Introduction
Worldwide, hepatocellular carcinoma (HCC) is the
fifth most prevalent type of cancer and, after lung and stomach
cancer, is the third most common cause of cancer-related
mortalities (1). Resection and
liver transplantation are generally regarded as curative treatments
for early-stage HCC and have exhibited effective results (2,3).
However, the majority of patients diagnosed with intermediate- to
advanced-stage HCC receive only palliative treatment, such as
transcatheter arterial chemoembolization (TACE). As the technology
has developed, three-dimensional conformal radiotherapy (3DCRT) has
allowed for high-dose radiation to be delivered to the target
volume accurately, while minimizing the dose to normal liver
tissues. TACE, combined with conventional external radiotherapy,
has become the main treatment option for intermediate- to
advanced-stage HCC, and the associated studies have reported safe
and effective outcomes (4,5).
γ-glutamyl transferase (GGT) is a cell surface
heterodimeric glycoprotein, which is routinely tested for in
clinical examinations. It is a simple biological marker which can
be easily obtained from the patient at a low cost. High expression
is observed in the biliary epithelium, brain capillaries and kidney
tubules (6). According to Griffith
et al (7), serum GGT can be
used as a diagnostic biomarker for hepatobiliary disease, and GGT
has been confirmed to be a major prognostic factor for survival in
cirrhosis (8). Studies have
demonstrated that serum GGT can predict tumor response and survival
after TACE and surgery (9,10); however, little is known regarding
the prognostic role of GGT in treatment with combined TACE and
3DCRT. In the current study, 154 intermediate [Barcelona Clinic
Liver Cancer (BCLC) stage B] (11)
HCC patients were retrospectively investigated and the predictive
value of the baseline serum GGT level with regard to overall
survival (OS) was analyzed following the combined treatment.
Patients and methods
Study design
The current retrospective study was conducted at the
Department of Radiation Oncology at Shandong Cancer Hospital
(Jinan, China). The criteria for entry into this study were as
follows: i) HCC confirmed by liver biopsy or with the clinical
features defined by the American Association for the Study of Liver
Diseases [persistently elevated α-fetoprotein (AFP) levels (>400
ng/ml) in conjunction with characteristic abdominal computed
tomography (CT) or magnetic resonance imaging (MRI) with arterial
phase enhancement and venous phase washout] (12); ii) all patients of intermediate
stage (BCLC stage B) with Child-Pugh grade A according to the BCLC
staging system (11); iii) Eastern
Cooperative Oncology Group performance status of 0–1 (13); and iv) available follow-up data. The
study protocol was approved by Shandong Tumor Prevention and
Control Institutional Ethics Committee, Shandong Cancer Hospital
and all patients provided written informed consent.
The clinical features of all patients included age,
gender, tumor size, gross tumor volume (GTV), hepatitis virus
infection, radiotherapy dose and total number of TACE treatments.
The blood samples were obtained the morning prior to the TACE.
Indicators of liver damage, including alanine transferase (ALT),
GGT, albumin (ALB) and AFP, were systematically analyzed. The
baseline imaging results (CT or MRI) of the liver were assessed
within a week prior to TACE. For continuous variables, including
age, GGT, tumor size, GTV and total number of TACE treatments,
patients were divided into two groups according to the median
values.
TACE procedures
TACE was performed using the conventional Seldinger
technique (14). Hepatic and
superior mesenteric artery angiographies were performed to identify
the tumor vessel anatomy, tumor staining and the tumor-feeding
artery. The catheter was superselectively inserted into the
tumor-feeding artery in as close proximity as possible to the
tumor. Chemotherapeutic agents, including 1.0 g 5-fluorouracil and
80 mg cisplatin were infused, following which, an emulsion of 10 mg
mitomycin C and 5–30 ml lipiodol was administered. The dosage of
chemotherapeutic agents or lipiodol was selected based on the tumor
size, liver function and routine blood analysis. For large tumors
that were hypervasculature in nature, a gelatin sponge was used for
the further embolization of the tumor-feeding artery. TACE was
performed every 1.5–2.0 months if required, on the basis of the
tumor response and patient health.
3DCRT procedure
3DCRT was performed two to four weeks after the
final TACE course. A CT scan was initially performed for treatment
planning. The patient position was fixed using vacuum casts in a
supine position, with the arms raised above the head. GTV was
delineated according to the primary lesion or lipiodol deposit from
TACE. The clinical target volume was expanded by 5 mm on the basis
of GTV and the planning target volume (PTV) was defined as GTV plus
a 5-mm radial expansion, as well as a 10-mm craniocaudal expansion
to account for daily setup error and respiratory organ motion
(15). Organs at risk were also
delineated, including the whole liver, non-target liver (whole
liver minus PTV), stomach, kidney and spinal cord. Aided by the
beam’s eye view, four to six coplanar or non-coplanar fields were
designed. A cumulative dose-volume histogram was used to evaluate
each treatment plan, and the target delineation was conducted by
the same experienced oncologist. The median radiation dose was 45
Gy (range, 10–60 Gy) and the mean dose to normal liver was limited
to ≤30 Gy.
