Introduction
At present, non-small cell lung cancer (NSCLC) is
one of the most common types of malignant tumor. The US Centers for
Disease Control and Prevention have shown that the number of
mortalities from lung cancer is higher than any other type of
cancer (1). Lung adenocarcinoma
accounts for 40% of all lung cancers, the incidence is predominant
in the female popluation and is not generally attributed to
cigarette smoking. Lung adenocarcinoma has an five-year survival
rate of 11.6% in the USA (2) due to
the fact that numerous patients are not diagnosed early and
therefore lose the opportunity to undergo complete surgical
resectioning. Treatment usually involves surgery, however, this is
often inadequate. Studies regarding NSCLC have been exhaustive and
research has progressed, particularly in the search for natural
anti-tumor treatments (3). Cobra
neurotoxin (cobrotoxin), derived from cobra venom, mainly
consisting of a postsynaptic nerve toxin. Cobrotoxin is capable of
combining with acetylcholine receptors, which may be the underlying
mechanism for its anti-inflammatory, immune adjustment and pain
easing qualities, additionally it has been shown to exhibit
antineoplastic activity (4,5). In the present study, the effect of
cobrotoxin on human lung cancer A549 cells was investigated to
determine a potential molecular mechanism, in order to provide a
theoretical basis for its application in clinical use.
Materials and methods
Cell culture
Human A549 lung glandular cancer cells and human
lung fibroblast cells that demonstrated adherent growth were
obtained from the Shanghai Institute of Cell Biology (Shanghai,
China). The A549 cells were maintained in RPMI-1640 culture medium
containing 10% fetal bovine serum. Human lung fibroblasts were
maintained in Dulbecco’s modified Eagle’s medium containing 10%
calf serum. The two cell lines were incubated at 37°C in a
humidified atmosphere of 5% CO2, and were harvested when
they reached 80–90% fusion with 0.25% trypsin digestion. Cells in
the logarithmic phase were used for this study. The study was
approved by the ethics committee of Soochow University (Suzhou,
China).
Drugs, reagents and equipment
Cobrotoxin was a gift from the Department of
Pharmacy at the Suzhou University Medical College (Suzhou, Jiangsu,
China), while the 3-methyl adenine (3-MA), dimethyl sulfoxide
(DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO,
USA). SB203580 was purchased from Beyotime Institute of
Biotechnology (Haimen, China) and an automatic enzyme standard
instrument (Benchmark) was purchased from Bio-Rad (Hercules, CA,
USA). A Heracell 150 carbon dioxide incubation box was purchased
from Thermo Fisher Scientific (Rockford, IL, USA). SDS-PAGE
apparatus (Mini-PROTEAN Tetra Electrophoresis System and
Mini-PROTEAN 3 Dodeca Cell, Bio-Rad) and a FEI TECNAI 10
transmission electron microscope (TEM; Philips, Amsterdam, Holland)
were also utilized.
Cell growth inhibition (MTT assay)
A single cell suspension (1×105 cells/ml)
of HFL1 and A549 cells in the logarithmic phase was prepared
following 0.25% trypsin digestion and seeded on a 96-well culture
plate (100 μl/well). Adherent cells were observed subsequent to 24
h. Cobrotoxin concentrations of 5, 10 and 20 μg/ml were added to 6
wells in each row, leaving one negative control group and one set
of blank wells. After 48 h, an MTT (5 mg/ml) assay was performed
and the cells were incubated for 4 h. Next, DMSO (150 μl) was added
to each well and the plates were agitated gently for 10 min.
Optical density (OD) was then measured at a wavelength of 570 nm.
Assays were performed in triplicate and the mean values were
calculated. The cell proliferation inhibition rate (%) was
calculated as follows: Cell proliferation inhibition rate (%) = (1
- mean OD value / control mean OD value) × 100.
Cell colony tablet cloning
The A549 cells were harvested at the logarithmic
phase following 0.25% trypsin digestion into a single cell
suspension (400 cells/ml), and placed into 24 wells (200
cells/well). The cells were then incubated for 24 h, following
which, 5, 10, and 20 μg/ml cobrotoxin was added, and a negative
control group was created. After 48 h, RPMI-1640 medium was
replaced (once every 2–3 days). After two weeks, the cells were
fixed with methanol, stained with Giemsa and observed
microscopically for a cell count of >50 cell colonies. The
colony formation inhibition rate (%) was calculated as follows:
Colony formation inhibition rate (%) = (control colony formation
rate - experimental colony formation rate / control colony
formation rate) × 100.
