Introduction
Desmoplastic small round cell tumor (DSRCT) is a
rare type of mesenchymal tumor that was first described as a
separate tumor type in 1989 by Gerald and Rosai (1). Worldwide, <200 cases are reported
in the literature. DSRCT typically affects adolescents and young
adults (predominantly males) and develops in the abdominal cavity.
Despite the availability of multimodal treatment, DSRCT has a
highly aggressive clinical course. In addition, the majority of
patients experience resistant and recurrent disease as they
approach the end of life, the three-year survival rate is 44% and
the five-year survival rate is 15% (2). However, >50% of the patients
reported by Lal et al (2)
did not exhibit distant metastasis. There is no standard therapy
for patients with DSRCT, particularly for those patients with
metastatic DSRCT, and there are few reports of metastatic DSRCT
treatment. Kushner et al (3)
reported 12 DSRCT patients, with a median survival time of 19
months. A patient reported by Mrabti et al (4) has a survival period of 26 months
following the diagnosis of DSRCT.
Renin-producing tumors are rare, and cases of
extrarenal renin-producing tumors are particularly rare. The
current study presents a case of a renin-producing DSRCT. Written
informed consent was obtained from the patient’s family.
Case report
In January 2011, a 20-year-old male was admitted to
the Department of Internal Medicine, Chosun University Hospital
(Gwangju, Korea) with a complaint of abdominal distension and a
palpable mass in the abdomen. The symptoms had begun two months
previously and the palpable mass had gradually grown over the two
weeks prior to presentation. The patient’s personal and family
medical histories were non-specific with the exception of high
blood pressure (BP; 150/100 mmHg; normal range, 100–120/70–80
mmHg).
The patient’s vital signs were as follows: Body
temperature, 36.6°C (normal range, 36.5–37.5°C); BP, 180/110 mmHg;
pulse, 108 beats per min (normal range, 60–100 beats per min); and
