Introduction
Polycythemia is one of the paraneoplastic syndromes
associated with renal cell carcinoma (RCC), which has been
associated with erythropoietin (EPO) production from renal
carcinoma cells (1). Although the
serum EPO (sEPO) level is reportedly elevated in 33–38% of patients
with RCC, it is relatively rare that patients with RCC manifest
polycythemia (2). The sEPO level is
used as a tumor marker in patients with RCC, as it has been found
to correlate with the stage and grade of RCC and provides
prognostic information (2,3). A previous study has confirmed that the
expression of EPO receptors and endogenous EPO by RCC cells
stimulates RCC cell proliferation (4). In addition to RCC, several types of
cancer cells reportedly use the EPO system for cell growth and
angiogenesis (5).
sEPO levels are generally lower in patients
undergoing chronic dialysis than in healthy individuals due to
impaired EPO production by the renal cells. Five cases of
EPO-producing RCC have previously been reported in patients
undergoing chronic hemodialysis (HD) (6–9). These
cases exhibited elevated hematocrit (Hct) and hemoglobin (Hgb)
levels, but did not manifest polycythemia. Polycythemia in patients
with RCC arising in end-stage kidney disease is a considerably rare
event. Four of the five cases were associated with acquired cystic
disease of the kidney (ACDK), and none of the cases were associated
with autosomal dominant polycystic kidney disease (ADPKD) (6–9). RCC
arising from ADPKD is an extremely rare condition (10).
We have previously reported the radiological finding
of an ADPKD patient with RCC who manifested polycythemia (11). Although the polycythemia was
diminished following the removal of the affected kidney, the sEPO
levels remained elevated in the patient. This clinical course led
us to consider EPO production in the contralateral polycystic
kidney, as it was possible that not only RCC, but also renal cysts
in ADPKD produce EPO. Therefore, in the current study, EPO
production by RCC and renal cysts was analyzed in the surgically
resected polycystic kidney, by immunohistochemistry and enzyme
immunoassay (EIA). EPO production was observed in RCC and the renal
cysts in ADPKD. This study also discussed the implications of sEPO
levels at each time-point of the clinical course. As polycythemia
diminished following nephrectomy, EPO production from the resected
kidney appeared to cause polycythemia. Positive EPO staining of
renal cysts in the resected polycystic kidney and sustained sEPO
elevation following the nephrectomy allowed us to predict EPO
production in the renal cysts of the contralateral polycystic
kidney. Written informed consent was obtained from the patient.
Case report
Materials
Polyclonal goat anti-human EPO antibody was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The ChemMate ENVISION kit for immunohistochemical analysis
was purchased from DakoCytomation (Kyoto, Japan).
Patient clinical course
This study presents the case of a 67-year-old female
undergoing chronic HD due to ADPKD manifesting polycythemia in
2003. As the patient’s sEPO level was elevated and abdominal
computed tomography (CT) indicated an enhanced lesion that was 3 cm
in diameter in the lower part of the left kidney, the patient was
referred to the Department of Urology at the National Defense
Medical College (Tokorozawa, Japan). The patient had a previous
history of HD that began at the age of 58 years due to ADPKD. The
patient presented with dilated cardiomyopathy and moderate grade
mitral regurgitation, and thus, experienced severe heart
dysfunction, including a decreased ejection fraction (25–30%) and
diffuse hypokinesis of the cardiac walls. Prior to 2003,
recombinant human EPO (rHuEPO) had been intermittently used to
treat renal anemia. Although rHuEPO treatment was stopped due to
the improvement of anemia in June 2003, the patient’s Hgb and Hct
levels remained elevated. The Hct and Hgb levels reached 53.4%
(normal range, 34.0–44.0%) and 16.5 g/dl (normal range, 12.0–15.0
g/dl), respectively, in September 2003 (Table I). The EPO level was 372 mU/ml
(normal range, 8–36 mU/ml), and CT exhibited a considerably
enhanced lesion in the left polycystic kidney. The radiological
finding of this case has been previously reported (11).
 | Table ILevels of sEPO, Hct and Hgb at various
time-points. |
Table I
Levels of sEPO, Hct and Hgb at various
time-points.
