role as a prognostic marker.
Acknowledgements
This study was supported by a grant from Shanghai
Pujiang Talent Program, Shanghai Science and Technology Committee
(grant no. 08PJ14026), awarded to Ms. Weiwei Feng.
References
|
1
|
Ferlay J, Shin HR, Bray F, et al: GLOBOCAN
2008 v1.2, Cancer Incidence and Mortality wWorldwide: IARC
CancerBase No. 10 [Internet]. International Agency for Research on
Cancer; Lyon, France: 2010, http://globocan.iarc.fr.
Accessed October 1, 2013
|
|
2
|
Sorosky JI: Endometrial cancer. Obstet
Gynecol. 120:383–397. 2012.
|
|
3
|
Singh S, Raidoo S, Pettigrew G and
Debernardo R: Management of early stage, high-risk endometrial
carcinoma: preoperative and surgical considerations. Obstet Gynecol
Int. 2013:7572492013.
|
|
4
|
Albertini AF, Devouassoux-Shisheboran M
and Genestie C: Pathology of endometrioid carcinoma. Bull Cancer.
99:7–12. 2012.
|
|
5
|
Matias-Guiu X and Davidson B: Prognostic
biomarkers in endometrial and ovarian carcinoma. Virchows Arch.
464:315–331. 2014.
|
|
6
|
Yoo KH and Hennighausen L: EZH2
methyltransferase and H3K27 methylation in breast cancer. Int J
Biol Sci. 8:59–65. 2012.
|
|
7
|
Choi JH, Song YS, Yoon JS, et al: Enhancer
of zeste homolog 2 expression is associated with tumor cell
proliferation and metastasis in gastric cancer. APMIS. 118:196–202.
2010.
|
|
8
|
Fan T, Jiang S, Chung N, et al:
EZH2-dependent suppression of a cellular senescence phenotype in
melanoma cells by inhibition of p21/CDKN1A expression. Mol Cancer
Res. 9:418–429. 2011.
|
|
9
|
Lu C, Han HD, Mangala LS, et al:
Regulation of tumor angiogenesis by EZH2. Cancer Cell. 18:185–197.
2010.
|
|
10
|
Varambally S, Dhanasekaran SM, Zhou M, et
al: The polycomb group protein EZH2 is involved in progression of
prostate cancer. Nature. 419:624–629. 2002.
|
|
11
|
Kleer CG, Cao Q, Varambally S, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:11606–11611. 2003.
|
|
12
|
Weikert S, Christoph F, Köllermann J, et
al: Expression levels of the EZH2 polycomb transcriptional
repressor correlate with aggressiveness and invasive potential of
bladder carcinomas. Int J Mol Med. 16:349–353. 2005.
|
|
13
|
Bachmann IM, Halvorsen OJ, Collett K, et
al: EZH2 expression is associated with high proliferation rate and
aggressive tumor subgroups in cutaneous melanoma and cancers of the
endometrium, prostate, and breast. J Clin Oncol. 24:268–273.
2006.
|
|
14
|
Mannelqvist M, Stefansson IM, Wik E, et
al: Lipocalin 2 expression is associated with aggressive features
of endometrial cancer. BMC Cancer. 12:169–175. 2012.
|
|
15
|
Eskander RN, Ji T, Huynh B, et al:
Inhibition of enhancer of zeste homolog 2 (EZH2) expression is
associated with decreased tumor cell proliferation, migration, and
invasion in endometrial cancer cell lines. Int J Gynecol Cancer.
23:997–1005. 2013.
|
|
16
|
No authors listed. FIGO Committee for the
Ethical Aspects of Human Reproduction and Women’s Health Committee
Statement on Ethical Guidelines (Cairo, March 1998). J Obstet
Gynaecol. 19:444–447. 1999.
|
|
17
|
Trimble CL, Method M, Leitao M, et al:
Society of Gynecologic Oncology Clinical Practice Committee:
Management of endometrial precancers. Obstet Gynecol.
120:1160–1175. 2012.
|
|
18
|
Samarnthai N, Hall K and Yeh IT: Molecular
profiling of endometrial malignancies. Obstet Gynecol Int.
2010:1623632010.
|
|
19
|
Crea F, Fornaro L, Bocci G, et al: EZH2
inhibition: targeting the crossroad of tumor invasion and
angiogenesis. Cancer Metastasis Rev. 31:753–761. 2012.
|
|
20
|
Liang BJ, Li XP, Lu J, et al: Effects of
enhancer of zeste homolog (EZH2) downregulation on the
proliferation and invasion of nasopharyngeal carcinoma cell and the
possible mechanism. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
47:298–304. 2012.(In Chinese).
|
|
21
|
Wang C, Liu X, Chen Z, et al: Polycomb
group protein EZH2-mediated E-cadherin repression promotes
metastasis of oral tongue squamous cell carcinoma. Mol Carcinog.
52:229–236. 2013.
|
|
22
|
Leng L, Huang Q, Dong Y, et al: EZH2 gene
silenced by siRNA suppresses the growth and invasion of endometrial
carcinoma cells. Nan Fang Yi Ke Da Xue Xue Bao. 33:866–869.
2013.(In Chinese).
|
|
23
|
Lin YW, Ren LL, Xiong H, et al: Role of
STAT3 and vitamin D receptor in EZH2-mediated invasion of human
colorectal cancer. J Pathol. 230:277–290. 2013.
|
|
24
|
Li H, Cai Q, Godwin AK and Zhang R:
Enhancer of zeste homolog 2 promotes the proliferation and invasion
of epithelial ovarian cancer cells. Mol Cancer Res. 8:1610–1618.
2010.
|
|
25
|
Zhou J, Roh JW, Bandyopadhyay S, et al:
Overexpression of enhancer of zeste homolog 2 (EZH2) and focal
adhesion kinase (FAK) in high grade endometrial carcinoma. Gynecol
Oncol. 128:344–348. 2013.
|