respiratory rate, 18 breaths per min (normal range, 12–20 breaths
per min).
Physical examination revealed a 1-cm non-tender,
hard and fixed nodule surrounding the umbilicus with abdominal
distension and without fluid shifting.
The laboratory results were as follows: White blood
cell count, 5,740/mm3 (normal range,
4,000–8,000/mm3); hemoglobin, 14.3 g/dl (normal range,
14.0–18.0 g/dl); platelet count, 282×103/mm3
(normal range, 150–400×103/mm3); total
protein, 7.83 g/dl (normal range, 5.3–7.4 g/dl); albumin, 4.49 g/dl
(normal range, 3.5–5.2 g/dl); aspartate aminotransferase, 19 IU/l
(normal range, 5–40 IU/l); alkaline aminotransferase, 14 IU/l
(normal range, 5–40 IU/l); alkaline phosphatase, 115 IU/l (normal
range, 35–123 IU/l); serum lactate dehydrogenase level, 530 IU/l
(normal range, 200–450 IU/l), blood urea nitrogen, 12.3 mg/dl
(normal range, 8.0–20 mg/dl); creatinine, 1.12 mg/dl (normal range,
0.5–1.3 mg/dl); serum sodium, 138 mEq/l (normal range, 136–146
mEq/l); serum potassium, 3.0 mEq/l (normal range, 3.5–5.0 mEq/l);
and chloride level, 97 mEq/l (normal range, 98–110 mEq/l). In
addition, metabolic alkalosis was observed in the arterial blood
gas analysis test (pH 7.483; partial pressure of CO2
[pCO2], 42.3 mmHg; pO2, 95.8 mmHg;
HCO3, −31.0 mmol/l; and base excess, 7.6 mmol/l).
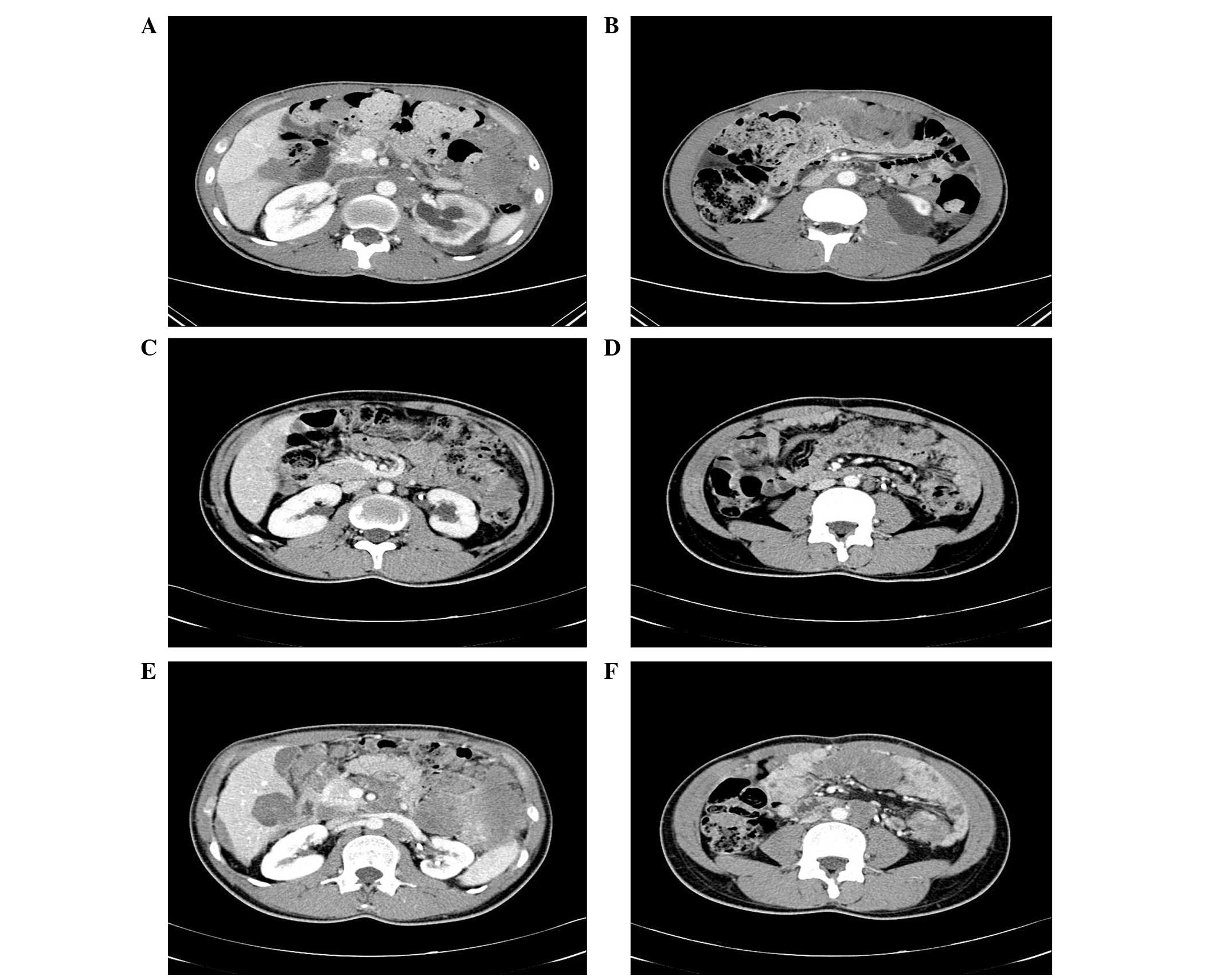
Computed tomography (CT; Fig. 1) revealed multiple large masses that
were composed of fused lymph nodes (LNs) of the mesenteric,
paraaortic and inferior vena cava, as well as metastatic nodules in
the liver, spleen and intraperitoneal space (Fig. 1A and B).
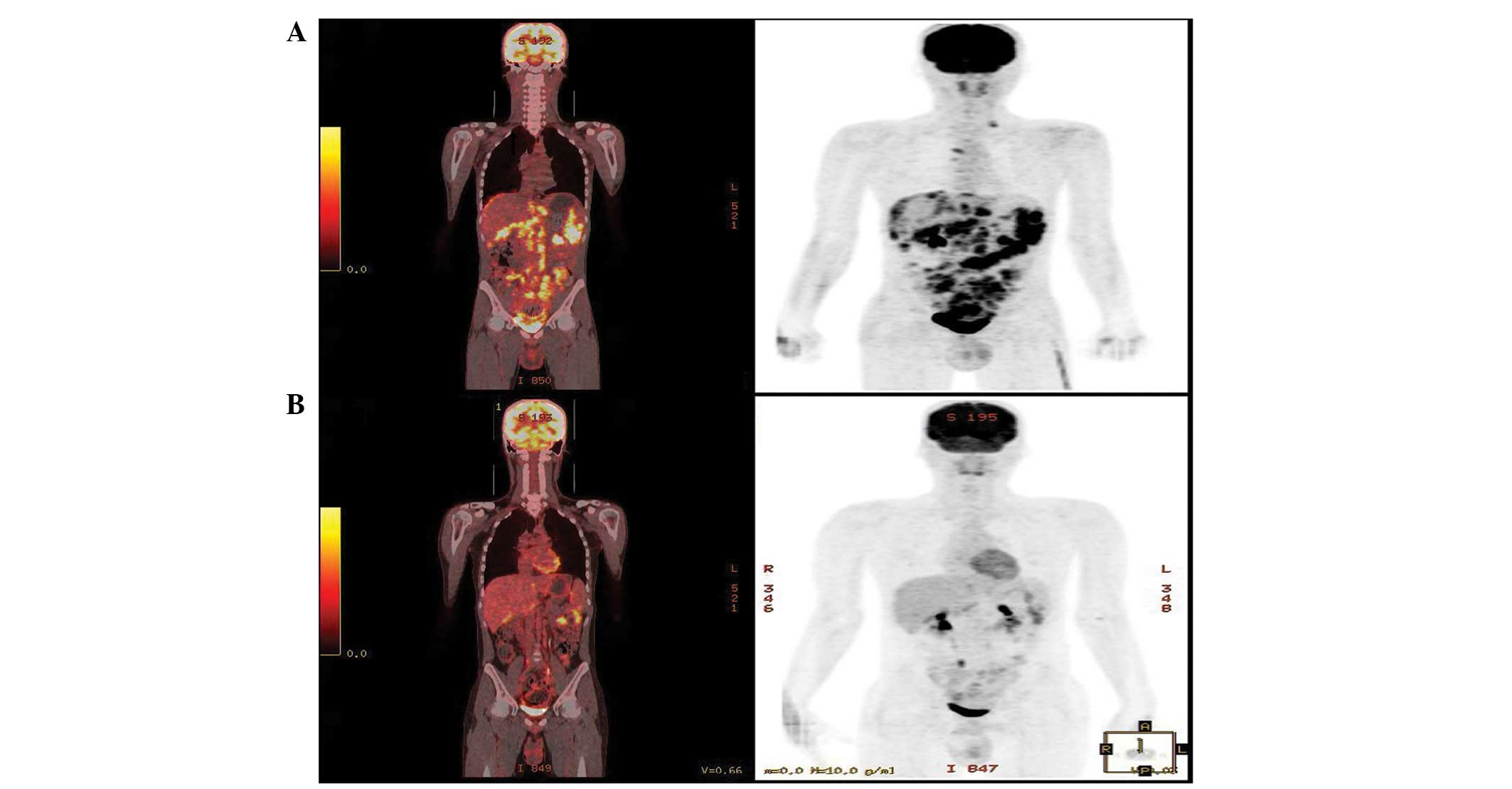
Hypermetabolism was observed by positron emission
tomography-CT in the left supraclavicular LN, right internal
mammary artery, retrosternal area and conglomerated LNs of the
mesentery, aortocaval, paraaortic and pericaval areas (Fig. 2A). Physical and imaging examinations
indicated the malignant nature of the tumor to be consistent with
malignant lymphoma or a germ cell tumor.