| Blood tests | Mar 2003 | Sept 2003 | Oct 2003
(Admission) | Nov 17, 2003 (4 days
post-EMB) | Nov 21, 2003 (8 days
post-EMB) | Dec 16, 2003
(post-RNx) | Jan 2004 (1 month
post-RNx) | Mar 2004 (3 months
post-RNx) |
|---|
| sEPO, mU/ml | NE | 372 | 893 | 61.2 | 1740 | 243 | 241 | NE |
| Hct, % | 29.2 | 53.4 | 57.1 | 45.9 | NE | 35.0 | 35.0 | 31.7 |
| Hgb, g/dl | 8.3 | 16.5 | 17.4 | 14.3 | NE | 10.6 | 10.3 | 9.2 |
At the time of admission, the patient exhibited
erythema on the face and hands, possibly due to polycythemia. The
laboratory results indicated high levels of Hgb (17.4 g/dl), Hct
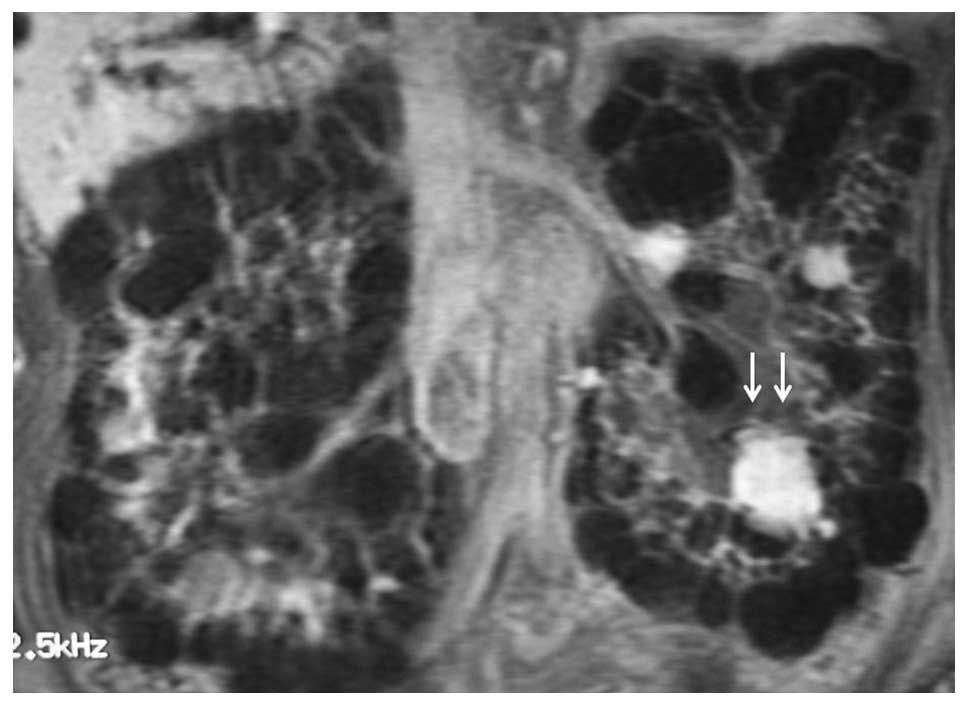
(57.1%), and an elevated EPO level (893 mU/ml) (Table I). CT, magnetic resonance imaging
(Fig. 1) and Doppler
ultrasonography indicated a hypervascular tumor (11). Although RCC was suspected, the
possibility of a benign tumor, such as renal hemangioma, could not
be excluded. Venous samples from bilateral renal veins were
obtained, and as the EPO levels from the two renal veins were
similarly elevated (right renal vein, 173 mU/ml; left, 159 mU/ml),
the origin of EPO production could not be determined. For
therapeutic and diagnostic purposes, an embolization and subsequent
needle biopsy of the tumor were performed. Immediately after
feeding, the artery was completely embolized by the superselective
method (11) and a fine-needle
biopsy was performed under ultrasonography. Four days after the
embolization, the EPO level had decreased to 61.2 mU/ml. However,
this level increased again to 1740 mU/ml eight days after the
embolization (Table I). As the CT
that was performed immediately after the marked increase in EPO
level indicated a highly enhanced tumor, and the blood flow of the
artery feeding the tumor appeared to have returned. With regard to
the decrease in EPO level following the embolization, it was
suggested that perhaps the renal lesion was producing EPO.
