Introduction
Retinoblastoma (RB) is a rare malignant tumor in
infants and young children that, if left untreated, has the
potential to greatly endanger vision and life (1). Numerous genes have been implicated in
the genesis and development of RB (2). Suppressor of Zeste 12 homolog (SUZ12),
is an important component of polycomb group protein (PcG), and is
essential in cell proliferation, cell cycle and embryonic
development processes (3,4). SUZ12 is also known to regulate tumor
phenotype through altering gene expression, with an important
regulatory role in tumor genesis and development, and this has been
widely investigated (5). However,
to the best of our knowledge, no studies have analyzed the role of
SUZ12 in RB and the underlying mechanism of action. The present
study aimed to define the impact of SUZ12 on RB cell invasive
ability, along with the potential underlying regulatory mechanism,
with the aid of an SUZ12 RNA interference technique in the SO-RB50
RB cell strain.
Materials and methods
Experimental materials
The SO-RB50 human RB cell strain was obtained from
the Cell Bank of the Chinese Academy of Sciences (Beijing, China)
and stored under liquid nitrogen in the laboratory. Dulbecco’s
modified Eagle’s medium supplemented with 10% fetal bovine serum
was purchased from Gibco-BRL (Carlsbad, CA, USA). The rabbit
anti-human SUZ12 (75 kDa), matrix metalloproteinase (MMP)-2 and
MMP-9 antibodies, and the vascular endothelial growth factor (VEGF)
polyclonal antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). The liposome Oligofectamine (Invitrogen
Life Technologies, Carlsbad, CA, USA), SUZ12 small interfering
(si)RNA double-stranded oligonucleotides (Invitrogen Life
Technologies), Transwell chamber models (Chemicon, Temeluca, CA,
USA) and western blotting kits (Boster Bio-company, Beijing, China)
were also used in the present study.
SUZ12 siRNA sequence construction and
transfection into the SO-RB50 RB cell strain
The purchased SUZ12 siRNA oligonucleotide sequences
used in gene sequencing were as follows: S1, UUA UUG GAC AAC UUA
CAU CCU UCC U; S2, AAU UCA UUA CUG GAA ACU GCC AGG G and S3, UAA
AUU CUC UUC UUC CUG GAC GAG U. These oligonucleotides were matched
with GeneBank human SUZ12 cDNA sequences using Basic Local
Alignment Search Tool contrast (http://blast.ncbi.nlm.nih.gov/Blast.cgi). In addition,
the following negative control sequence: Sn, UUC UCC GAA CGU GUC
ACG UUU GUG C was designed and synthesized. SO-RB50 cells were
divided into six groups: Blank control (Con-B), empty vector
(Con-N), S1 transfection (S1), S2 transfection (S2), S3
transfection (S3) and Sn transfection (Sn). Each sequence (100 nm)
was transfected into the SO-RB50 cells (1×105 cells/ml)
using Oligofectamine, with phosphate-buffered saline and empty
vector at the same concentrations transferred to the Con-B and
Con-N cell groups, respectively. The subsequent procedures did not
differ among groups. The most effective siRNA for SUZ12 knockdown
was selected for subsequent experiments.
Western blot analysis of protein
expression levels
Exponentially growing SO-RB50 cells were lysed in
RIPA buffer and centrifuged at 4,472 × g for 5 min at 4°C. The cell
supernatants were collected and the protein levels were determined
using the bicinchoninic acid protein quantity detection kit
(AR0146; Boster Bio-company) according to the manufacturer’s
instructions. Subsequently, 50 μg protein extract was added to 2X
sample buffer and denatured at 100°C for 5 min. The proteins were
separated by SDS-PAGE and then transferred to nitrocellulose
membranes. The membranes were incubated with specific primary
antibodies (1:100) at 4°C overnight and mouse anti-rabbit secondary
antibodies (1:1,000) (Boster Bio-company) for 4 h and washed with
Tris-buffered saline for 5 min. Protein bands were developed with
enhanced chemiluminescence (Biosdec Biocompany) and exposed to
X-ray films. The captured images underwent grayscale analysis using
BandScan software (Glyko, Novato, CA, USA).