Evaluation of GGT and follow-up
The serum concentrations of GGT were analyzed using
a Hitachi 917 machine (Roche Diagnostics, Mannheim, Germany). Tumor
responses were evaluated with contrast-enhanced CT or MRI one month
following TACE or 3DCRT. For patients without a complete response
(CR), TACE was repeated. If the patients achieved a CR,
contrast-enhanced ultrasound, AFP test, CT and MRI were performed
within three months following the treatment, and then routinely
performed every six months until December 2013. In addition,
routine blood analysis was conducted and liver function and serum
tumor markers were also analyzed.
Statistical analysis
The software used for statistical analysis was SPSS
13.0 for Windows (SPSS Inc., Chicago, IL, USA). All consecutive
results were presented as the mean ± standard deviation. Comparison
of variables was performed by the Mann-Whitney U test,
χ2 test or Fisher’s exact test. Variables that achieved
statistical significance in the univariate analysis were
subsequently included in a multivariate analysis using a stepwise
forward Cox regression procedure to identify factors independently
associated with mortality. OS was calculated as the interval
between the time of the initiation of treatment and the time of
mortality. The optimal threshold for GGT was identified by the
receiver operating characteristic (ROC) curve, derived from a
univariate logistic regression model predicting patient mortality
prior to the median OS. This threshold served in all further uni-
and multivariate analyses. Cumulative survival curves for each
variable were obtained by using the Kaplan-Meier method and the
difference was compared using the log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Prognostic factors affecting
survival
A total of 154 patients with intermediate HCC (71
females and 83 males) between January 2004 and December 2010 were
included in the study. The median age and GTV were 55 years (range,
23–71 years) and 200 cm3. At the time of the analysis,
the median number of TACE procedures performed for all patients was
four (range, one to 10). According to the ROC analysis (Fig. 1), the optimal threshold for GGT was
85 U/l. This resulted in a sensitivity of 75.13% and a specificity
of 69.81% [area under the ROC curve, 0.763; 95% confidence interval
(CI), 0.645–0.880]. Furthermore, 115 patients (74.7%) were included
in the high GGT group, according to the cut-off level, and 39
patients (25.3%) were included in the low GGT group.
The baseline characteristics of the 154 patients are
summarized in Table I. The results
indicated that GGT levels (P=0.003), ALT levels (P=0.012), ALB
levels (P=0.038), GTV (P=0.002), AFP levels (P=0.01), total number
of TACE procedures (P=0.039) and radiation dose (P=0.044) were all
associated with OS. Factors exhibiting a significant difference by
univariate analysis were adopted when multivariate Cox
proportional-hazards analysis was performed. The results
demonstrated that GGT levels [P=0.001; hazard ratio (HR), 2.32; 95%
CI, 1.133–3.643], GTV (P=0.007; HR, 1.263; 95% CI, 1.361–7.401),
AFP levels (P=0.006; HR, 1.84; 95% CI, 1.218–3.059) and radiation
dose (P=0.035; HR, 1.75; 95% CI, 1.157–2.998) were independent risk
factors for OS (Table II). A
comparison of the clinical results between patients with low and
elevated GGT expression is summarized in Table III. The results indicated that
patients with elevated GGT usually had higher serum ALT, AFP and
total bilirubin levels, as well as lower ALB and shorter
prothrombin time.
 | Table IUnivariate analysis of factors
associated with overall survival. |
Table I
Univariate analysis of factors
associated with overall survival.
