Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide (1). Approximately 80% of lung cancers are
non-small cell lung carcinomas (NSCLC), for which surgery
represents the major curative treatment. However, only 30% of NSCLC
patients are able to receive surgery. Furthermore, in the majority
of cases, the disease is overtly or covertly metastatic on
presentation and consequently is currently incurable. Although
chemotherapy has been increasingly adopted for advanced NSCLC
treatment, the five-year survival rate of NSCLC remains <15% and
has not improved in recent years. Therefore, the development of
more efficacious targeted therapeutic agents for NSCLC is
required.
Aurora B is one of the major protein kinases
involved in the execution and fidelity of mitosis. Aurora-B is a
member of the chromosomal passenger complex and is involved in
numerous mitotic functions, including chromosome-microtubule
interactions, sister chromatid cohesion, the spindle-assembly
checkpoint mechanism and cytokinesis. Previous studies have shown
that Aurora-B is considered to be an important anti-tumor target
(2–6). Li et al (7) revealed that the downregulation of
Aurora-B inhibited proliferation and metastasis, induced
G2/M phase arrest in clear cell renal cell carcinoma
cells and exerted antitumor activity in an SN12C xenograft model.
Furthermore, previous studies have shown that the nuclear Aurora-B
expression level is significantly associated with metastasis in
tumors (8–12). However, whether Aurora-B is involved
in NSCLC metastasis remains unclear.
In the present study, the effect of Aurora-B
inhibition on A549 cell invasion and migration was investigated
in vitro. In addition, the effect of silencing Aurora-B on
the phosphoinositide 3-kinase (PI3K)/Akt/nuclear factor-κB (NF-κB)
signaling pathway was investigated. The aim of the present study
was to investigate whether knockdown of Aurora B inhibits A549 cell
invasion and migration via downregulation of the PI3K/Akt/NFκB
signaling pathway in vitro.
Materials and methods
Cell cultures
The human NSCLC A549 cell lines (Shanghai Cell Bank,
Chinese Academy of Sciences, Shanghai, China) were cultured in
Dulbecco’s modified Eagle’s medium (HyClone, Thermo Fisher
Scientific, Waltham, MA, USA) with 10% fetal bovine serum (FBS;
Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37°C in an
atmosphere of 5% CO2.
Patients and specimens
A total of 67 NSCLC tissue samples were obtained
from patients who underwent surgery at The First Affiliated
Hospital of Nanchang University (Nanchang, China). A total of 28
cases exhibited lymph node metastasis, and 39 cases were identified
without lymph node metastasis. The lymph node metastasis survey was
performed via histopathological detection of the lymph node. No
patient had a history of receiving any prior therapeutic treatment
with anti-tumor agents or via radiotherapy. Informed consent was
obtained from all patients and the study was approved by the ethics
committee of Nanchang University (Nanchang, China).
Immunohistochemical analysis
Histological sections (4 μm) were stained with
hematoxylin and eosin and detected by immunohistochemical analysis,
which was performed using the streptavidin-peroxidase procedure.
Briefly, antigen retrieval was performed by heating the
deparaffinized rehydrated sections in 10 mm citrate buffer (pH 6.0;
Abcam, Cambridge, UK) for 20 min, followed by blocking with 10%
goat serum (Sigma-Aldrich). Next, sections were incubated overnight
at 4°C with the primary rabbit anti-Aurora-B monoclonal antibody
(Abcam) at a final dilution of 1:500. For negative controls,
sections were incubated with phosphate-buffered saline (PBS;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) rather than with antibodies. Following three washes with
PBS, the sections were incubated with biotinylated secondary
monoclonal mouse anti-rabbit antibody (1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 40 min, followed by
incubation with horseradish peroxidase (HRP)-conjugated
streptavidin (Beijing Solarbio Science & Technology Co., Ltd.)
for 30 min. The sections were then chemiluminescence-stained and
counterstained using hematoxylin. The stained sections were
subsequently scored by two pathologists that were blinded to the
clinicopathological features of the patients. The staining
intensity was analyzed (by examining ≥500 cells from five
representative areas), the expression level of Aurora-B was
evaluated and the intensity scores were recorded as follows: No
staining, 0; weak staining, 1; moderate staining, 2; and intense
staining, 3. According to the percentage of tumor cells exhibiting
a positive expression of Aurora-B, the following percentage scores
were used: 0%, score 0; <10%, score 1; 47–50%, score 2; 51–80%,
score 3; and 81–100%, score 4. The final score was averaged
according to the scores that were determined by the two
pathologists according to the percentage scores. The intensity
score was then added to the percentage score; a final score of
<4 was defined as (−), 4 as (+), 5 as (++) and ≥6 as (+++).