TEM
The A549 cells in the logarithmic phase were
harvested, digested with 0.25% and prepared in a single cell
suspension, whereby cell density was adjusted to 1.0×105
cells/ml and the cells were plated. RPMI-1640 culture medium
containing 10% fetal bovine serum was added and the cells were left
overnight. Culture medium was added to create the following groups:
10 μg/ml cobrotoxin, 10 μg/ml cobrotoxin + 5 mmol/ml 3-MA and 10
μg/ml cobrotoxin + 10 μmol/ml SB203580. The negative control
contained only RPMI-1640 medium. After 48 h in the nutrient
solution 2–3 drops of 2.5% glutaraldehyde were added, followed by
centrifugation at 3,000 rpm, at a centrifugal radius of 16 cm.
Following separation of the supernatant, 2.5% glutaraldehyde was
added, microwaved for 5–10 sec and fixed for 1 h at 4°C. Using a
hook anatomical needle, the cell mass was removed and cut into
1-mm3 blocks.
The generation of tissue blocks was performed by
fixing samples with 2.5% glutaraldehyde and refrigerating at 4°C
for >2 h, followed by three washes with 0.1 mol/l phosphate
buffer for 15 min each and fixation with 1% osmic acid for 2 h.
This was followed by a second rinse with 0.1 mol/l phosphate buffer
three times for 15 min each, dehydration for 15 min and three
washes with 50% ethanol, followed by drying with 3% acetic acid
(70% ethanol uranium) overnight. Next, gradient dehydration (90%
ethanol:90% acetone, 1:1) was performed for 15 min each, followed
by 100% acetone ketal dehydration at room temperature (15 minutes
each) three times, and embedding (pure acetone:embedding liquid,
1:1) at room temperature overnight (pure liquid embedding at 37°C
for 3 h). Finally, the blocks were cured at 45°C for 6 h, then at
60°C for 24 h. Using an LKB-1 (Pharmacia, Stockholm, Sweden) type
ultra-thin slicing machine, each sample was then cut at a thickness
of 50–60 nm, stained with a citrate lead piece and visualized.
Images were captured using TEM.
Western blotting determination of Beclin
1, LC3, p62, p38 and phosphorylated (p)-p38 protein expression
The A549 cells were harvested in the logarithmic
phase, digested with 0.25% trypsin, prepared in a single cell
suspension, with the cell density adjusted to 1.0×105
cells/ml, and plated. Cobrotoxin was added in the following
concentrations: 5, 10 or 20 μg/ml, 10 μg/ml cobrotoxin + 5 mmol/ml
3-MA and 10 μg/ml cobrotoxin + 10 μmol/ml SB203580 culture. A
negative control containing only RPMI-1640 medium was created. The
mixture was transferred to an Eppendorf tube (Eppendorf, Hamburg,
Germany) on ice and subjected to ultrasonic irradiation (JY96-II,
Haishu Kesheng Ultrasonic Equipment Co., Ltd., Ningbo, China) for
30 min, followed by centrifugation at 12,000 rpm, at a centrifugal
radius of 16 cm, for 5 min at 4°C. The protein concentration in the
supernatant was determined using the BCA method (Pierce, Rockford,
IL, USA). The samples were boiled, and the proteins were separated
using SDS-PAGE. Blots were subsequently transferred to
nitrocellulose membranes (Bio-Rad) and hybridized. The primary
antibodies used were rabbit anti-human Beclin-1 (1:500; BioVision,
Inc., Milpitas, CA, USA), LC3 (1:500; BioVision, Inc.), p38
(1:1,000; Cell Signaling Technology, Inc., Beverly, MA, USA) and
p-p38 (1:1,000; Cell Signaling Technology, Inc.) monoclonal
antibodies, with β-actin used as an internal control. Secondary
antibodies were used at a 1:5,000 diultion and labeled with
horseradish peroxidase in blocking solution for 1 h at room
temperature. A chemiluminescence assay was performed to determine
protein expression. Optical densities of respective protein bands
were analyzed using Sigma Scan Pro 5 (Sigma-Aldrich) and normalized
with loading control.