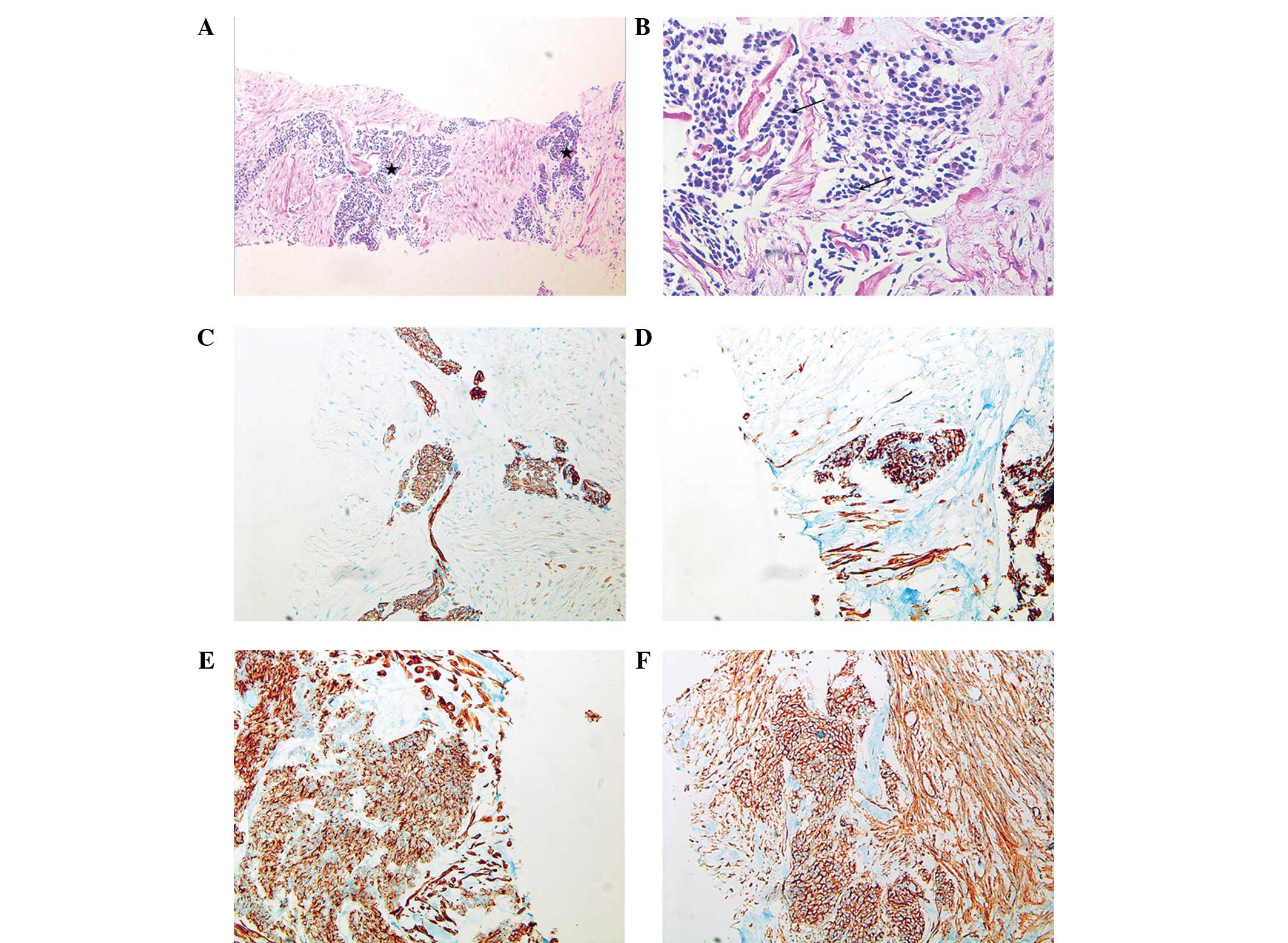
First, a percutaneous needle biopsy of a palpable
peritoneal nodule was performed. The biopsy specimen revealed the
presence of a poorly differentiated tumor of variable size and
shape composed of nests of small round cells surrounded by a
prominent desmoplastic stroma (Fig. 3A
and B). Immunohistochemically, the tumor cells coexpressed an
epithelial marker (cytokeratin), a mesenchymal marker (desmin and
vimentin), chromogranin A, and cluster of differentiation (CD)
antigens, CD99 and CD56 (Fig.
3C–F). The sample was negative for Wilms’ tumor 1 (WT-1)
protein and synaptophysin. Upon Ewing sarcoma (EWS)-fluorescence
in situ hybridization analysis, the EWSR1 gene (22q12)
rearrangement was detected in 93% of the cells.
Thus, malignant cells of the small round cell type,
which were expressing CD99, desmin, cytokeratin and the EWSR1 gene
(22q12) rearrangement were identified, as well as a malignant mass
that was predominantly in the abdominal area of the young, male
patient.