Recombinant lentivirus-vector (LV)
construction and cell transfection
The human mRNA sequence (NM_004217) encoding the
Aurora-B protein was obtained from GenBank (http://www.ncbi.nlm.nih.gov/pubmed/). An interfering
short hairpin RNA (shRNA) targeting Aurora-B was designed and
synthesized, as well as a negative shRNA, which served as a
negative control. shRNA sequences were inserted into the LV, GV115
(Aurora-B/LV and negative [Neg]/LV) and transfected into the A549
cells (multiplicity of infection = 20). The transfection efficiency
was evaluated using a fluorescence microscope (BX51M, Olympus
Corporation, Tokoyo, Japan).
Quantitative polymerase chain reaction
(qPCR) assays
Total RNA from the A549 cells was extracted using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA).
qPCR was used to detect Aurora-B mRNA expression, with β-actin
serving as the endogenous reference gene. All procedures were
performed according to the manufacturer’s instructions and the
following primers were used: Forward, 5′-AGAAGGAGAACTCCTACCCCT-3′
and reverse, 5′-CGCGTTAAGATGTCGGGTG-3′ for Aurora-B (product
length, 202 bp). Six independent experiments were performed over
numerous days.
Western blot analysis
Total protein from the cells was extracted using
radio-immunoprecipitation lysis buffer containing 60 lg/ml
phenylmethylsulfonyl fluoride [Tiangen Biotech (Beijing) Co., Ltd.,
Beijing, China]. The protein concentration was determined by
Bradford assay (Sigma-Aldrich). Western blot analysis was conducted
using the following primary monoclonal anti-human antibodies:
Rabbit anti-Aurora-B IgG, (1:200), rabbit anti-PI3K IgG (1:1,000),
rabbit anti-phosphorylated (p)Akt IgG (1:800) goat anti-Akt IgG
(1:1,000), rabbit anti-NF-κB (p65) IgG (1:400), rabbit anti-matrix
metalloproteinase (MMP)-2 (1:1,000) and rabbit anti-MMP-9 IgG
(1:1,000), which were all purchased from Abcam and mouse
anti-glyceraldehyde 3-phosphate dehydrogenase (1:5,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and the corresponding
mouse anti-rabbit, mouse anti-goat and goat anti-mouse monoclonal
secondary antibodies (ZSGB-BIO, Beijing, China). The immune
complexes were detected using the pro-light Streptavidin-HRP kit
(Pierce Biotechnology, Inc., Rockford, IL, USA). Six independent
experiments were performed over numerous days.
Transwell assays
The invasion of A549 cells was measured using the BD
BioCoat™ BD Matrigel™ Invasion Chamber (BD Biosciences, Franklin
Lakes, NJ, USA) according to the manufacturer’s instructions. The
medium in the lower chamber contained 5% fetal calf serum as a
source of chemoattractants. The cultures were rinsed with PBS and
replaced with fresh quiescent medium alone or containing 10% FBS,
following which the cells were incubated at 37°C for 24 h. Cells
that had passed through the matrigel-coated membrane were stained
using Diff-Quik (Sysmex, Kobe, Japan) and images were captured
using a camera (S200, Canon Inc., Tokoyo, Japan) and an inverted
microscope (Olympus Corporation). Cell migration was quantified by
direct microscopic visualization and counting. The rate of invasion
was calculated by counting three fields per membrane and presented
as the mean of six independent experiments performed over various
days.
Wound healing assay
Cell migration was analyzed by determining the
ability of the cells to move into a cellular space in a
two-dimensional in vitro wound healing assay. Briefly, cells
were grown to confluence in six-well tissue culture plates at a
density of ~5×106 cells/well. The cells were then
denuded by dragging a rubber policeman (Thermo Fisher Scientific
Inc., Rockford, IL, USA) through the center of the plate. Cultures
were then rinsed with PBS and replaced with fresh quiescent medium
alone or containing 10% FBS, following which the cells were
incubated at 37°C for 24 h. Images were captured at 0 and 24 h, and
the migrated distance was measured in five independent wound sites
per group. Six independent experiments were performed over numerous
days.