Soft agar assay of cell
anchorage-independent growth
A suspension of exponentially growing cells
(1×103 cells/ml) was prepared. Soft agar (5%) was mixed
with medium at a ratio of 1:9 and added to a plate, which was
cooled at room temperature. Subsequently, 1.5 ml cell suspension
was added to an equal volume of this plated 0.5% soft agar, and the
mixture was agitated and incubated at 37°C with 5% CO2
for two weeks. The cell colony formation rate was calculated
according to the following formula: Colony formation rate (%) =
(number of colonies/number of cells incubated) ×100.
Ex vivo invasion assay
Cell invasive ability was analyzed using a Transwell
chamber model (Chemicon). The cell suspension was adjusted to a
concentration of 1×105 cells/ml and 50 μl was placed in
the top chamber. After 24 h incubation, the cells that had migrated
to the lower chamber were fixed with 10% formalin and stained with
Giemsa to quantify the number of transmigrated cells.
Statistical analysis
Data are expressed as the mean ± standard deviation
and were processed using SPSS 16.0 software (SPSS, Inc., Chicago,
IL, USA). Comparisons of groups were performed using Student’s
t-test and P<0.05 was considered to indicate a statistically
significant difference.
Results
Efficiency of siRNA-mediated SUZ12
knockdown
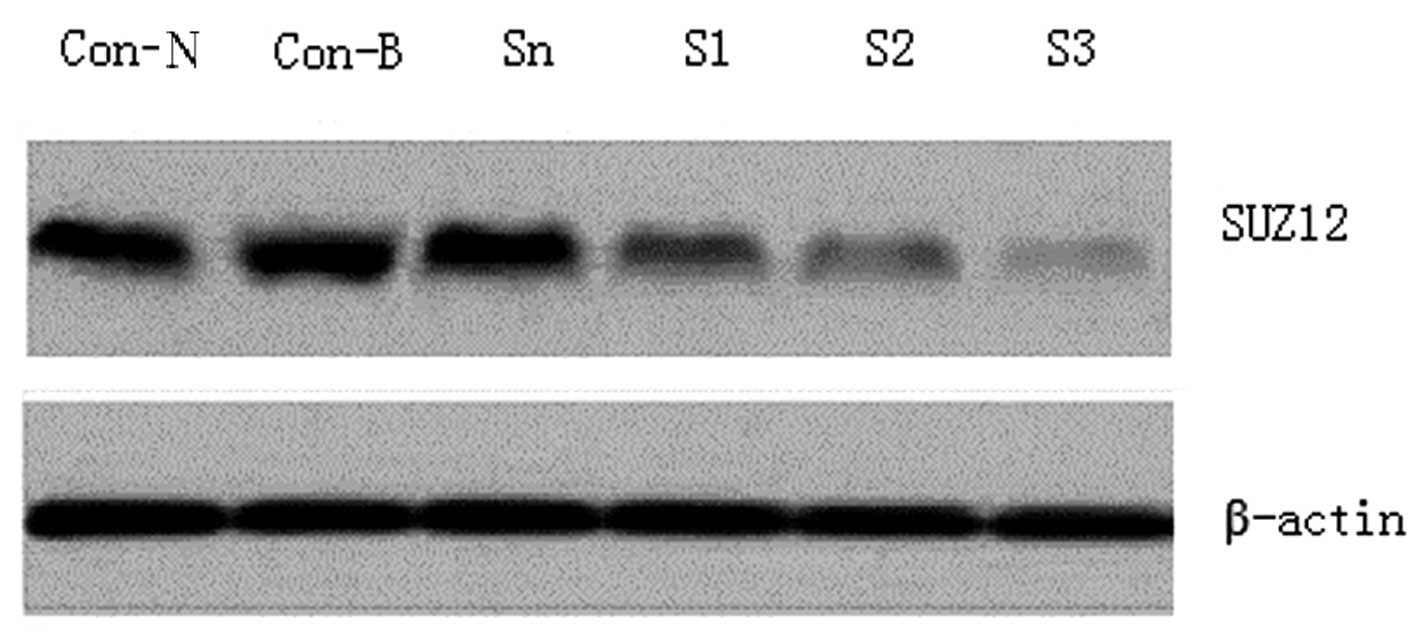
The SUZ12 expression levels in the SO-RB50 cells in
each group were determined using western blotting. SUZ12 exhibited
high expression levels in the Con-B, Con-N and Sn groups, but no
significant differences were detected among the three groups
(P>0.05). By contrast, SUZ12 was significantly downregulated in
the SUZ12-siRNA transfection groups (P<0.01); the reduction was
particularly marked in the S3 transfection group, which exhibited
an ~92.6% decline (Fig. 1).