A diagnosis of DSRCT was determined based on these
results, and the patient was treated with multiagent chemotherapy
using the VAC/IE regimen (vincristine, adriamycin,
cyclophosphamide, ifosfamide and etoposide). Vincristine 2 mg on
day 1 for every three weeks, cyclophosphamide 900 mg/m2
on day 1 every three weeks, adriamycin 37.5 mg/m2 on day
1 and 75 mg/m2 on day 2 every three weeks. Subsewuently,
etoposide 100 mg/m2 was administered on days 1–5 every
three weeks and ifosfamide 1,800 mg/m2 was administered
on days 1–5 every three weeks. However, the patient complained of a
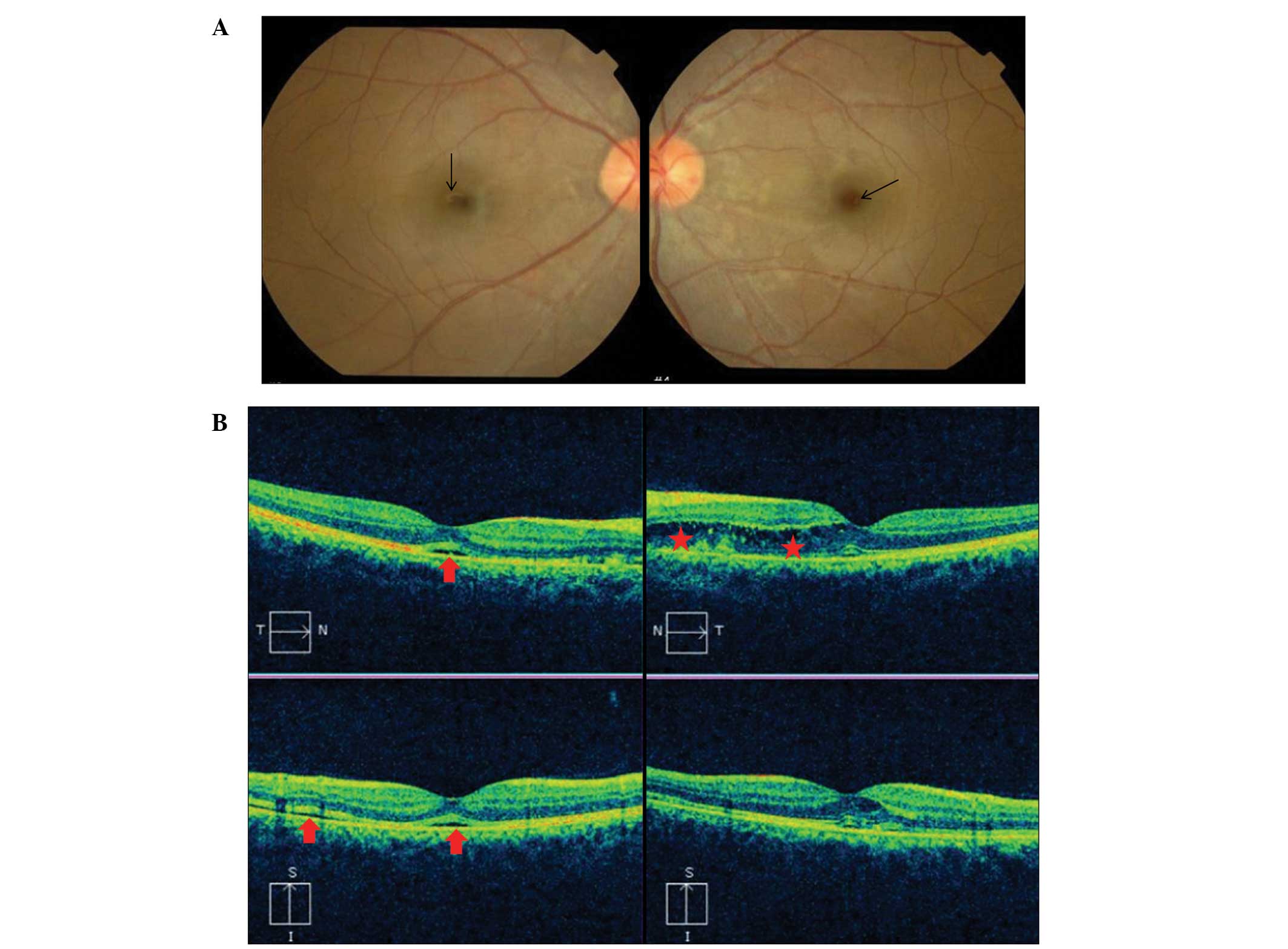
sudden visual disturbance during hospitalization, and therefore,
underwent an ophthalmic examination and brain magnetic resonance
imaging (MRI). During the ophthalmic examination, macular edema and
macular detachment were observed, which were considered to be
secondary hypertensive changes (Fig. 4A
and B). The brain MRI did not demonstrate acute bleeding, or
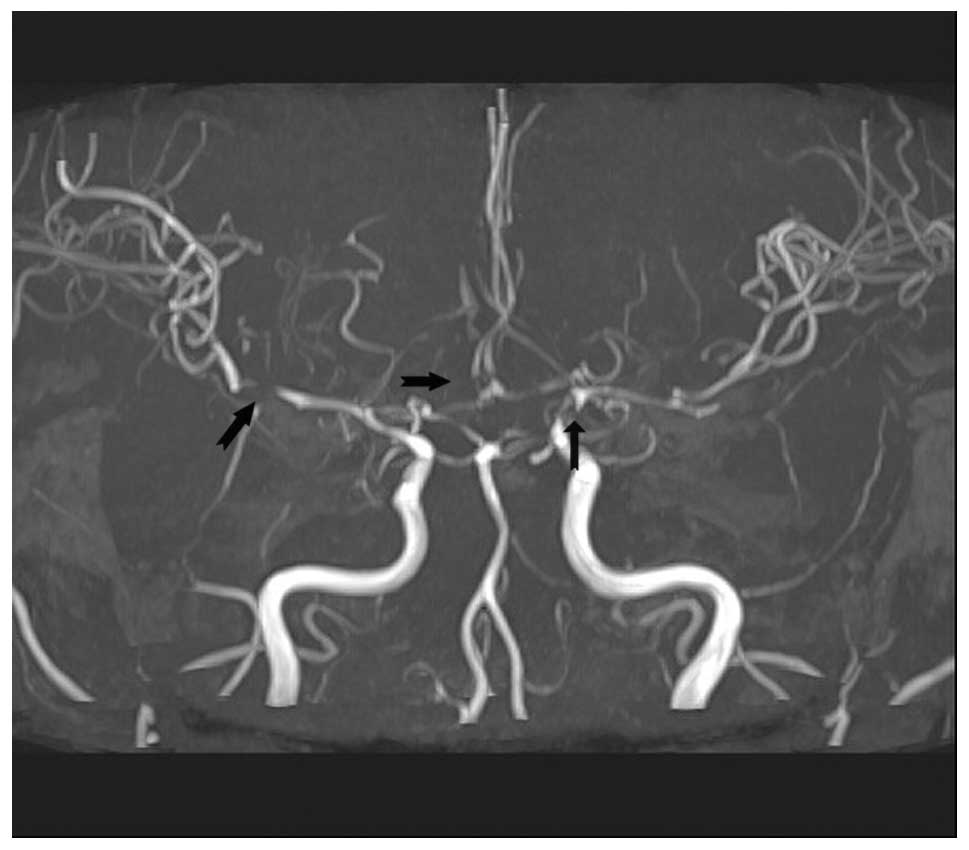
cerebral or cerebellum infarction; however, blood vessel damage due
to severe hypertension was revealed (Fig. 5).
Additional hormone tests were performed during the
secondary hypertension evaluation due to the severe hypertension
(BP, 180/110 mmHg), hypokalemia, metabolic alkalosis, and
hypertensive ophthalmic and cerebral vascular changes that were
observed in this young patient that did not have a family history
of hypertension.
Serum renin activity was 31.9 ng/ml/h in the supine
position (normal range standing, 1.3–4.0 ng/ml/h and normal range
in supine position, 0.15–2.33 ng/ml/h), while the aldosterone level
was greatly increased to 64 ng/dl (normal range standing, 4.0–31.0
ng/dl and normal range in supine position, 1.0–1.6 ng/dl). The
patient’s urinary vanillylmandelic acid level was 5.8 mg/day
(normal range, 0–8 mg/day), metanephrine was 0.7 mg/day (normal