| | Overall survival
rate, % | |
|---|
| |
| |
|---|
| Risk factors | n | 1-year | 3-year | 5-year | P-value |
|---|
| Age, years | | | | | 0.772 |
| ≤55 | 60 | 70.0 | 31.7 | 13.3 | |
| >55 | 94 | 58.5 | 21.3 | 9.6 | |
| Gender | | | | | 0.144 |
| Male | 83 | 71.1 | 42.2 | 14.5 | |
| Female | 71 | 46.5 | 12.7 | 5.6 | |
| Total bilirubin,
μmol/l | | | | | 0.586 |
| ≤17.1 | 60 | 61.7 | 31.7 | 8.3 | |
| >17.1 | 94 | 43.6 | 19.1 | 7.4 | |
| GGT, U/l | | | | | 0.003 |
| ≤85 | 39 | 79.5 | 48.7 | 17.9 | |
| >85 | 115 | 52.2 | 21.7 | 8.7 | |
| Prothrombin time,
sec | | | | | 0.388 |
| ≤14 | 121 | 66.9 | 38.8 | 9.1 | |
| >14 | 33 | 33.3 | 15.2 | 3.0 | |
| ALT, U/l | | | | | 0.012 |
| ≤40 | 74 | 63.5 | 31.1 | 9.5 | |
| >40 | 80 | 45.0 | 20.0 | 8.8 | |
| ALB, g/l | | | | | 0.038 |
| ≤35 | 43 | 32.6 | 18.6 | 4.7 | |
| >35 | 111 | 57.7 | 33.3 | 7.2 | |
| AFP, ng/ml | | | | | 0.010 |
| A≤400 | 92 | 60.9 | 51.1 | 14.1 | |
| B>400 | 62 | 40.3 | 11.3 | 3.2 | |
| GTV,
cm3 | | | | | 0.002 |
| ≤200 | 73 | 72.6 | 60.3 | 24.7 | |
| >200 | 81 | 61.7 | 27.2 | 14.8 | |
| Radiation dose,
Gy | | | | | 0.044 |
| ≤45 | 82 | 56.1 | 18.3 | 4.9 | |
| >45 | 72 | 61.1 | 36.1 | 16.7 | |
| TACE, n | | | | | 0.039 |
| 1–4 | 70 | 61.4 | 11.4 | 8.6 | |
| >4 | 84 | 64.3 | 30.9 | 12.9 | |
| HBV | | | | | 0.054 |
| Positive | 89 | 49.4 | 20.2 | 4.5 | |
| Negative | 65 | 50.8 | 38.5 | 12.3 | |
 | Table IIMultivariate analysis of factors
associated with overall survival. |
Table II
Multivariate analysis of factors
associated with overall survival.
| Risk factors | Hazard ratio | 95% CI | P-value |
|---|
| GGT (≤85 vs >85
U/l) | 2.320 | 1.133–3.643 | 0.001 |
| ALT (≤40 vs >40
U/l) | 1.263 | 0.599–2.092 | 0.545 |
| ALB (≤35 vs >35
g/l) | 0.721 | 0.509–1.021 | 0.065 |
| GTV (≤200 vs
>200 cm3) | 1.263 | 1.361–7.401 | 0.007 |
| TACE, n | 0.648 | 0.381–1.101 | 0.109 |