Effect of SUZ12 siRNA on
anchorage-independent SO-RB50 cell growth
Due to the above finding that the S3 sequence was
the most effective in silencing SUZ12, this sequence was selected
as the SUZ12-specific interference sequence in subsequent
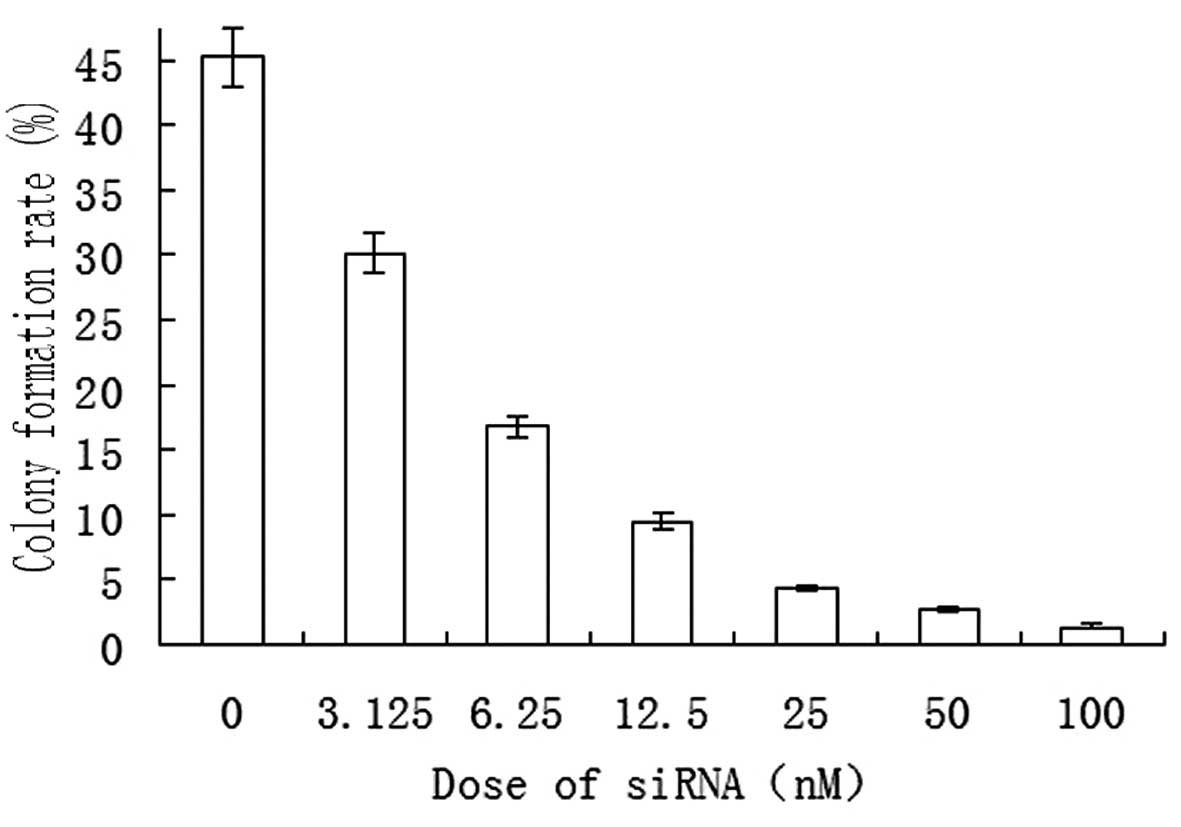
experiments. A soft agar colony formation assay revealed that
SO-RB50 cells formed colonies spontaneously in the in vitro
culture system. Following transfection of the cells with S3 siRNA
(at doses of 0, 3.125, 6.25, 12.5, 25, 50 and 100 nM), a gradually
reduced colony formation rate was observed as the transfection dose
was increased (Fig. 2).