range, 0–1.0 mg/day) and cortisol was 75.2 μg/day (normal range,
20–90 μg/day); these levels were all within the normal range.
Doppler sonography of the kidney revealed normal blood flow without
renal artery stenosis and a CT examination revealed no masses or
vessel abnormalities in the kidney. However, mild hydronephrosis
was observed in the left kidney. The 24-h BP monitoring revealed an
average BP of 167/123 mmHg.
These findings were strongly indicative of a
renin-secreting DSRCT. The patient was prescribed an aldosterone
antagonist (spironolactone; 100 mg), an angiotensin II antagonist
(olmesartan; 20 mg) and a calcium channel blocker (amlodipine; 5
mg). Upon treatment, the patient’s systolic BP decreased to 100–120
mmHg.
Following a second cycle of the VAC/IE regimen, a
partial response was achieved (Fig. 1C
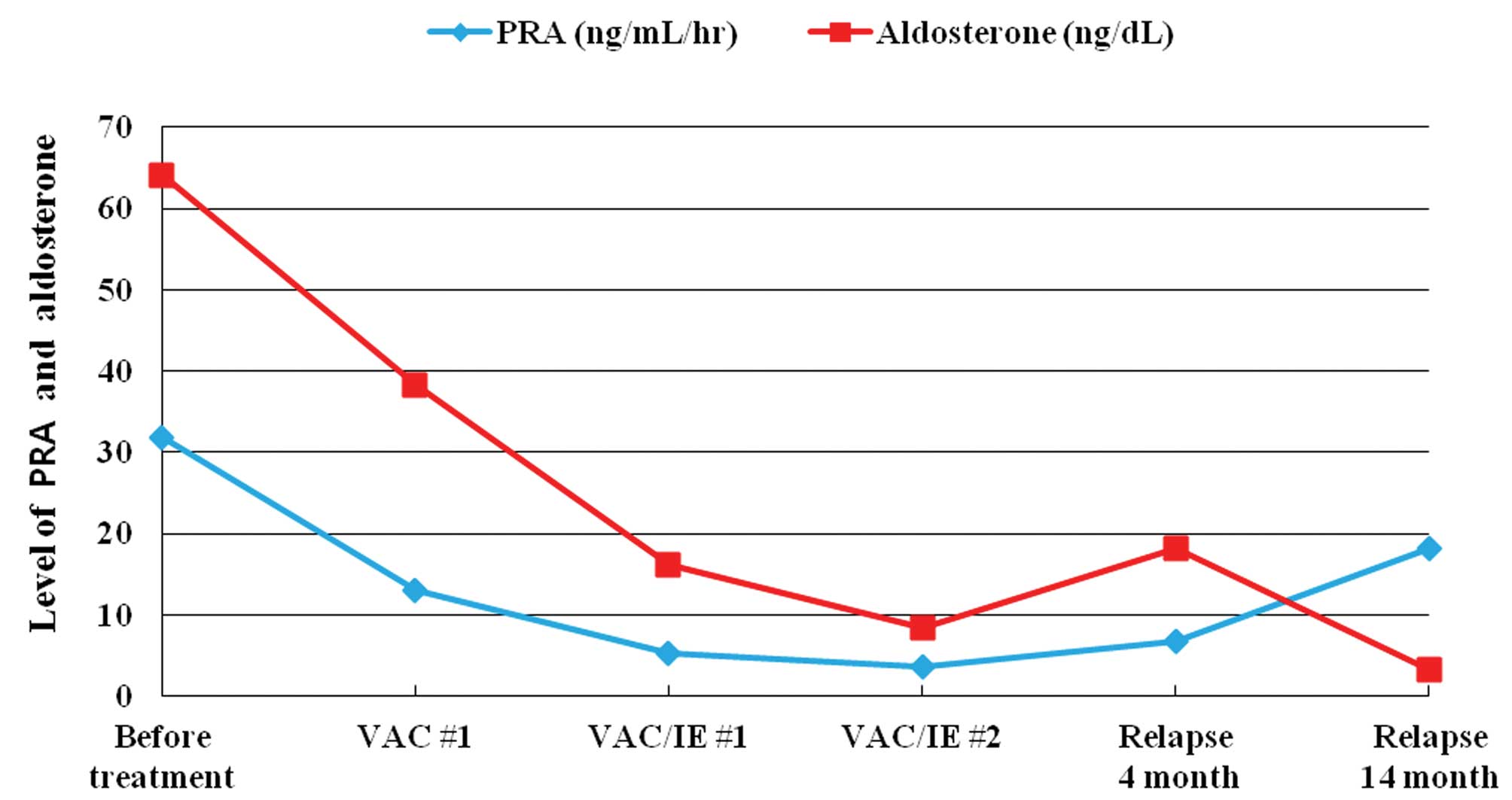
and D). The renin and aldosterone levels were gradually
decreased to the normal range (renin activity decreased from 31.9
to 5.2 ng/ml/h, while the aldosterone levels decreased from 64 to
16.2 ng/dl). The patient’s BP was normalized without
antihypertensive medication (Fig.
6).
The patient was treated with a total of six cycles
of the VAC/IE regimen and achieved a partial response (Fig. 2B). However, further chemotherapy was
not administered due to the patient’s asymptomatic state and
concerns that the patient was too weak to receive further
chemotherapy. At this time, the patient stopped attending the
Chosun University Hospital.
Following a four-month break from chemotherapy, the
patient was readmitted to the Chosun University Hospital
complaining of abdominal discomfort. It was identified that the
tumor size had increased (Fig. 1E and
F) and the renin level was re-elevated (Fig. 6). Based on these results, it was
determined that the disease had progressed following cessation of
chemotherapy. Second-line chemotherapy was recommended, however,