Statistical analysis
All data are presented as the mean ± standard
deviation. The two independent-samples t-test was used to analyze
the difference between Aurora-B protein expression levels in NSCLC
patients with and without lymph node metastasis. P<0.05 was
considered to indicate a statistically significant difference. All
analyses were performed using SPSS version 13.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
Aurora-B protein may be involved in lymph
node metastasis in NSCLC
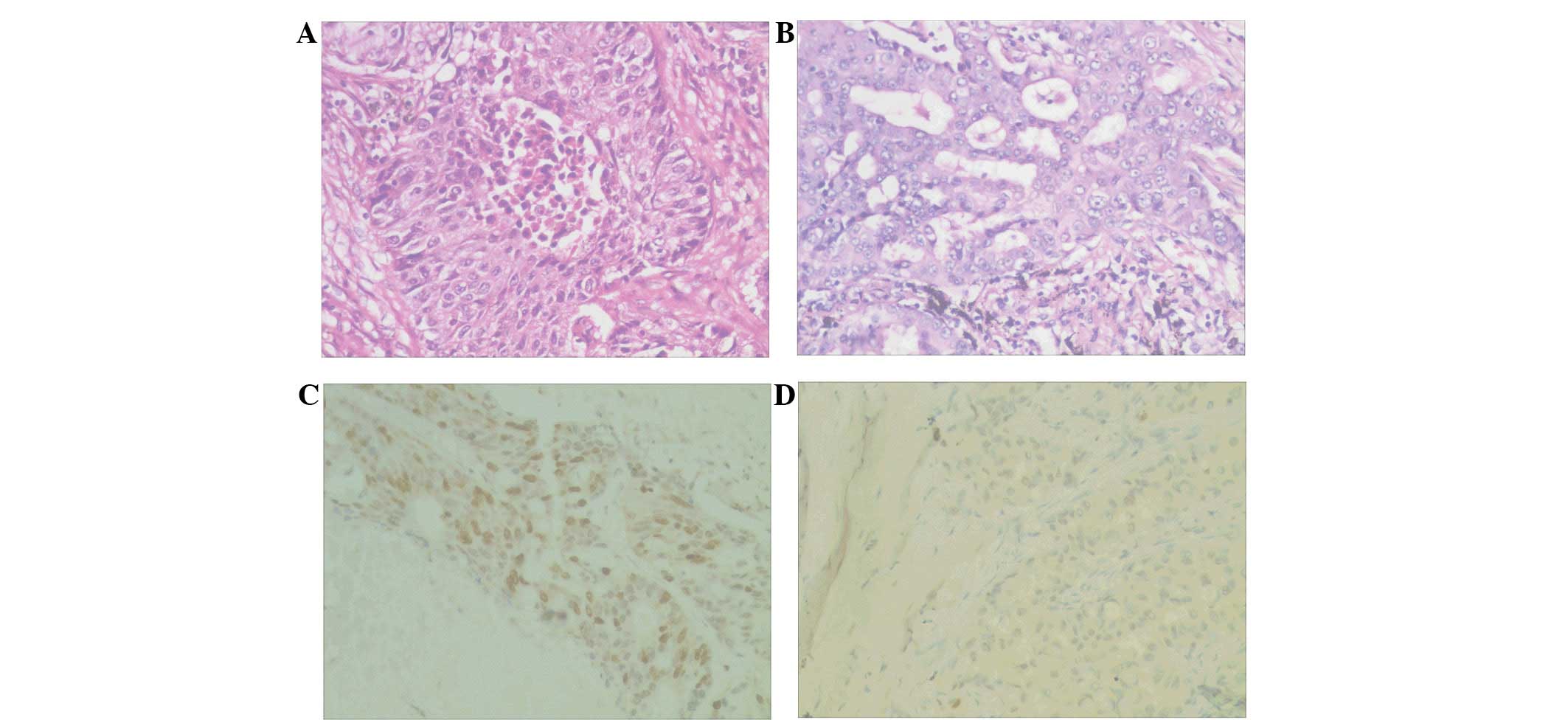
Aurora-B was expressed in the nucleus (Fig. 1) and the positive expression rate in
the samples with metastatic disease was 82.1% (23/28), however, in
those without lymph node metastasis the rate was only 43.6%