Nude mice transplantation tumor
model
Following disinfection of the dorsal skin, 28 female
nude mice (age, 35 days; weight, 18–24 g) were injected with A549
cells in the logarithmic phase, with a cell density adjusted to
l.0×108 cell/ml, from a cell suspension of 100 μl (total
of 1×107 cells). Following injection, the animals were
closely observed each day for their activity levels, mental state,
food intake and defecation amounts. Vernier calipers were used to
measure the size of subcutaneous transplantation tumors and the
animals were weighed twice a week.
Calculation of tumor inhibition for each
intervention group
Nine days after inoculation, tumor nodules grew to
4–6 mm in size. Each group was randomly divided into four groups of
seven mice, and received 40 μg/kg cobrotoxin, 40 μg/kg cobrotoxin +
10 mmol/kg 3-MA, 40 μg/kg cobrotoxin + 5 mg/kg SB203580 or 0.9%
NaCl (negative control group). During the same week as cell
transplantation, the experimental animals received cobrotoxin doses
subcutaneously, as described, while control animals received saline
injections. The mice were sacrificed four weeks after treatment.
The nodules were excised and weighed, and tumor inhibition rates
were calculated as follows: Tumor inhibition rate (%) = (1 - weight
of experimental group tumor / weight of control group tumor) ×
100.
Statistical analysis
Data are presented as the mean ± standard deviation.
Experimental and control groups were compared using the two sample
t-test. Comparison between the groups was performed using single
factor analysis of variance. P<0.05 was considered to indicate a
statistically significant difference. SPSS version 15.0 (SPSS,
Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Cobrotoxin inhibition of A549 and HFL1