Histological analysis of the biopsy specimen indicated that
low-grade RCC was most likely. Although the patient had severe
cardiac dysfunction, a left radical nephrectomy was performed to
control the polycythemia. The surgical specimen exhibited a
yellowish solid tumor in the lower part of the polycystic kidney.
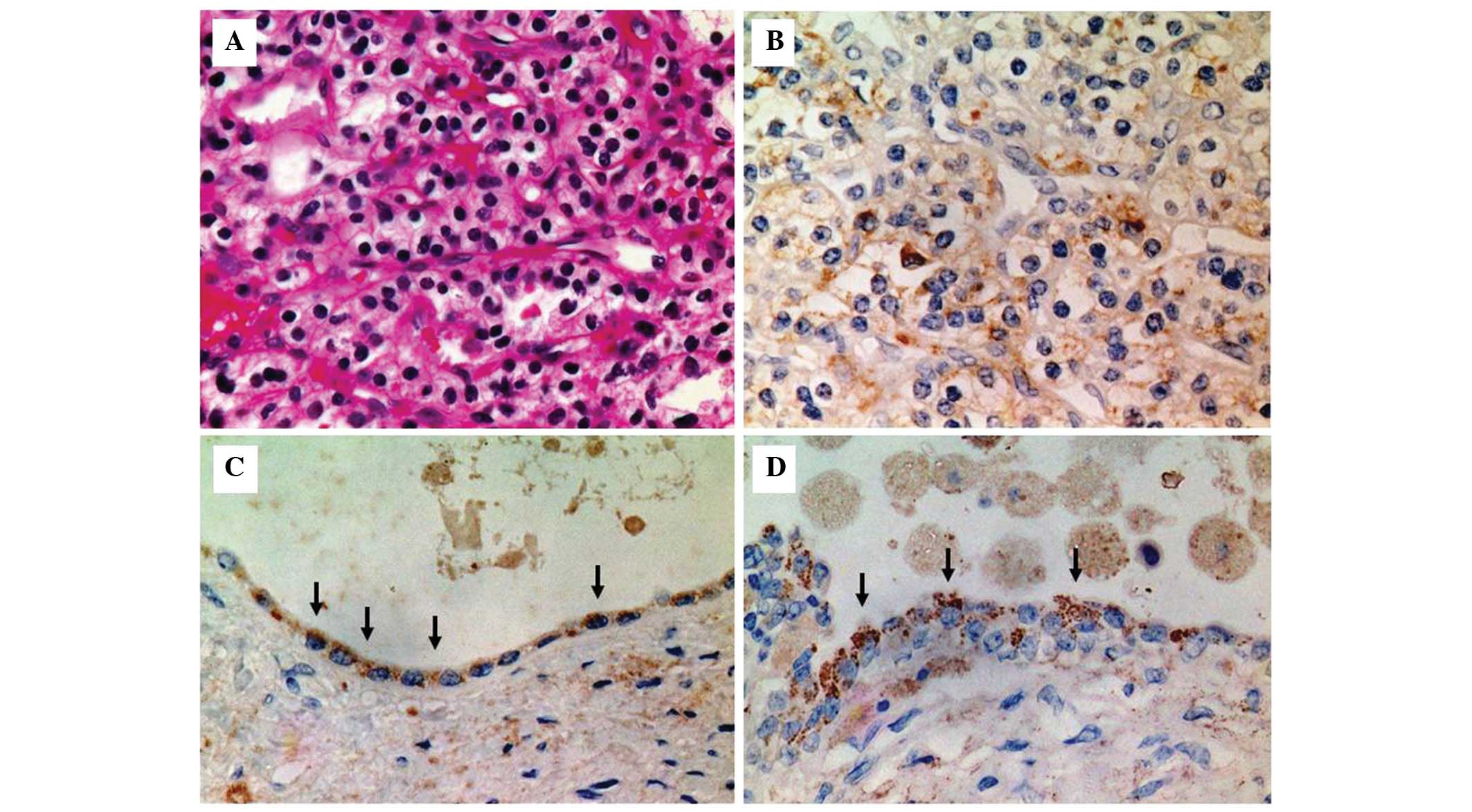
Microscopically, the tumor was diagnosed as RCC (clear-cell; grades
1 and 2; pT1a) (Fig. 2A).