Effect of SUZ12 interference on SO-RB50
cell invasion
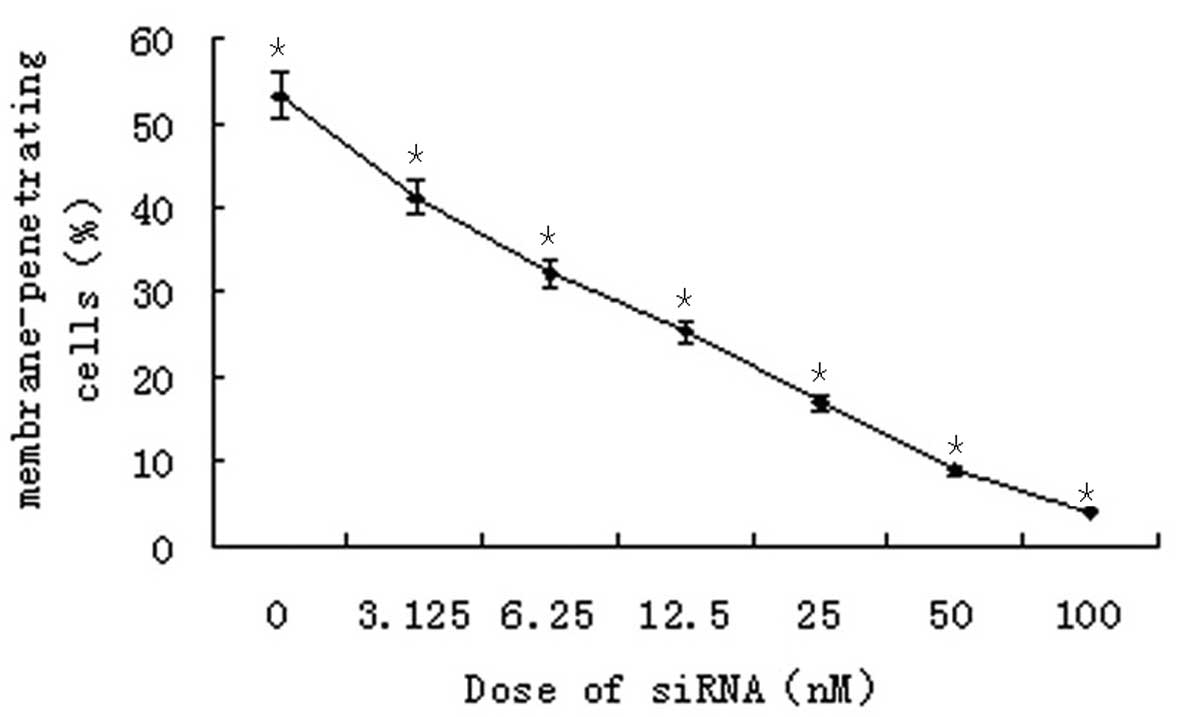
A Transwell chamber assay was employed to detect the
invasive ability of SO-RB50 cells 48 h after transfection with
different concentrations of the S3 siRNA. The results revealed that
siRNA-mediated knockdown of SUZ12 significantly reduced the number
of membrane-permeating cells in a concentration-dependent manner
(P=0.018; Fig. 3).