(17/39); the difference was identified to be significant
(P<0.05) (data not shown). These results indicated that Aurora-B
may be involved in lymph node metastasis in NSCLC.
Recombinant LV inhibits Aurora-B
expression in A549 cells
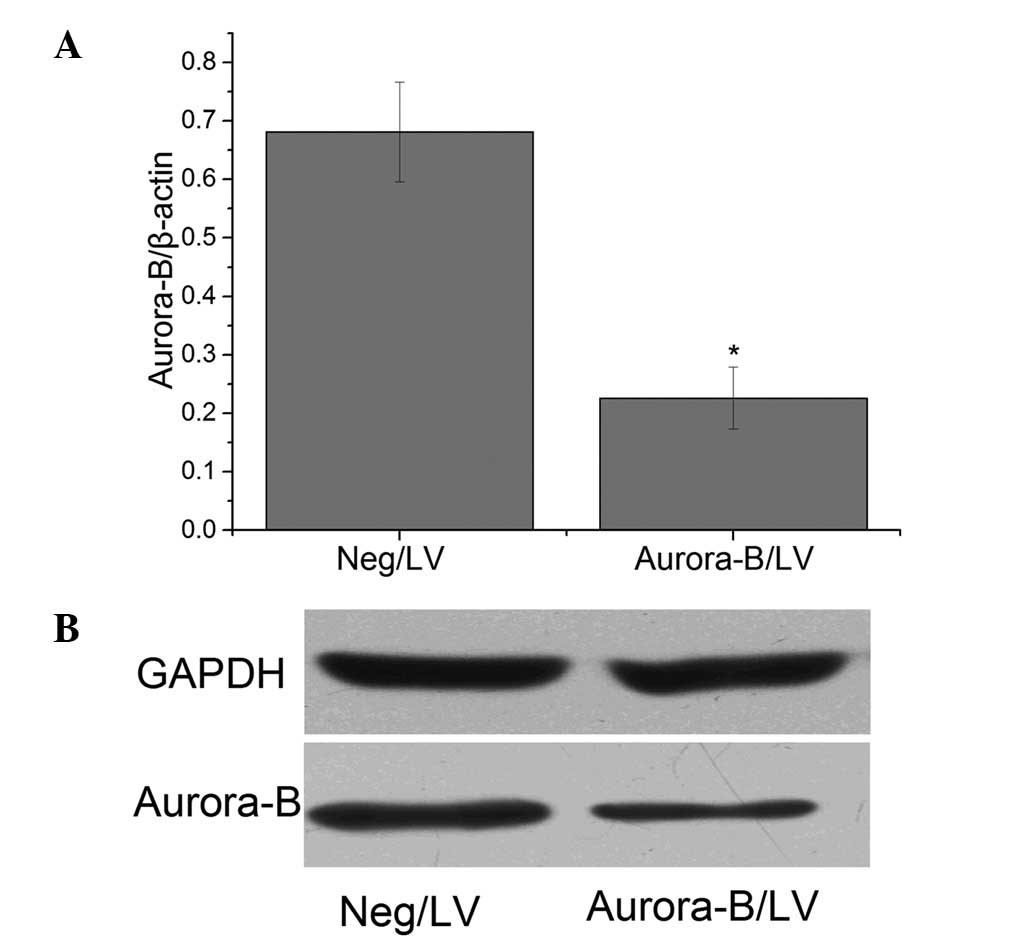
A549 cells were transfected with the recombinant LV
targeting Aurora-B. qPCR and western blot analysis revealed that
the level of Aurora-B protein expression was significantly lower in
cells transfected with Aurora-B/LV compared with in those
transfected with Neg/LV (Fig.
2).
Inhibition of Aurora-B suppresses A549
cell migration
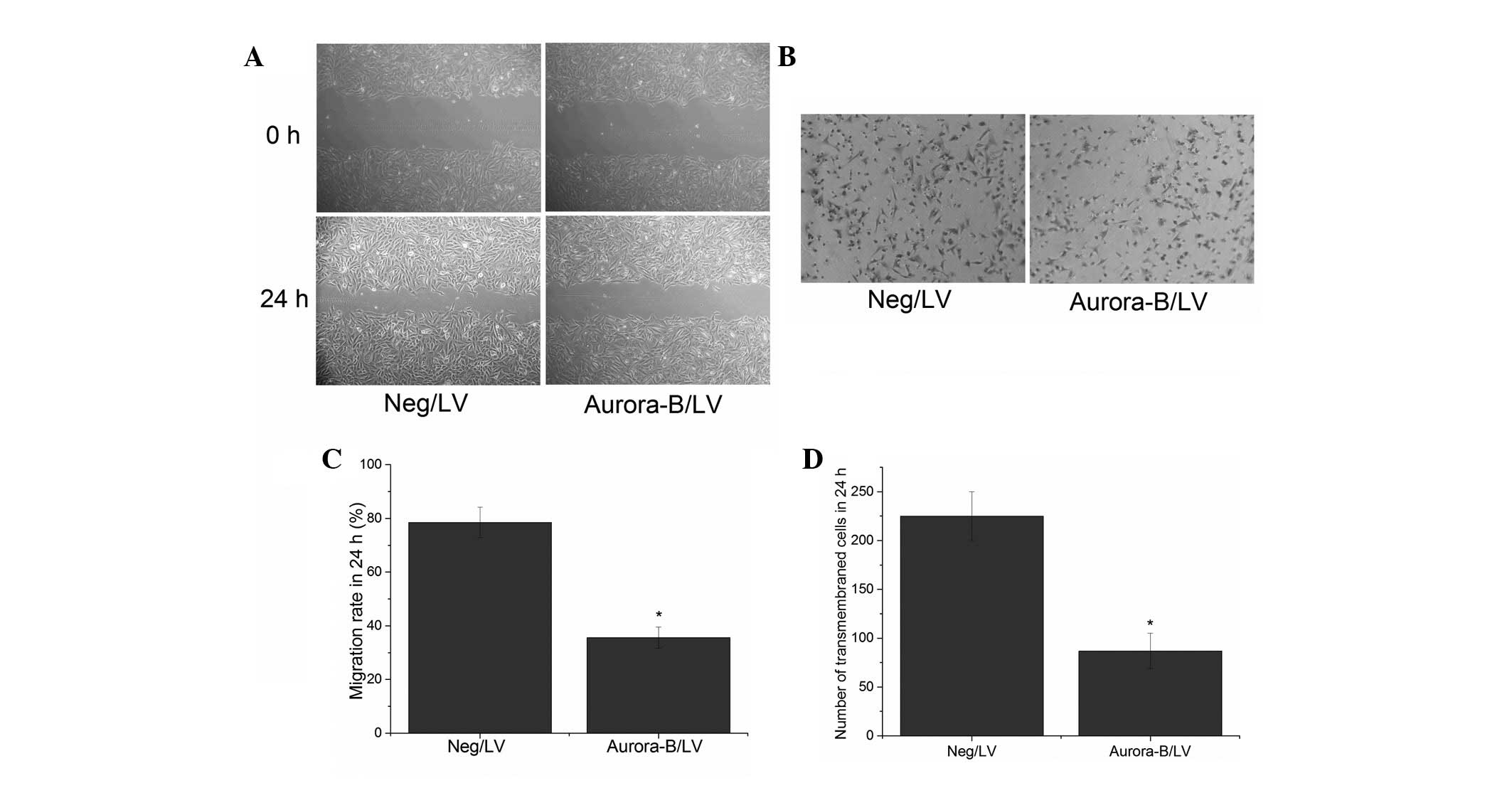
To investigate whether Aurora-B affects cellular
migration, an in vitro wound healing assay was performed.