cell growth
After 48 h, significant inhibition of the A549 cells
was exhibited, as determined by the MTT method (P<0.05), at
rates of 24.1, 32.3 and 56.4%, following treatment with 5, 10 and
20μg/ml cobrotoxin, respectively (Table
I). Cobrotoxin did not appear to affect HFL1 cell growth.
 | Table IEffect of cobrotoxin on the growth of
A549 cells. |
Table I
Effect of cobrotoxin on the growth of
A549 cells.
| Cobrotoxin
concentration, μg/ml | Optical density (mean
± SD) | P-value | Inhibition rate,
% |
|---|
| Control | 0.741±0.121 | | |
| 5 | 0.562±0.127 | 0.017 | 24.1 |
| 10 | 0.501±0.034 | 0.004 | 32.3 |
| 20 | 0.323±0.018 | 0.001 | 56.4 |
Cobrotoxin impact on A549 cell colony
formation
Compared with the controls, cobrotoxin significantly
inhibited A549 cell colony formation at 48 h in a dose-dependent
manner (Table II).
 | Table IIEffect of cobrotoxin on colony
formation. |
Table II
Effect of cobrotoxin on colony
formation.
| Cobrotoxin
concentration, μg/ml | Colony number (mean ±
SD) | P-value | Inhibition rate,
% |
|---|
| Control | 136.4±15.1 | | |
| 5 | 99.3±6.5 | 0.027 | 27.1 |
| 10 | 76.5±11.7 | 0.013 | 43.9 |
| 20 | 61.5±12.1 | 0.005 | 54.8 |
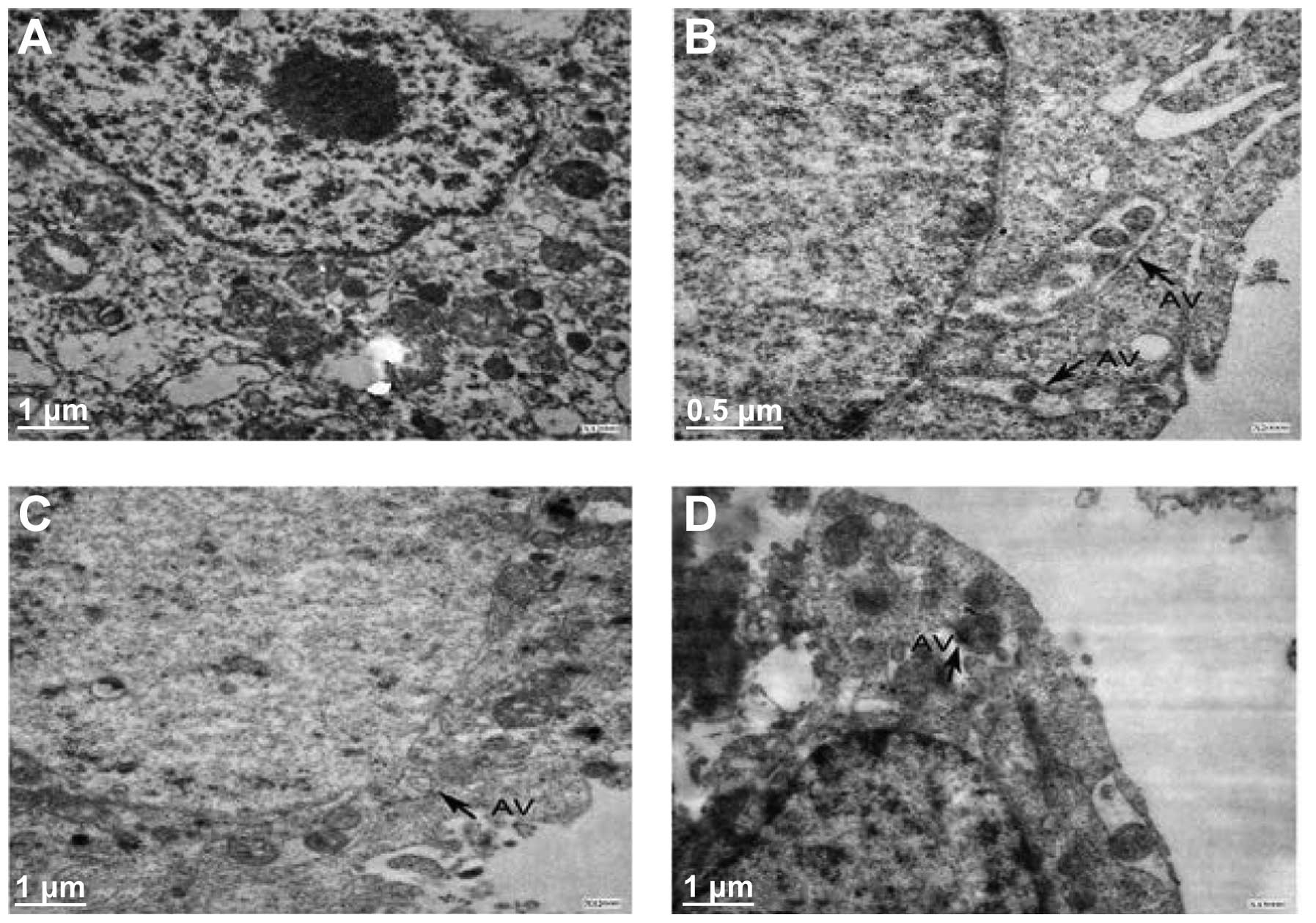
TEM analysis regarding A549 cell
morphology and autophagy
TEM was used to observe structural changes in the
A549 cells compared with the plasma controls. Following 48 h of
cobrotoxin treatment, a large number of cytoplasmic double-membrane
structures were observed, with gradual extension and bending,
containing cytoplasm components and lysosome organelles, with signs
of autophagy and glycogen accumulation in the cells. In the
cobrotoxin + 3-MA and cobrotoxin + SB203580 groups, autophagy was
significantly reduced (Fig. 1).