Effect of SUZ12 interference on VEGF,
MMP-2 and MMP-9 protein expression levels
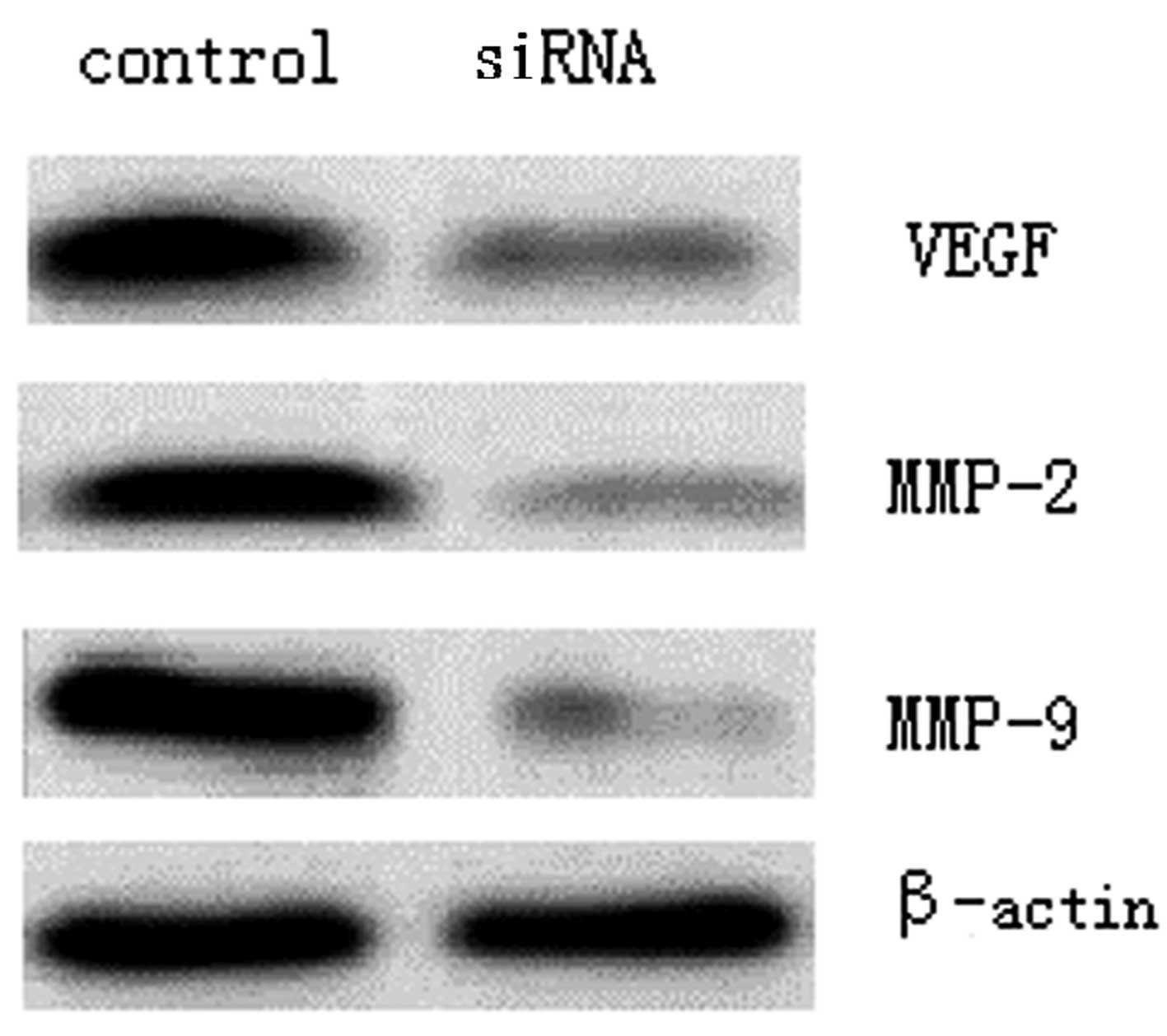
To identify the underlying mechanism of
SUZ12-mediated regulation of RB cell invasion, the VEGF, MMP-2 and
MMP-9 expression level changes following SUZ12 interference were
detected using western blotting, whereby the grey values
represented the level of protein expression. The results revealed
that the VEGF level (0.26±0.04) was significantly reduced compared
with that prior to SUZ12 knockdown (0.89±0.10) (P<0.01).