the patient refused further chemotherapy. Subsequently, supportive
therapy was administered, which included paracentesis. The focus of
the present case was adjusted, and the aim was to provide direct
evidence that the DSRCT was renin-secreting. Therefore, using
malignant cells obtained from the patient’s ascites, reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
analysis was performed. Total RNA was extracted using TRI reagent
(RNAiso Plus; Takara Bio, Inc., Shiga, Japan) and cDNA was
synthesized by RT (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. The cDNA was
amplified by RT-qPCR using a Roche LightCycler 2.0 system (Roche
Diagnostics, Mannheim, Germany) for 45 cycles. The PCR reaction
contained 4 μl cDNA (diluted to 1:5), 4 ml MgCl2, 10
pmol of each primer and 4 μl Fast Starter Mix buffer (4 mM
deoxynucleotide triphosphates, 2X SYBR® Green dye and
2.5 U Taq polymerase). The primers and conditions used for RT-qPCR
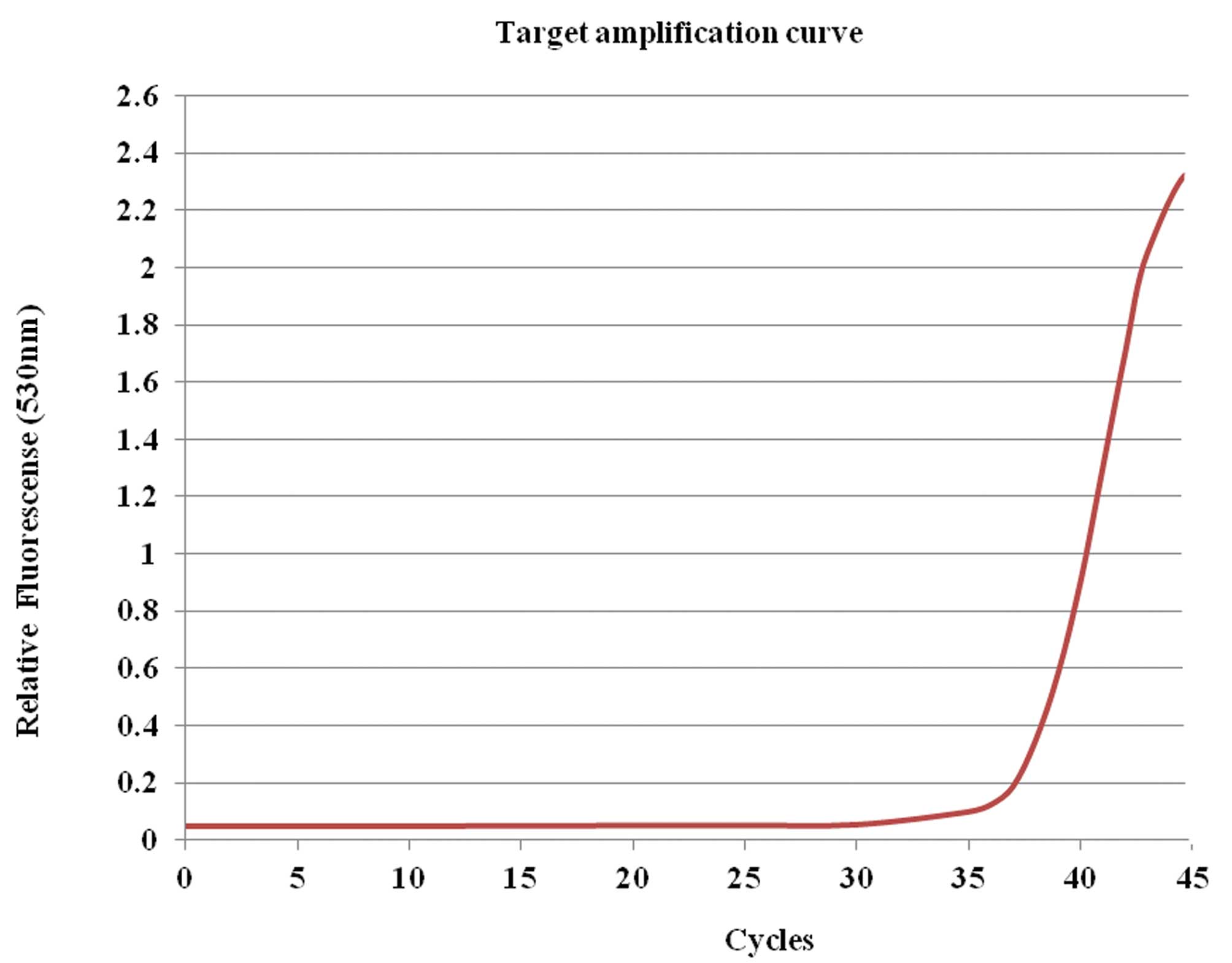
are shown in Tables I and II. RT-qPCR revealed that the patient’s
ascites contained renin (Fig. 7).
At 16 months, following relapse, the patient refused further
chemotherapy and succumbed.
 | Table IPrimers for reverse transcription
quantitative polymerase chain reaction. |
Table I
Primers for reverse transcription
quantitative polymerase chain reaction.
| Gene | Primer |
|---|
| Renin | Sense: tga cac tgg
ttc gtc caa tg
Antisense: agc tgg agg aat ccg aag c |
| β-actin | Sense: gac tat gac
tta gtt gcg tt
Antisense: gtt gaa ctc tac ata ctt ccg |
 | Table IIConditions for reverse transcription
quantitative polymerase chain reaction. |
Table II
Conditions for reverse transcription
quantitative polymerase chain reaction.
| Gene | Hot start | Denaturation | Annealing | Extension |
|---|
| Renin | 95°C, 10 min | 95°C, 15 sec | 60°C, 5 sec | 72°C, 10 sec |
| β-actin | 95°C, 10 min | 95°C, 15 sec | 55°C, 5 sec | 72°C, 21 sec |