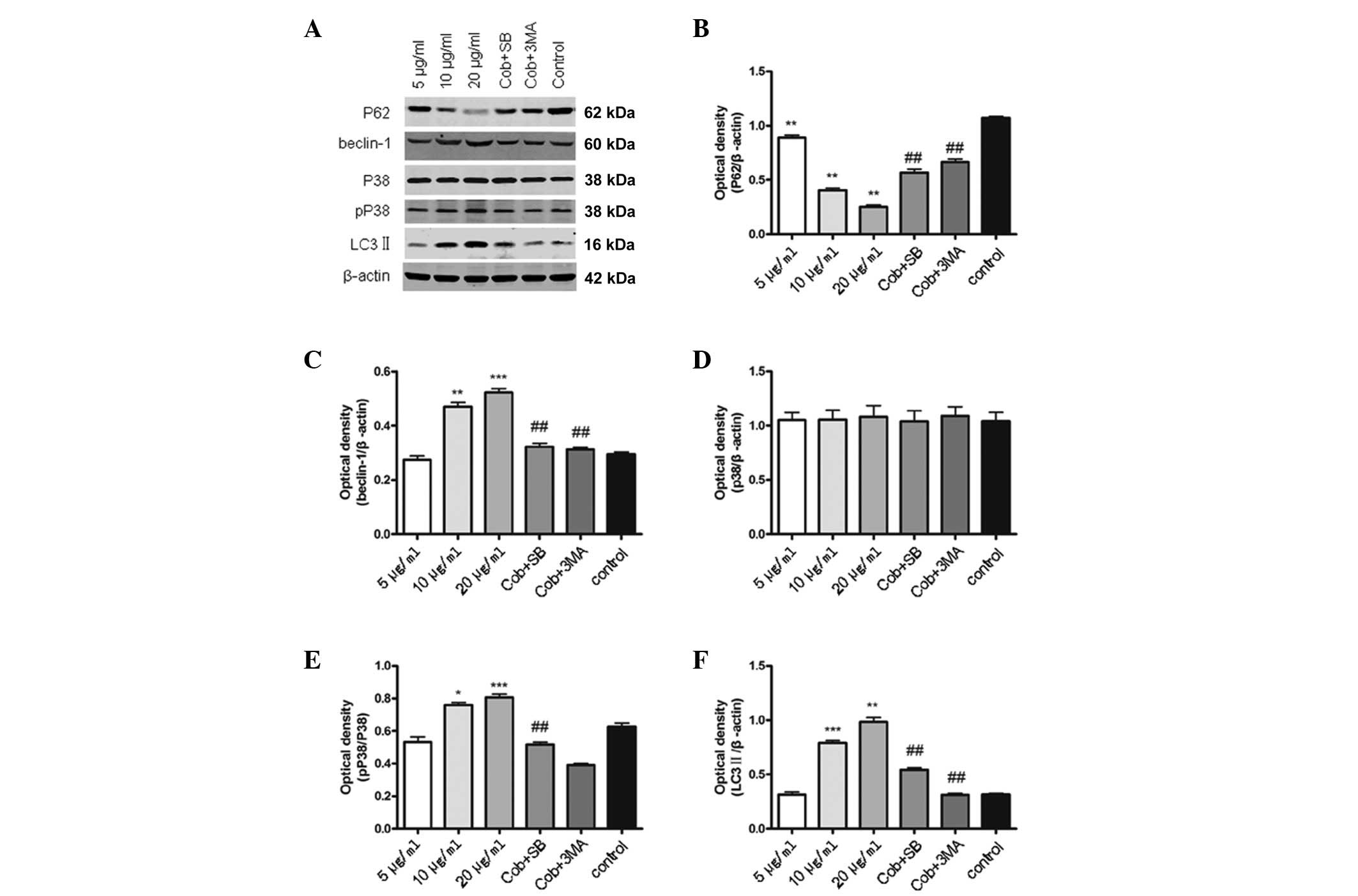
Western blot analysis of p62, Beclin 1,
LC3, p-p38 and p38 protein expression levels
Compared with the controls, Beclin 1, p-p38 and
LC3-II protein expression in the cobrotoxin treated group was
significantly increased, and p62 protein expression decreased
significantly, in a dose dependent manner. Compared with the
cobrotoxin group (10 μg/ml), in the cobrotoxin + SB203580
intervention group, Beclin 1, p-p38 and LC3-II protein expression
decreased, and p62 protein expression was increased significantly,
however, no significant differences were identified in p38 protein
expression. Compared with cobrotoxin group (10 μg/ml), in the
cobrotoxin + 3-MA intervention group, Beclin 1 and LC3-II protein
expression were decreased significantly, whereas p62 was increased
significantly (Fig. 2).
In vivo tumor suppression of human lung
cancer A549 cells transplanted in nude mice
A total of 28 Balb/c nude mice were subcutaneously
injected with the A549 cells. After 9 days, the mice grew
subcutaneous round or oval tumor nodules with clear palpable
boundaries, and demonstrated skin aging. There was no evident
change in weight, however, activity declined with tumor growth.