Furthermore, the levels of MMP-2 (0.16±0.02) and MMP-9 (0.12±0.02)
were also lowered significantly from those prior to SUZ12 knockdown
(0.94±0.16 and 1.15±0.18, respectively) (P<0.01; Fig. 4).
Discussion
SUZ12 is a component of the PcG complex that, along
with zeste 2 enhancer and embryonic ectoderm development, is
involved in cell proliferation, differentiation and aging via
inactivating target gene promoters (6). SUZ12 is critical for tumor
pathogenesis and development (7),
and may be involved in the regulation of tumor stem cells (8). Previous studies demonstrated high
SUZ12 expression levels in aggressive tumors, such as prostatic
carcinoma (9), breast carcinoma
(10) and nervous system carcinoma
(11), and a marked correlation
between SUZ12 expression levels and tumor malignancy. However, to
the best of our knowledge, no studies have analyzed the role of
SUZ12 in RB and the underlying mechanism of action.
In the present study, siRNA-mediated knockdown of
SUZ12 was performed, and the cell anchorage independence and
invasive ability were observed using soft agar colony formation
assay and Transwell chamber models, respectively. Anchorage
dependence refers to the finding that certain cells require
anchorage with a specific substrate to suppress apoptosis and
survive; conversely, tumor cells are characterized by
anchorage-independent growth. The soft agar colony formation assay
is able to measure tumor cell anchorage-independent growth and
tumor malignancy (12). Greater
invasive ability in tumor cells is associated with a higher number
of cell colonies. The present study revealed that SUZ12-specific
siRNA suppressed SO-RB50 cell colony formation in soft agar in a
concentration-dependent manner (Fig.
2), demonstrating that SUZ12 interference hindered SO-RB50 cell
invasion. The ability of tumors to migrate and invade is associated
with the microenvironment and the extracellular matrix (ECM);
therefore, a Transwell chamber model that imitates the ECM is thus
far a reliable method for assaying cell invasive ability (13). In the present study, a marked
reduction in the number of SO-RB50 cells that had passed through
the Transwell chamber was detected following SUZ12 silencing, and
the reduction appeared to be siRNA concentration-dependent
(Fig. 3). Preliminarily, these
findings suggest that interference with SUZ12 suppresses RB cell
invasive ability.
The invasive and migratory abilities of tumor cells
are closely associated with the capacity of the cells to induce
proteinase production that may degrade the ECM and the basement
membranes (14). A substantial
number of molecules are involved in the regulation of tumor cell
invasion and migration. Among these, VEGF, which mediates tumor
vascularizaion, is important in tumor formation, invasion and
metastasis, and may be a promising target in tumor therapy
(15). The present study
demonstrated that SUZ12 interference suppressed SO-RB50 cell
invasion as well as reducing VEGF activity. Another type of
molecule that is associated with tumor invasion is the MMPs. Among
these, MMP-9 and MMP-2 regulate vascular endothelial cell activity,
induce neovascularization, and exert an important role in RB
hyperplasia and differentiation (16). Furthermore, upregulation of MMP-9
and MMP-2 is associated with poorer outcomes in RB (17). The present study revealed that SUZ12
interference inhibited MMP-2 and MMP-9 activity, and thus hindered
cell invasion.