The results showed that the migration rates of cells transfected
with Aurora-B/LV and Neg/LV were 35.6±3.98% and 78.5±5.66%,
respectively. The difference was identified to be significant
(Fig. 3A and B). These results
indicated that Aurora-B inhibition may suppress A549 cell migration
in vitro.
Inhibition of Aurora-B suppresses A549
cell invasion
A Transwell assay was performed to evaluate the
effect of Aurora-B inhibition on the invasion of A549 cells. The
results revealed that the number of transmembrane cells was lower
in the cells that had been transfected with Aurora-B/LV (87±18
cells per high power field) when compared with that of the cells
transfected with Neg/LV (225±25 cells per high power field
[P<0.05]; Fig. 3C and D). These
results indicated that the inhibition of Aurora-B may suppress the
invasion of A549 cells in vitro.
Silencing Aurora-B inhibits
PI3K/Akt/NF-κB signaling in A549 cells
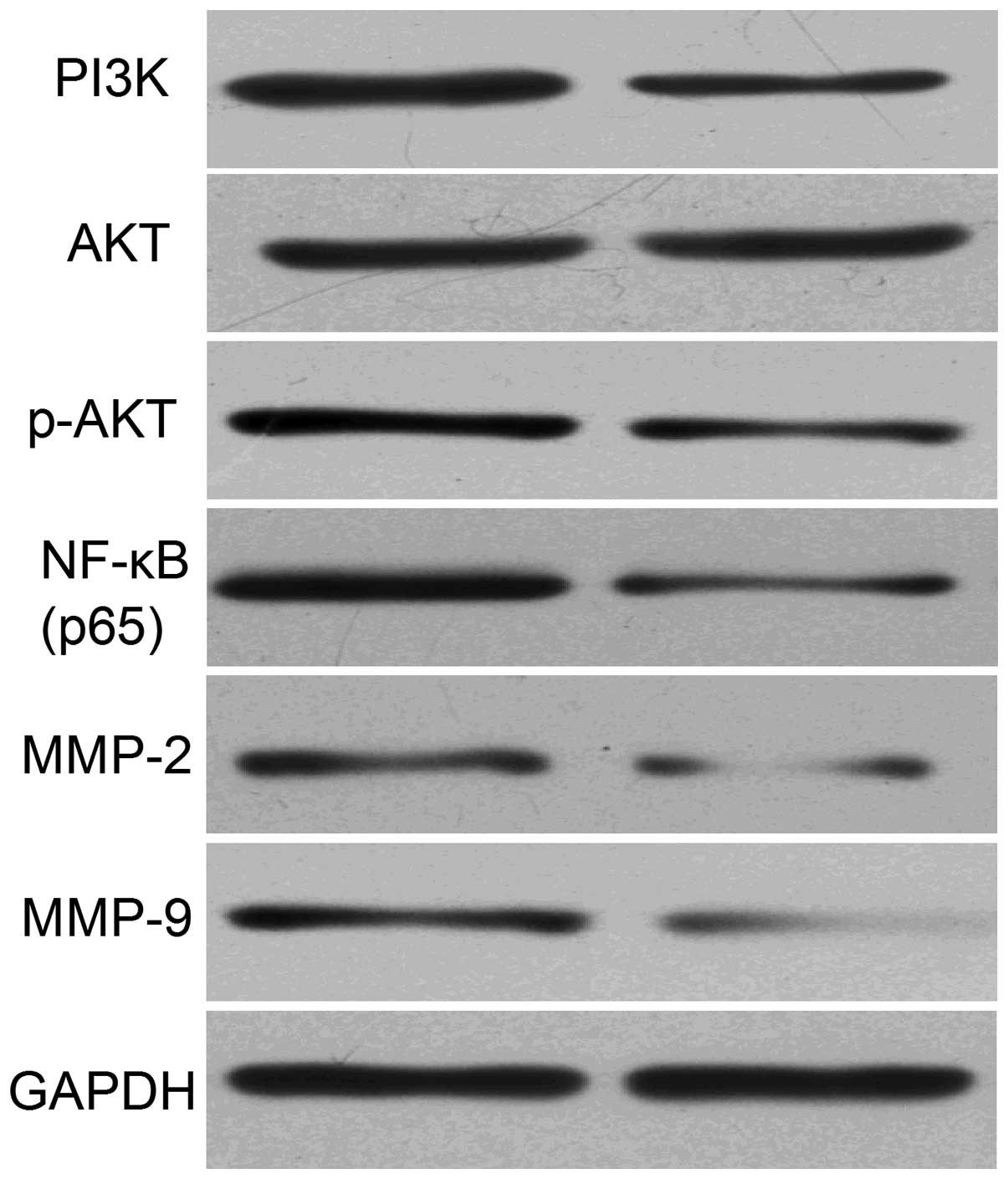
In order to investigate the effect of Aurora-B
inhibition on the activity of the PI3K/Akt signaling pathway in
A549 cells, the expression levels of PI3K, Akt, p-Akt, NF-κB (p65)
as well as the MMP-2 and -9 proteins was measured using western
blot analysis. The results revealed that the level of PI3K, p-Akt,
NF-κB (p65), MMP-2 and -9 protein expression in cells transfected
with Aurora-B/LV was significantly lower than that of cells
transfected with Neg/LV (Fig. 4).
This indicated that the inhibition of Aurora-B may inhibit the
activity of the PI3K/Akt/ NF-κB signaling pathway in A549
cells.
Discussion
Aurora kinases are serine/threonine kinases, which
are crucial for cell cycle control and mitosis. Three Aurora kinase
family members (A, B and C) have been identified in mammals and are
expressed at maximal levels during mitosis. Aurora-B, a component
of the chromosome passenger complex, is located on the chromosome
arms during prophase, and at the centromeres during the
prometaphase and metaphase. Aurora-B subsequently localizes to the
midbody during cytokinesis. Previous studies have shown Aurora-B to
be overexpressed in numerous types of cancer (9,10,13).
In the present study, the expression levels of Aurora-B protein in
NSCLC tissues were detected by immunohistochemistry, which revealed
that the Aurora-B protein was expressed in the nucleus.
Furthermore, the positive expression rate of Aurora-B protein in
the NSCLC tissues with lymph node metastasis was significantly
higher when compared with the tissue samples without lymph node
metastasis. These results are consistent with those reported by