There was no significant difference identified between the groups
with regard to body morphology or tumor size at 12–16 days
post-transplantation. At 20 days post-cobrotoxin administration,
tumor growth slowed, the animals were lively, no significant change
in weight was identified and there were no deaths. After 30 days,
the growth of the tumor nodules was more rapid, and the animals
lost weight and appeared lifeless with decreased activity, although
without any deaths. In the cobrotoxin + 3-MA and cobrotoxin +
SB203580 subcutaneous transplantation groups, the tumor nodules of
the animals grew slightly faster. The animals were sacrificed and
the subcutaneous tumor nodules were removed; no distant metastases
were observed. The tumor inhibition rate of the tumors transplanted
into the nude mice were 43.4, 25.0 and 31.5%, for the cobrotoxin,
cobrotoxin + 3-MA and cobrotoxin + SB203580 groups, respectively
(P<0.01 compared with the control group; Table III).
 | Table IIIEffects of each intervention group on
tumor weight and tumor inhibition in a nude mouse subcutaneous
transplantable tumor model. |
Table III
Effects of each intervention group on
tumor weight and tumor inhibition in a nude mouse subcutaneous
transplantable tumor model.
| Group | n | Average tumor weight,
g (mean ± SD) | Tumor inhibition
rate, % |
|---|
| Control | 7 | 0.76±0.13 | |
| Cobrotoxin | 7 | 0.43±0.24 | 43.4 |
| Cobrotoxin +
3-MA | 7 | 0.57±0.15 | 25.0 |
| Cobrotoxin +
SB203580 | 7 | 0.52±0.07 | 31.5 |
The intervention and control groups differed
significantly with regard to tumor weight (P<0.01 for the
cobrotoxin + 3-MA intervention group and P<0.05 for the
cobrotoxin + SB203580 intervention group, compared with the
cobrotoxin group).
Discussion
Cobrotoxin exhibits an analgesic effect and has been
approved by the Food and Drug Administration for clinical
application. Recently, cobrotoxin has been reported to exhibit an
antitumor effect, however, the specific mechanism remains unclear.
It has been speculated that its antineoplastic effect may be
associated with the N-type acetylcholine receptor (6). In the present study, cobrotoxin was
found to exhibit an evident anti-tumor effect in the A549 cells
(inhibition rates of 24.1% at 5 μg/ml, 32.3% at 10 μg/ml and 56.4%
at 20 μg/ml). In addition, cobrotoxin inhibited A549 cell colony
formation (27.1% at 5 μg/ml, 43.9% at 10 μg/ml and 54.8% at 20
μg/ml). Furthermore, TEM revealed the separation of autophagy and
cytoplasm components. 3-MA and SB203580 autophagy following
intervention was reduced, demonstrating that cobrotoxin antitumor
effects, the induction of autophagy and the activated
p38-mitogen-activated protein kinase (MAPK) pathways are closely
associated.
Autophagy is a lysosomal function of the cell
degradation process, however, the mechanism of autophagy is unclear
(7). In autophagy, LC3-I is
converted to LC3-II and results in a novel autophagy membrane,
therefore, LC3, particularly type II, is usually used to indicate
autophagy in mammalian cell proteins (8,9).
Autophagy genes, such as Beclin 1, are specific to autophagy in
mammals and also reflect its occurrence (10). The present study observed that
following treatment of the A549 cells with cobrotoxin, Beclin 1 and
LC3-II protein expression was increased, and p62 protein-induced
autophagy was also confirmed. 3-MA inhibited autophagy and
increased p62, Beclin 1 and LC3-II protein expression, further
confirming autophagy.
Autophagy exhibits a dual role in tumor development;
in the case of an insufficient blood supply autophagic tumor cells
receive energy metabolism, which maintains their growth and
protects the cells. However, autophagic cell death may also occur
(11,12). The MAPK signal transduction pathway
is an important signaling system that stimulates signal
transduction of extracellular stimuli to cells, mediating
proliferation, differentiation, transformation, apoptosis and
autophagy (13–16). The present study observed that p38
and p62 protein expression increased following MAPK
pathway-specific SB203580 inhibition, whereas Beclin 1 and LC3-II
protein expression decreased, and thus, we hypothesize that
cobrotoxin-induced autophagy of A549 cells may activate the
p38-MAPK signaling pathway.