Discussion
DSRCT is a rare and aggressive malignant neoplasm
that occurs in adolescents and young adults. The mean age at
diagnosis is ~22 years, and ranges between six and 49 years; the
male to female ratio is 4:1 (5).
Clinical manifestations are often associated with widespread
abdominal disease and distant metastases are frequently present at
the time of diagnosis. Symptoms include abdominal pain and
distension and potentially nausea or emesis. DSRCT is a member of
the large family of small round cell tumors of childhood, along
with the primitive neuroectodermal tumor, alveolar and embryonal
rhabdomyosarcoma, and poorly differentiated synovial sarcoma and
rhabdoid tumors. However, DSRCT is characterized by a distinct
immunohistochemical pattern and a recurrent, specific chromosomal
translocation (5–7). The tumor is composed of desmoplastic
stroma with nests of small round blue cells, which show
immunohistochemical reactivity for epithelial (cytokeratin and
epithelial membrane antigen), mesenchymal (vimentin), neural
(neuron-specific enolase) and muscle (desmin) markers when
visualized using light microscopy (5). DSRCT is associated with a unique
chromosomal translocation, t(11;22)(p13;q12), that fuses the
N-terminus of the EWS gene to the C-terminus of the WT-1 gene
(6,7). The presence of this translocation
provides confirmation of the diagnosis of DSRCT. The current case
presented the characteristic morphological, immunohistochemical
(cytokeratin, vimentin, desmin and CD99) and molecular features of
DSRCT.
Despite their rarity, paraneoplastic syndromes must
always be considered in cancer patients with unusual clinical
findings. The current patient exhibited hypokalemia at
presentation, therefore, an endocrine cause for the hypertension
was sought out. Serum renin and aldosterone were found to be
markedly elevated, which explained the abnormal clinical findings.