| Radiation dose,
Gy | 1.750 | 1.157–2.998 | 0.035 |
| AFP (≤400 vs
>400 ng/ml) | 1.840 | 1.218–3.059 | 0.006 |
 | Table IIIComparison of clinicopathological
factors between patients with low and high γ-glutamyl transferase
levels. |
Table III
Comparison of clinicopathological
factors between patients with low and high γ-glutamyl transferase
levels.
| Risk factors | Low GGT(≤85
U/l) | High GGT (>85
U/l) | P-value |
|---|
| Gender, n | | | 0.173 |
| Male | 23 | 60 | |
| Female | 27 | 44 | |
| Age, years | 50.2±12.1 | 51.7±14.5 | 0.087 |
| ALT, U/l | 39.7±18.9 | 57.3±38.5 | 0.023 |
| HBV | | | 0.088 |
| Positive | 24 | 65 | |
| Negative | 26 | 39 | |
| AFP, ng/ml | 3112.1±3840.3 |
21831.2±18723.5 | 0.020 |
| Prothrombin time,
sec | 12.53±1.75 | 11.22±2.24 | 0.238 |
| ALB, g/l | 40.1±5.8 | 35.3±4.1 | 0.185 |
| Total bilirubin,
μmol/l | 16.8±6.8 | 18.7±8.7 | 0.021 |
OS of patients with various GGT
levels
In Table IV, the
median OS time following TACE combined with 3DCRT was 24.3 months
(95% CI, 12.84–35.16), with one-, three- and five-year OS rates of
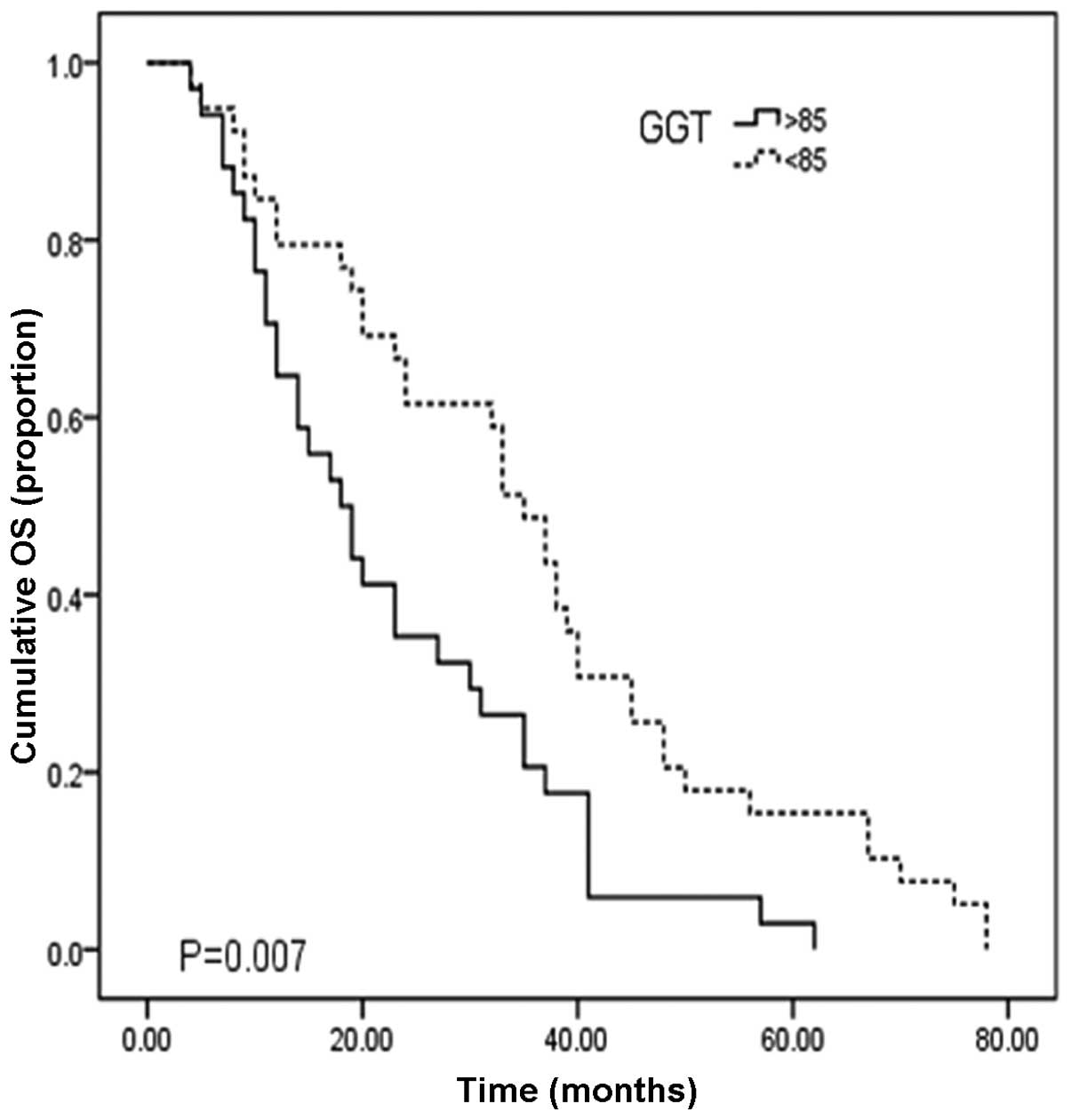
62.1, 27.5 and 10.9%, respectively. Fig. 2 shows the cumulative overall
survival curve for patients with low (≤85 U/l) and high GGT levels
(>85 U/l). For HCC patients with low GGT levels (n=39), the
median OS time was 35.0 months (95% CI, 29.9–40.1) with 1-, 3- and
5-year survival rates of 79.9, 49.7 and 17.2%, respectively. For
patients with high GGT levels (n=115), the median OS time was 18.0
months (95% CI 12.3–23.7) with 1-, 3-, and 5-year survival rates of
52.3, 22.1 and 8.5%, respectively. The OS time of low GGT patients
was significantly longer than that of the elevated GGT group
(Fig. 2; P=0.007).
 | Table IVDifferent γ-glutamyl transferase
levels associated with OS. |
Table IV
Different γ-glutamyl transferase
levels associated with OS.