In conclusion, in the present study, SUZ12 knockdown
attenuated the invasive ability of RB SO-RB50 cells and suppressed
VEGF, MMP-2 and MMP-9 expression. Therefore, SUZ12 is of great
importance in regulating RB invasion and metastasis, and is
expected to be involved in targeted molecular therapy in RB.
References
|
1
|
Temming P, Eggert A, Bornfeld N, et al:
Diagnosis and treatment of retinoblastoma: current strategies for
effective tumour control and preservation of vision. Klin Monbl
Augenheilkd. 230:232–242. 2013.(In German).
|
|
2
|
Reis AH, Vargas FR and Lemos B: More
epigenetic hits than meets the eye: microRNAs and genes associated
with the tumorigenesis of retinoblastoma. Front Genet.
3:2842012.
|
|
3
|
Pasini D, Bracken AP, Jensen MR, et al:
Suz12 is essential for mouse development and for EZH2 histone
methytransferase activity. EMBO J. 23:4061–4071. 2004.
|
|
4
|
Pasini D, Bracken AP, Hansen JB, Capillo M
and Helin K: The polycomb group protein Suz12 is required for
embryonic stem cell differentiation. Mol Cell Biol. 27:3769–3779.
2007.
|
|
5
|
Kirmizis A, Bartley SM and Farnham PJ:
Identification of the polycomb group protein SU(Z)12 as a potential
molecular target for human cancer therapy. Mol Cancer Ther.
2:113–121. 2003.
|
|
6
|
Kirmizis A, Bartley SM, Kuzmichev A, et
al: Silencing of human polycomb target genes is associated with
methylation of histone H3 Lys 27. Genes Dev. 18:1592–1605.
2004.
|
|
7
|
Hahn MA, Hahn T, Lee DH, et al:
Methylation of polycomb target genes in intestinal cancer is
mediated by inflammation. Cancer Res. 68:10280–10289. 2008.
|
|
8
|
Lliopoulos D, Lindahl-Allen M, Polytarchou
C, et al: Loss of miR-200 inhibition of Suz12 leads to
polycomb-mediated repression required for the formation and
maintenance of cancer stem cells. Molecular Cell. 39:761–772.
2010.
|
|
9
|
Freedland SJ, Humphreys EB, Mangold LA, et
al: Risk of prostate cancer-specific mortality following
biochemical recurrence after radical prostatectomy. JAMA.
294:433–439. 2005.
|
|
10
|
Kleer CG, Cao Q, Varambally S, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:11606–11611. 2003.
|
|
11
|
Crea F, Hurt EM and Farrar WL: Clinical
significance of polycomb gene expression in brain tumors. Mol
Cancer. 9:2652010.
|
|
12
|
Yu JD, Yu JJ, Rhodes DR, et al: A polycomb
repression signature in metastatic prostate cancer predicts cancer
outcome. Cancer Res. 67:10657–10663. 2007.
|
|
13
|
Marshall J: Transwell(®)
Invasion Assays. Methods Mol Biol. 769:97–110. 2011.
|
|
14
|
Duffy MJ, McGowan PM and Gallagher WM:
Cancer invasion and metastasis: changing views. J Pathol.
214:283–293. 2008.
|
|
15
|
Yancopoulos GD: Clinical application of
therapies targeting VEGF. Cell. 143:13–16. 2010.
|
|
16
|
Kim JH, Kim JH, Cho CS, et al:
Differential roles of matrix metalloproteinase-9 and -2, depending
on proliferation or differentiation of retinoblastoma cells. Invest
Ophthalmol Vis Sci. 51:1783–1788. 2010.
|
|
17
|
Long H, Zhou B and Jiang FG: Expression of
MMP-2 and MMP-9 in retinoblastoma and their significance. Int J
Ophthalmol. 4:489–491. 2011.
|