Post-operatively, the sEPO level decreased to 243 mU/ml (Table I), and although the post-operative
EPO level remained higher than the normal range, the Hct level
gradually decreased and rHuEPO was required again three months
after the nephrectomy. Eight months after the nephrectomy, the Hct
level was 30.2% with the administration of rHuEPO.
Samples
Serum samples were obtained from the patient at each
time-point of the clinical course and stored at −80°C until use.
Cyst fluid was obtained immediately from the surgically resected
specimen and stored at −80°C until use. A surgically resected
tissue sample was fixed in 10% neutral-buffered formalin (Wako Pure
Chemical Industries, Ltd., Osaka, Japan) for immunohistochemical
analysis.
Detection of EPO levels in serum and cyst
fluid
A 50 ml aliquot of the serum samples and cyst fluid
was used for the EPO immunoassay, which was performed in duplicate.
EPO levels were quantified using an EIA kit (Diagnostic Products
Corporation, Los Angeles, CA, USA) according to the manufacturer’s
instructions.
Immunohistochemical analysis in
paraffin-embedded tissues
Immunohistochemical analyses to detect EPO were
performed on RCC and renal cysts. A surgically resected left
polycystic kidney was fixed in 10% neutral-buffered formalin and
embedded in paraffin. Paraffin-embedded sections (4 μm) were
mounted onto glass slides. The sections were dewaxed in xylene
(Wako Pure Chemical Industries, Ltd.), rehydrated in decreasing
concentrations of ethanol (Wako Pure Chemical Industries, Ltd.) and
washed three times in phosphate-buffered saline (PBS) (Nissui
Pharmaceutical Co., Ltd., Tokyo, Japan) for 10 min. Endogenous
peroxidase was quenched for 45 min with 0.6% hydrogen peroxide in
methanol (Wako Pure Chemical Industries, Ltd.). For antigen
retrieval, subsequent to being washed with filtered water, the
slides were boiled in 10 mmol/l citrate buffer (pH 6.0) for 20 min
in an autoclave (121°C). Subsequent to being washed with PBS, a
blocking step was performed using 3% bovine serum albumin (Wako
Pure Chemical Industries, Ltd.) for 10 min. The primary goat
polyclonal anti-human EPO antibody (Santa Cruz Biotechnology, Inc.)
was subsequently incubated at 4°C overnight. The primary antibody
was used at a dilution of 1:100. Following a second wash with PBS,
the ChemMate Envision antibody (DakoCytomation) against goat
immunoglobulins was used as a secondary antibody for 1 h. Sections
were developed with diaminobenzidine and counterstained using 10%
hematoxylin. Samples incubated without the primary antibody
followed the same staining steps and were used for baseline
staining.
Results
Detection of EPO concentration in
serum and cyst fluid
The sEPO levels determined by EIA are shown in
Table I. The sEPO level
significantly decreased following the embolization of the renal
tumor, suggesting EPO production by the renal tumor. However, the
sEPO level increased significantly several days later, suggesting
that the blood flow to the tumor had been restarted. Although the
EPO level decreased following the nephrectomy, the post-operative
sEPO levels remained higher than the normal range. In addition, the
renal cyst fluid exhibited an extremely high EPO concentration of
2,680 mU/ml in the surgically resected polycystic kidney,
suggesting EPO production from the renal cysts.
Immunolocalization of EPO in renal
cancer cells and cyst epithelial cells
EPO-positive staining was exhibited in the renal
cancer cells (Fig. 2B) and the
cells lining the cyst wall (Fig. 2C and
D), as shown by immunohistochemical analysis using an anti-EPO
antibody. Clear cell renal cancer and positive EPO staining were
observed in the cytoplasm of the tumor cells (Fig. 2B). In the renal cysts of ADPKD,
positive staining was located in the cytoplasm of the cyst
epithelial cells (Fig. 2C and D)
and positively stained particles were observed in the cytoplasm of
the cells lining the cyst (Fig.
2D). Samples incubated without the primary antibody did not
exhibit any staining (data not shown).