Takeshita et al (9) and Wang
et al (14). The results of
the present study indicated that Aurora-B may be involved in the
development and progression of lymph node metastasis, and may
present a novel diagnostic and therapeutic target for NSCLC.
Various studies have revealed that the inhibition of
Aurora-B blocked cell proliferation and induced cell apoptosis in a
variety of tumors (15–17). These findings highlighted Aurora-B
as a potential molecular target for cancer treatment. Notably,
recent studies have shown that the upregulation of Aurora-B
expression was associated with tumor cell metastasis, and the
downregulation of Aurora-B inhibited cell invasion and migration in
various tumors (18,19). However, the effect of Aurora-B
inhibition in NSCLC malignancies remains to be fully elucidated. In
the present study, to investigate the effect of Aurora-B inhibition
on NSCLC cell migration and invasion, the recombinant LV targeting
Aurora-B was constructed to inhibit Aurora-B expression in A549
cells. Furthermore, the migration and invasion of A549 cells was
investigated by wound healing and Transwell assays, and the results
revealed that the migration and invasion rate of cells was
significantly lower in cells that were transfected with Aurora-B/LV
than those that were transfected with Neg/LV. This indicated that
the inhibition of Aurora-B may suppress A549 cell migration and
invasion in vitro.
In addition, the potential molecular mechanisms
associated with the inhibition of Aurora-B expression, and A459
cell migration and invasion suppression were analyzed. The role of
the PI3K/Akt/NF-κB signaling pathway in tumor cell invasion and
migration was investigated (20–25).
Long et al (26)
demonstrated that ZM447439, an inhibitor of Aurora-B, was
significantly associated with a decrease in Akt phosphorylation (at
Ser473) and a decrease in the phosphorylation of its substrates,
glycogen synthase kinase 3-α and -β (at Ser21 and Ser9) in Hep2
cancer cells. Akt is essential for NF-κB activation via the
stimulation of the IκB kinase complex, which phosphorylates and
inactivates IκB, an inhibitor of NF-κB. Previous studies have
demonstrated that NF-κB upregulates MMP-9 (27) and the inhibition of NF-κB was found
to downregulate MMP-2 (28). During
the development of metastases, cancer cells must degrade the
components of the extracellular matrix. MMPs, in particular MMP-2
and -9, are markedly associated with this process due to their
capacity to degrade the extracellular matrix, which promotes tumor
invasion.