| GGT | Median OS,
months | OS rate, % | 95% CI |
|---|
|
|---|
| 1-year | 3-year | 5-year |
|---|
| All patients | 24.3 | 62.1 | 27.5 | 10.9 | 12.8–35.2 |
| ≤85 U/l | 35.0 | 79.9 | 49.7 | 17.2 | 29.9–40.1 |
| >85 U/l | 18.0 | 52.3 | 22.1 | 8.5 | 12.3–23.7 |
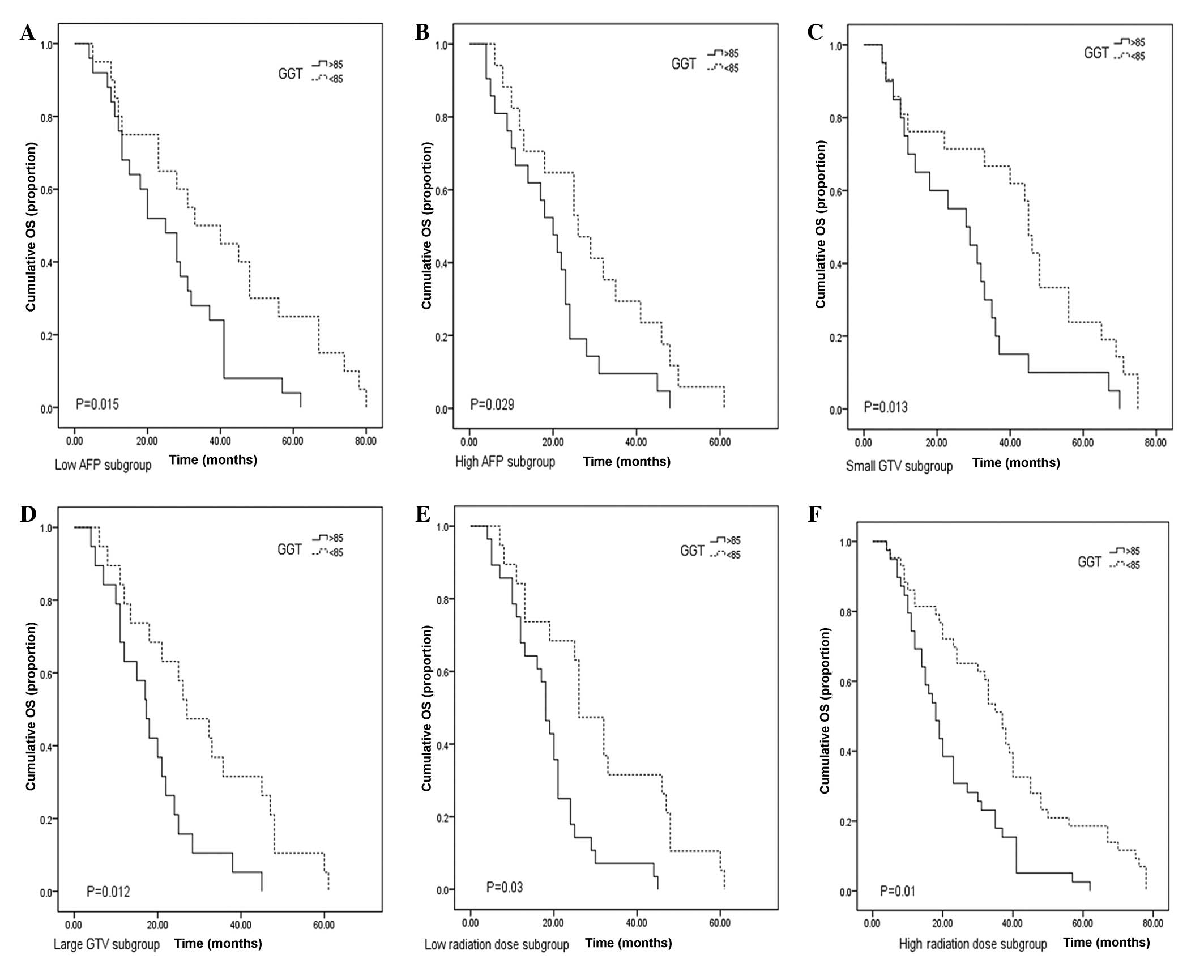
Considering the effects of the high AFP levels,
large GTV and high radiation dose on OS, these factors were
stratified to further clarify the prognostic significance of GGT
levels. The results demonstrated that serum GGT levels correlated
with OS time in the subgroup of low (≤400 ng/ml) and high (>400
ng/ml) serum AFP levels (P=0.015 and 0.029, respectively; Fig 3A and B). When the results were
stratified according to GTV, patients with low serum GGT levels had
a longer OS time compared with that of the high GGT level group
(P=0.013 and 0.012, respectively; Fig
3C and D). For the patients receiving a low radiation dose
(≤45Gy), those with high GGT levels exhibited a shorter OS time
compared with that of the low GGT group (P=0.03; Fig. 3E). In the high radiation dose group,
a significant difference was also observed in OS time between
patients with low and high GGT levels (P=0.01; Fig. 3F).
Discussion
Measurement of GGT levels has been investigated and
developed as a liver function test for several decades (14,15).