Discussion
EPO production has been reported in several
diseases, including hepatocellular carcinoma, hemangioblastoma of
the cerebellum, gastric and pancreatic cancer, and in kidney
lesions, such as RCC, renal cysts (6,9) and
hemangioma (12). In the present
case, no suspicious lesion was observed on imaging examination that
could have produced EPO in other organs. However, the exact origin
of the abnormal EPO production could not be determined by blood
sampling from the bilateral renal veins. In addition, as the lesion
in the left polycystic kidney was extremely hypervascular, there
was also a possibility of a benign renal tumor, such as a
hemangioma. EPO production from a renal hemangioma has been
previously reported (12),
therefore, a biopsy of the lesion was performed. Superselective
embolization of the tumor significantly decreased the sEPO level,
suggesting that the hypervascular lesion in the left kidney may be
the origin of abnormal EPO production. As the lesion was suspected
to be RCC and the likely cause of polycythemia, a surgical
resection was indicated.
Following the superselective embolization, the
decline of the EPO level with time, and its subsequent elevation,
were observed. This may be due to EPO release by destroyed cells
into the blood stream following embolization, or the induction of
EPO production by hypoxia in renal cancer cells and/or other renal
cells, such as cyst epithelial cells. Hypoxia inducible factor-1
(HIF-1) is reportedly upregulated in renal cancer cells and is
important in cell proliferation and angiogenesis (13). HIF-1 transactivates genes encoding
target proteins, including EPO, vascular endothelial growth factor
and inducible nitric oxide synthase (14–16).
The expression of HIF-1 and its target proteins, including EPO, may
be increased by embolization. Considering the decline in EPO levels
following the embolization and then the subsequent increase, the
renal lesion was considered to be at least one of the causes of EPO
elevation in the present study.
The EPO level remained above the normal range
following the nephrectomy. As the production of EPO was observed in
the cyst walls of the nephrectomized kidney and as bilateral
elevation of sEPO level was demonstrated in the renal vein
sampling, it is possible that the EPO may have been produced by
cysts in the contralateral kidney. In addition, the EPO level
following the left nephrectomy was higher than that four days after
the embolization. Therefore, certain compensatory changes may have
occurred in the contralateral polycystic kidney following the
nephrectomy. Under normal conditions, compensatory changes
following uninephrectomy of the contralateral kidney, including
hypertrophy, are observed (17).
Compensatory changes in the contralateral kidney of patients with
ADPKD have not been previously evaluated, thus, the phenomenon of
the sustained high EPO level following the nephrectomy is
noteworthy. Furthermore, the reason for the decrease in the
post-operative Hct level, in spite of an EPO level higher than the
normal range, is unknown. An extremely large amount of EPO may be
required to manifest polycythemia.
In normal kidneys, EPO is produced in the
peritubular cells under the control of an oxygen sensor located in
the epithelial cells of the proximal tubules (18). In previous studies, EPO production
has been observed in RCC cells and epithelial cells in the cyst
wall of patients with RCC arising from ACDK (6,9). In
the present case, EPO production was also demonstrated by
immunohistochemical analysis in the RCC and cyst epithelial cells.
The high EPO levels in the cyst fluid further confirmed EPO
production in the renal cysts. EPO production by RCC cells and cyst
epithelial cells in the left kidney may cause polycythemia.
Patients with ADPKD undergoing chronic dialysis have exhibited
significantly higher EPO levels compared with patients without
ADPKD (19). Furthermore, ACDK has
also been associated with the improvement of anemia in patients on
long-term HD (20). Therefore,
renal cysts occasionally produce EPO in patients undergoing chronic
dialysis. In addition, a high risk of RCC development has been
reported for ACDK patients (21,22),
indicating that EPO production in renal cysts may be involved in
RCC development.
In conclusion, the present study demonstrated EPO
production in RCC arising from ADPKD and the renal cysts of ADPKD.
The extremely high EPO concentration in the cyst fluid supported
the hypothesis of EPO production from the renal cysts in ADPKD.
Based on the results of immunohistochemistry, EIA and the clinical
course, polycythemia was caused by high EPO production from the
resected kidney. The sustained elevation of sEPO may have been due
to EPO production from the renal cysts in the contralateral
polycystic kidney.