The juxtaglomerular apparatus predominantly secretes renin into the
interstitium of the kidney, and not the lumen of the vessel. In
addition to the kidney, renin has also been identified in a number
of organs, including the brain, genital tract, salivary gland,
vessels, skeletal muscle and heart. Therefore, tumors arising from
these organs are capable of secreting renin. Renin-secreting tumors
are classified into the following three groups: i) Tumors arising
from the juxtaglomerular apparatus of the kidney; ii)
renin-secreting renal tumors, including Wilms’ tumor, clear
cell-type renal cell carcinoma, oncocytoma and mesoblastic
nephroma; and iii) extrarenal tumors, including granulosa cell
tumors, lung cancer and pancreatic cancer (8–11). In
total, ~40 patients with a renin-producing tumor (excluding lesions
of the juxtaglomerular apparatus) had been reported by 1988 and the
majority were of renal origin. The renin-secreting tumor triad
consists of hypertension, hypokalemia and elevated plasma renin
activity (PRA). Hypertension in patients with an extrarenal tumor
is more severe than hypertension in patients with a renal tumor. In
the present patient, the BP was 180/110 mmHg, the serum potassium
level was 3.0 mEq/l (normal range, 3.5–5.0 mEq/l) and the PRA had
increased by 8–10 times the upper limit of the normal range (normal
range standing, 1.3–4.0 ng/ml/h and normal range in supine
position, 0.15–2.33 ng/ml/h). In addition, the patient complained
of visual disturbance resulting from the hypertensive retinopathy.
The elevated renin level at presentation fell following successful
treatment of the primary tumor, which strongly indicates that the
tumor was the source of the renin. The diagnosis of a
renin-secreting tumor is usually determined by positive
immunostaining of renin or by electron microscopic identification
of renin granules (13–16). The predominant characteristic
finding from electron microscopy are two types of granules:
Rhomboid crystalline protogranules and amorphous homogeneous,
round, electron dense mature granules (13).
However, immunostaining and electron microscopic
identification are not readily available in clinical practice and
the use of these methods in the context of this case report is
experimental and not universal.
In conclusion, although immunostaining for renin or
electron microscopic examination were not performed in the present
study, the diagnosis of a renin-producing tumor was determined due
to the elevated serum renin and aldosterone levels, which
normalized during tumor regression and were re-elevated during
tumor progression. In addition, the biosynthesis of renin was
confirmed by the demonstration of mRNA in the patient’s ascites
that codes for the renin precursor. To the best of our knowledge,
the current report is the first to demonstrate an extremely rare
case of renin secretion from a rare type of cancer termed DSRCT.
The results of the current study indicated that DSRCT may be
considered as one of the differential diagnoses for extrarenal
renin producing tumors.
References
|
1
|
Gerald WL and Rosai J: Case 2.
Desmoplastic small cell tumor with divergent differentiation.
Pediatr Pathol. 9:177–183. 1989.
|
|
2
|
Lal DR, Su WT, Wolden SL, et al: Results
of multimodal treatment for desmoplastic small round cell tumors. J
Pediatr Surg. 40:251–255. 2005.
|
|
3
|
Kushner BH, LaQuaglia MP, Wollner N, et
al: Desmoplastic small round cell tumor: prolonged progression-free
survival with aggressive multimodality therapy. J Clin Oncol.
14:1526–1531. 1996.
|
|
4
|
Mrabti H, Kaikani W, Ahbeddou N, et al:
Metastatic desmoplastic small round cell tumor controlled by an
anthracycline-based regimen: review of the role of chemotherapy. J
Gastrointest Cancer. 43:103–109. 2012.
|
|
5
|
Lae ME, Roche PC, Jin L, Lloyd RV and
Nascimento AG: Desmoplastic small round cell tumor: a
clinopathologic, immunohistochemical, and molecular study of 32
tumors. Am J Surg Pathol. 26:823–835. 2002.
|
|
6
|
Gerald WL, Ladanyi M, de Alava E, et al:
Clinical, pathologic, and molecular spectrum of tumors associated
with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its
variants. J Clin Oncol. 16:3028–3036. 1998.
|
|
7
|
Ladanyi M and Gerald WL: Fusion of the EWS
and WT1 genes in the desmoplastic small round cell tumor. Cancer
Res. 54:2837–2840. 1994.
|
|
8
|
Bauer JH, Durham J, Miles J, Hakami N and
Groshong T: Congenital mesoblastic nephroma presenting with primary
reninism. J Pediatr. 95:268–272. 1979.
|
|
9
|
Tomita T, Poisner A and Inagami T:
Immunohistochemical localization of renin in renal tumors. Am J
Pathol. 126:73–80. 1987.
|
|
10
|
Têtu B, Lebel M and Camilleri JP:
Renin-producing ovarian tumor. A case report with
immunohistochemical and electron-microscopic study. Am J Surg
Pathol. 12:634–640. 1988.
|
|
11
|
Hauger-Klevene JH: High plasma renin
activity in an oat cell carcinoma: a renin-secreting carcinoma?
Cancer. 26:1112–1114. 1970.
|
|
12
|
Ruddy MC, Atlas SA and Salerno FG:
Hypertension associated with renin secreting adenocarcinoma of the
pancreas. N Engl J Med. 307:993–997. 1982.
|
|
13
|
Lindop GB, Stewart JA and Downie TT: The
immunohistochemical demonstration of renin in a juxtaglomerular
cell tumor by light and electron microscopy. Histopathology.
7:421–431. 1983.
|
|
14
|
Squires JP, Ulbright TM,
DeSchryver-Kesckemeti K and Engleman W: Juxtaglomerular cell tumor
of the kidney. Cancer. 53:516–523. 1984.
|
|
15
|
Valdés G, Lopez JM, Martinez P, et al:
Renin-secreting tumor. Case report. Hypertension. 2:714–718.
1980.
|
|
16
|
Dennis RL, McDougal WS, Glick AD and
MacDonell RC Jr: Juxtaglomerular cell tumor of the kidney. J Urol.
134:334–338. 1985.
|