Hann et al (18) reported
that serum GGT levels may predict HCC risk and mortality in
hepatitis B virus (HBV) patients. Guiu et al (19) suggested that a serum GGT level of
≥165 U/L was associated with shorter time to treatment failure and
OS time following TACE. Zhang et al (10) revealed that the predictive value was
stable, and even higher, when a threshold of between 60 and 300 U/L
was used in a large retrospective study (277 patients).
Furthermore, elevation of GGT levels was confirmed as a predictor
of poor clinical outcome for intrahepatic cholangiocarcinoma
patients (19). However, the
correlation between GGT levels and TACE combined with 3DCRT remains
unexplored. In the current study, the results demonstrated that GGT
levels of >85 U/l were associated with a shorter OS time
(P=0.007). The optimal threshold of GGT levels (85 U/l) was
identified by the ROC analysis (Fig.
1), derived from a univariate logistic regression model
predicting patient mortality prior to the median OS.
The molecular mechanisms of GGT in HCC development
remain unclear. It has been suggested that functions of the
oxidative stress pathways in cellular response may mediate the role
of GGT in tumorigenesis (21). The
membrane-bound enzyme, GGT, catalyzes the degradation of
extracellular glutathione (GSH), making the component amino acids
available for the resynthesis of intracellular GSH (6). GSH can protect cells from damage
induced by oxidants generated during normal metabolism. There is
extensive evidence to suggest that GGT and GSH can cooperatively
generate free radicals, subsequently leading to lipid peroxidation
(18,22,23).
An additional explanation for the predictive nature of GGT on OS of
HCC patients in the current study is the significant implication of
lipid peroxidation and other metabolisms in the tumorigenesis of a
number of malignancies, including HCC (24,25).
Furthermore, an increased level of intracellular GSH often
correlates with resistance to platinum-based drugs (26). Daubeuf et al (27) revealed that GGT activity may affect
the cytotoxicity of platinum drugs in two ways: i) Following a
reaction with the thiol group of cysteinylglycine, cisplatin can be
detoxified extracellularly; or ii) in the case of carboplatin, GCT
initiates the supply of GSH precursors, which subsequently
increases the intracellular level of the tripeptide and provides
enhanced defensive mechanisms to the cell. In the current study,
cisplatin was the chemotherapeutic agent used during the TACE
procedure. This may also explain the longer OS time of patients
with low GGT levels (≤85 U/l) compared with those with high GGT
levels (>85 U/l) as increased levels of intracellular GSH are
often found to correlate with resistance to platinum-based drugs
and a high level of GGT is associated with a higher concentration
of GSH. Furthermore, in subgroups stratified according to serum AFP
levels, GTV and radiation dose, GGT levels still had the power to
discriminate patients with good results from those with poor
outcomes.
In the present study, univariate analysis indicated
that ALT levels, GTV, AFP levels, radiation dose and the number of
TACE procedures all correlate with OS. In the multivariate
analysis, only radiation dose, GTV and AFP levels were independent
prognostic factors. The number of TACE procedures were not an
independent predictive factor as different numbers of TACE were
performed for each patient until the iodized oil deposited the
whole tumor.
To date, radiotherapy technology has evolved
markedly and is significant in the treatment of HCC. Kouloulias
et al (28) reported that a
high radiation dose (50–52 Gy) of 3DCRT can achieve a high local
control rate in advanced HCC patients and inferior vena cava tumor
thrombosis. In the current study, patients receiving a radiation
dose of >45 Gy may achieve improved survival compared with those
receiving a low radiation dose (P=0.035). According to Son et
al (29) a large volume of
liver receiving radiotherapy may lead to radiation-induced liver
disease (RILD), which may result in hepatic failure and mortality.
The authors suggested that in order to reduce the risk of RILD, the
total liver volume receiving <18 Gy must be >800
cm3; therefore, sparing more normal liver during
radiotherapy is essential for HCC patients. In the current study,
longer survival was observed in patients with smaller GTV (≤200
cm3) compared with that of larger GTV (P=0.013).
Cell proliferation and angiogenesis are promoted by
AFP, as well as the increased resistance of cells toward tumor
necrosis factor-associated, apoptosis-inducing ligand-induced
apoptosis (30–32). It is well reported that AFP levels
are a significant prognostic factor for patients following
radiofrequency ablation and resection (33,34).
Tsai et al (35) and Kohles
et al (36) demonstrated
that AFP levels can be used as a biomarker to predict poor response
following TACE. The current study indicated that serum AFP levels
were an independent prognostic factor (P=0.006) for intermediate
HCC patients treated with TACE combined with 3DCRT.
The present study had certain limitations, including
the retrospective design and small number of patients. Therefore,
further studies investigating larger patient populations are
required to validate the results of the study.