References
|
1
|
Da Silva JL, Lacombe C, Bruneval P, et al:
Tumor cells are the site of erythropoietin synthesis in human renal
cancers associated with polycythemia. Blood. 75:577–582. 1990.
|
|
2
|
Ljungberg B, Rasmuson T and Grankvist K:
Erythropoietin in renal cell carcinoma: evaluation of its
usefulness as a tumor marker. Eur Urol. 21:160–163. 1992.
|
|
3
|
Ito K, Yoshii H, Asano T, et al: Impact of
increased erythropoietin receptor expression and elevated serum
erythropoietin levels on clinicopathological features and prognosis
in renal cell carcinoma. Exp Ther Med. 3:937–944. 2012.
|
|
4
|
Westenfelder C and Baranowski RL:
Erythropoietin stimulates proliferation of human renal carcinoma
cells. Kidney Int. 58:647–657. 2000.
|
|
5
|
Yasuda Y, Fujita Y, Matsuo T, et al:
Erythropoietin regulates tumor growth of human malignancies.
Carcinogenesis. 24:1021–1029. 2003.
|
|
6
|
Hanada T, Mimata H, Ohno H, Nasu N,
Nakagawa M and Nomura Y: Erythropoietin-producing renal cell
carcinoma arising from acquired cystic disease of the kidney. Int J
Urol. 5:493–495. 1998.
|
|
7
|
Ohi K, Yamamoto H, Shigematsu T, et al:
Elevated serum erythropoietin as a marker of renal cell carcinoma
in a hemodialysis patient. Nihon Toseki Igakkai Zasshi. 30:141–145.
1997.(In Japanese).
|
|
8
|
Ooki R, Amamiya M, Umino T, et al:
Elevated serum erythropoietin and IAP as a marker of renal cell
carcinoma in an autosomal dominant polycystic kidney disease. Jin
Touseki. 45:709–711. 1998.(In Japanese).
|
|
9
|
Sakamoto S, Igarashi T, Osumi N, et al:
Erythropoietin-producing renal cell carcinoma in chronic
hemodialysis patients: a report of two cases. Int J Urol. 10:49–51.
2003.
|
|
10
|
Jacobs C, Reach I and Degoulet P: Cancer
in patients on hemodialysis. N Engl J Med. 300:1279–1280. 1979.
|
|
11
|
Hama Y, Kaji T, Ito K, et al:
Erythropoietin-producing renal cell carcinoma arising from
autosomal dominant polycystic kidney disease. Br J Radiol.
78:269–271. 2005.
|
|
12
|
Leak BJ, Javidan J and Dagher R: A rare
case of renal hemangioma presenting as polycythemia. Urology.
57:9752001.
|
|
13
|
Krieg M, Haas R, Brauch H, Acker T, Flamme
I and Plate KH: Up-regulation of hypoxia-inducible factors
HIF-1alpha and HIF-2alpha under normoxic conditions in renal
carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of
function. Oncogene. 19:5435–5443. 2000.
|
|
14
|
Bunn HF and Poyton RO: Oxygen sensing and
molecular adaptation to hypoxia. Physiol Rev. 76:839–885. 1996.
|
|
15
|
Semenza GL: Regulation of mammalian O2
homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol.
15:551–578. 1999.
|
|
16
|
Wenger RH and Gassmann M: Oxygen(es) and
the hypoxia-inducible factor-1. Biol Chem. 378:609–616. 1997.
|
|
17
|
Shohat J, Erman A, Boner G and Rosenfeld
J: Mechanisms of the early and late response of the kidneys to
contralateral nephrectomy. Ren Physiol Biochem. 14:103–111.
1991.
|
|
18
|
Lacombe C, Da Silva JL, Bruneval P, et al:
Peritubular cells are the site of erythropoietin synthesis in the
murine hypoxic kidney. J Clin Invest. 81:620–623. 1988.
|
|
19
|
Férnandez A, Hortal L, Rodríguez JC, et
al: Anemia in dialysis: its relation to acquired cystic kidney
disease and serum levels of erythropoietin. Am J Nephrol. 11:12–15.
1991.
|
|
20
|
Shalhoub RJ, Rajan U, Kim VV, Goldwasser
E, Kark JA and Antoniou LD: Erythrocytosis in patients on long-term
hemodialysis. Ann Intern Med. 97:686–690. 1982.
|
|
21
|
Hughson MD, Buchwald D and Fox M: Renal
neoplasia and acquired cystic kidney disease in patients receiving
long-term dialysis. Arch Pathol Lab Med. 110:592–601. 1986.
|
|
22
|
Levine E, Slusher SL, Grantham JJ and
Wetzel LH: Natural history of acquired renal cystic disease in
dialysis patients: a prospective longitudinal CT study. AJR Am J
Roentgenol. 156:501–506. 1991.
|