In the present study, PI3K, Akt, p-Akt and NF-κB
(p65) protein expression levels were detected by western blot
analysis to investigate whether inhibiting Aurora-B led to a
decrease in the activity of the PI3K/Akt/NF-κB signaling pathway.
The results revealed that the expression levels of the PI3K, p-Akt
and NF-κB (p65) proteins were significantly decreased in cells that
were transfected with Aurora-B/LV when compared with those in cells
that were transfected with Neg/LV. These results indicated that the
inhibition of Aurora-B downregulates the PI3K/Akt/NF-κB signaling
pathway in NSCLC cells. In addition, western blot analysis was
performed to investigate the expression levels of MMP-2 and -9
proteins. It was found that the protein expression levels were
reduced as a result of Aurora-B inhibition when compared with the
negative control cells, indicating that the inhibition of Aurora-B
attenuates the activation of MMP-2 and -9 proteins.
In conclusion, the present study demonstrates that
the inhibition of Aurora-B may suppress NSCLC cell invasion and
migration via modulation of the PI3K/Akt/NF-κB signaling pathway
in vitro. This indicates that targeting Aurora-B and the
PI3K/Akt/NF-κB signaling pathway may present a potential treatment
strategy for NSCLC.
Acknowledgements
The present study was supported by grants from
Jiangxi Province Education Department of Science and Technology
(grant no. GJJ12097).
References
|
1
|
Shi L, Tang J, Tong L and Liu Z: Risk of
interstitial lung disease with gefitinib and erlotinib in advanced
non-small cell lung cancer: a systematic review and meta-analysis
of clinical trials. Lung Cancer. 83:231–239. 2014.
|
|
2
|
Carmena M, Ruchaud S and Earnshaw WC:
Making the Auroras glow: regulation of Aurora A and B kinase
function by interacting proteins. Curr Opin Cell Biol. 21:796–805.
2009.
|
|
3
|
Ohi R, Sapra T, Howard J and Mitchison TJ:
Differentiation of cytoplasmic and meiotic spindle assembly MCAK
functions by Aurora B-dependent phosphorylation. Mol Biol Cell.
15:2895–2906. 2004.
|
|
4
|
Wang F, Ulyanova NP, Daum JR, Patnaik D,
Kateneva AV, Gorbsky GJ and Higgins JM: Haspin inhibitors reveal
centromeric functions of Aurora B in chromosome segregation. J Cell
Biol. 199:251–268. 2012.
|
|
5
|
Piekorz RP: Dissecting the role of
INCENP-Aurora B in spindle assembly checkpoint function,
chromosomal alignment and cytokinesis. Cell Cycle. 9:1678–1679.
2010.
|
|
6
|
Becker M, Stolz A, Ertych N and Bastians
H: Centromere localization of INCENP-Aurora B is sufficient to
support spindle checkpoint function. Cell Cycle. 9:1360–1372.
2010.
|
|
7
|
Li Y, Zhou W, Wei L, Jin J, Tang K, Li C,
et al: The effect of Aurora kinases on cell proliferation, cell
cycle regulation and metastasis in renal cell carcinoma. Int J
Oncol. 41:2139–2149. 2012.
|
|
8
|
Qi G, Ogawa I, Kudo Y, Miyauchi M,
Siriwardena BS, Shimamoto F, et al: Aurora-B expression and its
correlation with cell proliferation and metastasis in oral cancer.
Virchows Arch. 450:297–302. 2007.
|
|
9
|
Takeshita M, Koga T, Takayama K, Ijichi K,
Yano T, Maehara Y, et al: Aurora-B overexpression is correlated
with aneuploidy and poor prognosis in non-small cell lung cancer.
Lung Cancer. 80:85–90. 2013.
|
|
10
|
Hetland TE, Nymoen DA, Holth A, Brusegard
K, Flørenes VA, Kærn J, et al: Aurora B expression in metastatic
effusions from advanced-stage ovarian serous carcinoma is
predictive of intrinsic chemotherapy resistance. Hum Pathol.
44:777–785. 2013.
|
|
11
|
Bonet C, Giuliano S, Ohanna M, Bille K,
Allegra M, Lacour JP, et al: Aurora B is regulated by the
mitogen-activated protein kinase/extracellular signal-regulated
kinase (MAPK/ERK) signaling pathway and is a valuable potential
target in melanoma cells. J Biol Chem. 287:29887–29998. 2012.
|
|
12
|
Tuncel H, Shimamoto F, Kaneko Guangying Qi
H, Aoki E, Jikihara H, Nakai S, et al: Nuclear Aurora B and
cytoplasmic Survivin expression is involved in lymph node
metastasis of colorectal cancer. Oncol Lett. 3:1109–1114. 2012.
|
|
13
|
Honma K, Nakanishi R, Nakanoko T, Ando K,
Saeki H, Oki E, et al: Contribution of Aurora-A and -B expression
to DNA aneuploidy in gastric cancers. Surg Today. 44:454–461.