In conclusion, the results presented in this study
demonstrated that the baseline GGT levels of intermediate HCC
patients with Child-Pugh grade A is an independent prognostic
factor for OS following TACE combined with 3DCRT. In additon, the
results of the present study may aid to predict outcomes for
patients and may also be used to guide individualized treatment for
HCC patients that receive TACE in combination with 3DCRT.
References
|
1
|
Cárdenes HR: Role of stereotactic body
radiotherapy in the management of primary hepatocellular carcinoma.
Rationale, technique and results. Clin Transl Oncol. 11:276–283.
2009.
|
|
2
|
Nathan H, Schulick RD, Choti MA and Pawlik
TM: Predictors of survival after resection of early hepatocellular
carcinoma. Ann Surg. 249:799–805. 2009.
|
|
3
|
Cha CH, Saif MW, Yamane BH and Weber SM:
Hepatocellular carcinoma: current management. Curr Probl Surg.
47:10–67. 2010.
|
|
4
|
Zeng ZC, Tang ZY, Fan J, et al: A
comparison of chemoembolization combination with and without
radiotherapy for unresectable hepatocellular carcinoma. Cancer J.
10:307–316. 2004.
|
|
5
|
Xu LT, Zhou ZH, Lin JH, et al: Clinical
study of transarterial chemoembolization combined with
3-dimensional conformal radiotherapy for hepatocellular carcinoma.
Eur J Surg Oncol. 37:245–251. 2011.
|
|
6
|
Whitfield JB: Gamma glutamyl transferase.
Crit Rev Clin Lab Sci. 38:263–355. 2001.
|
|
7
|
Griffith OW, Bridges RJ and Meister A:
Transport of gamma-glutamyl amino acids: role of glutathione and
gamma-glutamyl transpeptidase. Proc Natl Acad Sci USA.
76:6319–6322. 1979.
|
|
8
|
Poynard T, Zourabichvili O, Hilpert G, et
al: Prognostic value of total serum bilirubin/gamma-glutamyl
transpeptidase ratio in cirrhotic patients. Hepatology. 4:324–327.
1984.
|
|
9
|
Ju MJ, Qiu SJ, Fan J, et al: Preoperative
serum gamma-glutamyl transferase to alanine aminotransferase ratio
is a convenient prognostic marker for Child-Pugh A hepatocellular
carcinoma after operation. J Gastroenterol. 44:635–642. 2009.
|
|
10
|
Zhang JB, Chen Y, Zhang B, et al:
Prognostic significance of serum gamma-glutamyl transferase in
patients with intermediate hepatocellular carcinoma treated with
transcatheter arterial chemoembolization. Eur J Gastroenterol
Hepatol. 23:787–793. 2011.
|
|
11
|
Llovet JM, Fuster J and Bruix J:
Barcelona-Clínic Liver Cancer Group: The Barcelona approach:
diagnosis, staging, and treatment of hepatocellular carcinoma.
Liver Transpl. 10:S115–S120. 2004.
|
|
12
|
Bruix J, Sherman M, Llovet JM, et al: EASL
Panel of Experts on HCC: Clinical management of hepatocellular
carcinoma. Conclusions of the Barcelona-2000 EASL Conference
European Association for the Study of the Liver. J Hepatol.
35:421–430. 2001.
|
|
13
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982.
|
|
14
|
Saheb SM, Nath VN, Kumar KP and Padmaja
PP: A novel method using Seldinger’s technique for submental
intubation in major craniomaxillofacial fractures: A case series.
Indian J Anaesth. 58:48–50. 2014.
|
|
15
|
Kuo YC, Chiu YM, Shih WP, et al:
Volumetric intensity-modulated Arc (RapidArc) therapy for primary
hepatocellular carcinoma: comparison with intensity-modulated
radiotherapy and 3-D conformal radiotherapy. Radiat Oncol.
6:762011.
|
|
16
|
Whitfield JB, Pounder RE, Neale G and Moss
DW: Serum-glytamyl transpeptidase activity in liver disease. Gut.
13:702–708. 1972.
|
|
17
|
Idéo G, Morganti A and Dioguardi N:
Gamma-glutamyl transpeptidase: a clinical and experimental study.
Digestion. 5:326–336. 1972.
|
|
18
|
Hann HW, Wan S, Myers RE, et al:
Comprehensive analysis of common serum liver enzymes as prospective
predictors of hepatocellular carcinoma in HBV patients. PloS one.
7:e476872012.
|
|
19
|
Guiu B, Deschamps F, Boulin M, et al:
Serum gamma-glutamyl-transferase independently predicts outcome
after transarterial chemoembolization of hepatocellular carcinoma:
external validation. Cardiovasc Intervent Radiol. 35:1102–1108.