Nude mice were injected with the A549 lung cancer
cells and tumor growth inhibition was observed following cobrotoxin
treatment, without evident adverse effects on the mice and with no
significant differences identified between the treatment and
control groups. The cobrotoxin + 3-MA and cobrotoxin + SB203580
intervention groups showed rapid tumor growth compared with the
cobrotoxin group, a further sign of cobrotoxin involvement in the
p38-MAPK pathway for A549 cell autophagy. Cobrotoxin inhibited A549
cell growth in vitro, and this effect may be due to the
activation of the p38-MAPK pathway autophagy process. The present
study aids in the understanding of the mechanism of
cobrotoxin-induced autophagy in A549 cells, and provides new
insight into antitumor combinations for lung cancer therapy.
Acknowledgements
This study was funded by Jiangsu Province’s Key
Provincial Talents Program (grant no. RC2011112).
References
|
1
|
US Cancer Statistics Working Group. United
States cancer statistics: 1999–2010 incidence and mortality
web-based report. http://198.246.124.29/cancer/npcr/pdf/USCS_FactSheet.pdf.
Accessed February 23, 2013
|
|
2
|
Kim MH, Shim HS, Kang DR, et al: Clinical
and prognostic implications of ALK and ROS1 rearrangements in
never-smokers with surgically resected lung adenocarcinoma. Lung
Cancer. 83:389–395. 2014.
|
|
3
|
Wu C, Jiang J, Shi L and Xu N: Prospective
study of chemotherapy in combination with cytokine-induced killer
cells in patients suffering from advanced non-small cell lung
cancer. Anticancer Res. 28:3997–4002. 2008.
|
|
4
|
Chen RZ and Robinson SE: The effect of
cholinergic manipulations on the analgesic response to cobrotoxin
in mice. Life Sci. 47:1949–1954. 1990.
|
|
5
|
Giozio A, Paleari L, Catassi A, et al:
Natural agents targeting the alpha7-nicotinic-receptor in NSCLC: a
promising prospective in anti-cancer drug development. Int J
Cancer. 122:1911–1915. 2008.
|
|
6
|
Alama A, Bruzzo C, Cavalieri Z, et al:
Inhibition of the nicotinic acetylcholine receptors by cobra venom
α-neurotoxins: is there a perspective in lung cancer treatment?
PLoS One. 6:e206952011.
|
|
7
|
Tang D, Kang R, Livesey KM, et al:
Endogenous HMGBl regulates autophagy. J Cell Biol. 190:881–892.
2010.
|
|
8
|
Gewirtz DA: Autophagy as a mechanism of
radiation sensitization in breast tumor cells. Autophagy.
3:249–250. 2007.
|
|
9
|
Lambert LA, Qiao N, Hunt KK, et al:
Autophagy: a novel mechanism of synergistic cytotoxicity between
doxorubicin and roscovitine in a sarcoma model. Cancer Res.
68:7966–7974. 2008.
|
|
10
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
|
|
11
|
Cao Y and Klionsky DJ: Physiologic
functions of Atg6/Beclin 1: a unique autophagy-related protein.
Cell Res. 17:839–849. 2007.
|
|
12
|
Sridhar S, Botbol Y, Macian F and Cuervo
AM: Autophagy and disease: always two sides to a problem. J Pathol.
226:255–273. 2012.
|
|
13
|
White E, Karp C, Strohecker AM, et al:
Role of autophagy in suppression of inflammation and cancer. Curr
Opin Cell Biol. 22:212–217. 2010.
|
|
14
|
Wu JG, Tang H, Liu ZJ, et al:
Angiotensin-(1–7) inhibits vascular remodelling in rat jugular vein
grafts via reduced ERK1/2 and p38 MAPK activity. Int J Med Res.
39:2158–2168. 2011.
|
|
15
|
Liu Z, Xu X, Chen L, et al: Helicobacter
pylori CagA inhibits the expression of Runx3 via Src/MEK/ERK and
p38 MAPK pathways in gastric epithelial cell. J Cell Biochem.
113:1080–1086
|
|
16
|
del Barco Barrantes I and Nebreda AR:
Roles of p38 MAPKs invasion and metastasis. Biochem Soc Trans.
40:79–84
|