2014.
|
|
14
|
Wang WR, Yang SS, Lin JX, Zeng ZY, Liu DM
and Liu HT: Expression of Aurora-B in non-small cell lung cancer
and its clinical significance. Nan Fang Yi Ke Da Xue Xue Bao.
29:1853–1856. 2009.(In Chinese).
|
|
15
|
Ma YX and Li XZ: Effect of aurora kinase B
inhibitor AZD1152 in the treatment of cisplatin-resistant ovarian
carcinoma. Zhonghua Fu Chan Ke Za Zhi. 48:46–50. 2013.(In
Chinese).
|
|
16
|
Sak A, Stuschke M, Groneberg M, Kübler D,
Pöttgen C and Eberhardt WE: Inhibiting the aurora B kinase potently
suppresses repopulation during fractionated irradiation of human
lung cancer cell lines. Int J Radiat Oncol Biol Phys. 84:492–499.
2012.
|
|
17
|
Qi W, Liu X, Cooke LS, Persky DO, Miller
TP, Squires M and Mahadevan D: AT9283, a novel aurora kinase
inhibitor, suppresses tumor growth in aggressive B-cell lymphomas.
Int J Cancer. 130:2997–3005. 2012.
|
|
18
|
Zhang L and Zhang S: ZM447439, the Aurora
kinase B inhibitor, suppresses the growth of cervical cancer SiHa
cells and enhances the chemosensitivity to cisplatin. J Obstet
Gynaecol Res. 37:591–600. 2011.
|
|
19
|
Pohl A, Azuma M, Zhang W, Yang D, Ning Y,
Winder T, et al: Pharmacogenetic profiling of Aurora kinase B is
associated with overall survival in metastatic colorectal cancer.
Pharmacogenomics J. 11:93–99. 2011.
|
|
20
|
Lin ML, Lu YC, Chen HY, Lee CC, Chung JG
and Chen SS: Suppressing the formation of lipid raft-associated
Rac1/PI3K/Akt signaling complexes by curcumin inhibits
SDF-1α-induced invasion of human esophageal carcinoma cells. Mol
Carcinog. 53:360–379. 2014.
|
|
21
|
Xu CL, Lu XL, Yan XN, Wang HL and Chen SQ:
Effects of PI3K/Akt/NF-κB signal pathway on FSH facilitation on
cell proliferation and invasion by human epithelial ovarian cancer.
Zhonghua Fu Chan Ke Za Zhi. 47:134–138. 2012.(In Chinese).
|
|
22
|
Lin X, Zhang X, Wang Q, Li J, Zhang P,
Zhao M and Li X: Perifosine downregulates MDR1 gene expression and
reverses multidrug-resistant phenotype by inhibiting PI3K/Akt/NF-κB
signaling pathway in a human breast cancer cell line. Neoplasma.
59:248–256. 2012.
|
|
23
|
Koumakpayi IH, Le Page C, Mes-Masson AM
and Saad F: Hierarchical clustering of immunohistochemical analysis
of the activated ErbB/PI3K/Akt/NF-kappaB signalling pathway and
prognostic significance in prostate cancer. Br J Cancer.
102:1163–1173. 2010.
|
|
24
|
Song L, Xiong H, Li J, Liao W, Wang L, Wu
J and Li M: Sphingosine kinase-1 enhances resistance to apoptosis
through activation of PI3K/Akt/NF-κB pathway in human non-small
cell lung cancer. Clin Cancer Res. 17:1839–1849. 2011.
|
|
25
|
Cortes-Sempere M, Chattopadhyay S, Rovira
A, Rodriguez-Fanjul V, Belda-Iniesta C, Tapia M, et al: MKP1
repression is required for the chemosensitizing effects of
NF-kappaB and PI3K inhibitors to cisplatin in non-small cell lung
cancer. Cancer Lett. 286:206–216. 2009.
|
|
26
|
Long ZJ, Xu J, Yan M, Zhang JG, Guan Z, Xu
DZ, et al: ZM 447439 inhibition of aurora kinase induces Hep2
cancer cell apoptosis in three-dimensional culture. Cell Cycle.
7:1473–1479. 2008.
|
|
27
|
Andela VB, Gordon AH, Zotalis G, Rosier
RN, Goater JJ, Lewis GD, et al: NFkappaB: a pivotal transcription
factor in prostate cancer metastasis to bone. Clin Orthop Relat
Res. (Suppl 415): S75–S85. 2003.
|
|
28
|
Felx M, Guyot MC, Isler M, Turcotte RE,
Doyon J, Khatib AM, et al: Endothelin-1 (ET-1) promotes MMP-2 and
MMP-9 induction involving the transcription factor NF-kappaB in
human osteosarcoma. Clin Sci (Lond). 110:645–654. 2006.
|