2012.
|
|
20
|
Hanigan MH: gamma-Glutamyl transpeptidase,
a glutathionase: its expression and function in carcinogenesis.
Chem Biol Interact. 111–112:333–342. 1998.
|
|
21
|
Pompella A, Corti A, Paolicchi A,
Giommarelli C and Zunino F: Gamma-glutamyltransferase, redox
regulation and cancer drug resistance. Curr Opin Pharmacol.
7:360–366. 2007.
|
|
22
|
Stark AA, Zeiger E and Pagano DA:
Glutathione metabolism by γ-glutamyl transpeptidase leads to lipid
peroxidation: characterization of the system and relevance to
hepatocarcinogenesis. Carcinogenesis. 14:183–189. 1993.
|
|
23
|
Paolicchi A, Tongiani R, Tonarelli P,
Comporti M and Pompella A: gamma-Glutamyl transpeptidase-dependent
lipid peroxidation in isolated hepatocytes and HepG2 hepatoma
cells. Free Radic Biol Med. 22:853–860. 1997.
|
|
24
|
Negre-Salvayre A, Auge N, Ayala V, et al:
Pathological aspects of lipid peroxidation. Free Radic Res.
44:1125–1171. 2010.
|
|
25
|
Zhao J, Zhao Y, Wang H, Gu X, Ji J and Gao
C: Association between metabolic abnormalities and HBV related
hepatocelluar carcinoma in Chinese: a cross-sectional study. Nutr
J. 10:492011.
|
|
26
|
Godwin AK, Meister A, O’Dwyer PJ, Huang
CS, Hamilton TC and Anderson ME: High resistance to cisplatin in
human ovarian cancer cell lines is associated with marked increase
of glutathione synthesis. Proc Natl Acad Sci USA. 89:3070–3074.
1992.
|
|
27
|
Daubeuf S, Balin D, Leroy P and Visvikis
A: Different mechanisms for gamma-glutamyltransferase-dependent
resistance to carboplatin and cisplatin. Biochem Pharmacol.
66:595–604. 2003.
|
|
28
|
Kouloulias V, Mosa E, Georgakopoulos J, et
al: Three-dimensional conformal radiotherapy for hepatocellular
carcinoma in patients unfit for resection, ablation, or
chemotherapy: A retrospective study. ScientificWorldJournal.
2013:7801412013.
|
|
29
|
Son SH, Choi BO, Ryu MR, et al:
Stereotactic body radiotherapy for patients with unresectable
primary hepatocellular carcinoma: dose-volumetric parameters
predicting the hepatic complication. Int J Radiat Oncol Biol Phys.
78:1073–1080. 2010.
|
|
30
|
Li M, Zhou S, Liu X, Li P, McNutt MA and
Li G: alpha-Fetoprotein shields hepatocellular carcinoma cells from
apoptosis induced by tumor necrosis factor-related
apoptosis-inducing ligand. Cancer Lett. 249:227–234. 2007.
|
|
31
|
Mitsuhashi N, Kobayashi S, Doki T, et al:
Clinical significance of alpha-fetoprotein: involvement in
proliferation, angiogenesis, and apoptosis of hepatocellular
carcinoma. J Gastroenterol Hepatol. 23:e189–e197. 2008.
|
|
32
|
Yang X, Zhang Y, Zhang L, Zhang L and Mao
J: Silencing alpha-fetoprotein expression induces growth arrest and
apoptosis in human hepatocellular cancer cell. Cancer Lett.
271:281–293. 2008.
|
|
33
|
Ho CM, Wu CY, Lee PH, Lai HS, Ho MC, Wu YM
and Hu RH: Analysis of the risk factors of untransplantable
recurrence after primary curative resection for patients with
hepatocellular carcinoma. Ann Surg Oncol. 20:2526–2533. 2013.
|
|
34
|
Siripongsakun S, Wei SH, Lin S, et al:
Evaluation of alpha-fetoprotein in detecting hepatocellular
carcinoma recurrence after radiofrequency ablation. J Gastroenterol
Hepatol. 29:157–164. 2014.
|
|
35
|
Tsai YJ, Hsu CY, Huang YH, et al: Early
identification of poor responders to transarterial
chemoembolization for hepatocellular carcinoma. Hepatol Int.
5:975–984. 2011.
|
|
36
|
Kohles N, Nagel D, Jüngst D, Durner J,
Stieber P and Holdenrieder S: Prognostic relevance of oncological
serum biomarkers in liver cancer patients undergoing transarterial
chemoembolization therapy. Tumor Biol. 33:33–40. 2012.
|