Introduction
Recurrent respiratory papillomatosis (RRP) is a
potentially devastating, incurable disease that is caused by
infection with the human papilloma virus (HPV), predominantly HPV-6
and -11. Primary benign tumorous manifestations of RRP occur
throughout the respiratory tract and may lead to laryngeal,
tracheal or bronchial obstructions, and pulmonary nodes and cystic
lesions due to valve effects (1).
The incidence of RRP in the USA is 4.3/100,000 children and
1.8/100,000 adults (2).
Furthermore, malignant transformation occurs in 3–5% of RRP cases
(3). When RRP progresses into the
pulmonary parenchyma, there are no effective therapeutic strategies
and it is almost invariably fatal (3,4).
Current standard management of RRP involves repeated local surgical
debulking by laser therapy, which is associated with a significant
risk of chronic morbidity, including voice mutilation. In total
>10,000 interventional treatment procedures for RRP are
performed each year in the USA; patients undergo a mean average of
4.4 procedures per year and 19.7 overall (2). The data regarding adjuvant antiviral
therapy, particularly intralesional cidofovir, remain inconsistent
(5).
A strong mRNA expression of VEGF-A and its
receptors, VEGFR-1 and -2 has been observed in tissue samples of
RRP patients (6). In 2009, Nagel
et al (7) reported a case of
systemic adjuvant therapy using the anti-VEGF antibody,
bevacizumab. In this case, bevacizumab appeared to delay the
requirement for further local interventions. In addition, a
previous study was conducted, which demonstrated a series of adults
exhibiting papillomatosis who were treated with combined laser
therapy and sublesionally injected bevacizumab (8); a second study was recently conducted
in a population of children (9).
The clinical courses of five patients with long
histories of RRP in whom the systemic administrations of
bevacizumab were evaluated are reported.
Patients and methods
Patient characteristics
Between April 2011 and May 2012 five patients aged
8–56 years (median, 43 years) presented at the Department of
Medicine (University Hospital, Muenster, Germany) with histories of
RRP ranging between 6 months and 37 years (median, 3 years). The
patient characteristics are presented in Table I. The manifestations of RRP were as
follows: Lung parenchyma, n=2; tracheobronchial involvement, n=2;
the larynx and vocal cords, n=3; and sinonasal inverted papilloma,
n=1. All of the patients had previously received multiple local
interventions (from three to >30) predominantly by laser
therapy. One patient suffering from papilloma involvement of the
paranasal sinuses had received a radical surgery by midfacial
degloving and adjuvant radiotherapy. One patient, who was
exhibiting lung parenchymal involvement, had received a middle lobe
resection and demonstrated evidence of malignant transformation of
a papilloma manifestation. In addition, the administration of
additional adjuvant medical therapeutic agents, such as cidofovir,
celecoxib and interferon had been tried in two patients. However,
all five patients presented with progressive disease at the time of
treatment initiation with systemic bevacizumab therapy. The report
is in accordance with the Declaration of Helsinki guidelines.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient | Age at initial
diagnosis (years) | Gender (M/F) | Total debulking
interventions pre-bevacizumab | Age at treatment
initiation (years) | Cycles (n) |
|---|
| 1 | 6 | M | >30 | 43 | 16 |
| 2 | 49 | M | 2 | 49 | 3 |
| 3 | 53 | F | 16 | 56 | 6 |
| 4 | 2 | F | >30 | 8 | 9 |
| 5 | 32 | M | 6 | 34 | 6 |
Treatment and response evaluation
Written informed consent was obtained from each
patient. Bevacizumab administration was initiated at a dose of
between 5 and 15 mg/kg intravenously every 2–3 weeks. Treatment
intervals were subsequently extended after achieving the maximum
response as demonstrated by endoscopy and/or bronchoscopy.
Endoscopy was performed prior to and between days three and seven
following treatment initiation. Follow-up endoscopies were
performed prior to every subsequent course of bevacizumab. Digital
endoscopic imaging documentation was conducted using a routine
documentation systems (BF Q180 video-bronchoscope; CV-160
video-processor; Olympus Corporation, Tokyo, Japan). Computed
tomography (CT) was performed in two patients to follow up the RRP
involvement of the lung parenchyma or paranasal sinuses.
Histopathological analyses
Papilloma tissue samples were obtained for standard
histological and immunohistological analyses prior to and following
the first infusion of bevacizumab in two patients. For
immunohistochemistry, formalin-fixed and paraffin-embedded tissues
were analyzed using primary polyclonal rabbit anti-human antibodies
specific to VEGF (Santa Cruz Biotechnology, CA, USA; working
dilution, 1:50), phosphorylated VEGFR-2 (Santa Cruz Biotechnology;
working dilution, 1:50) and the alkaline phosphatase anti-alkaline
phosphatase double-bridge technique (DakoCytomation, Glostrup,
Denmark). Briefly, tissue sections were deparaffinized in xylene
and rehydrated in a graded ethanol series. Following antigen
retrieval in a microwave oven at 450 W (twice for 7 min in 10 mM
sodium citrate [pH 6.0; DakoCytomation]) the primary antibodies
were applied to the tissue sections overnight at 4°C. Subsequent
steps were performed according to the manufacturer’s instructions.
The Fast-Red substrate (DakoCytomation) was used for detection of
phosphatase activity and sections were counterstained with
hematoxylin and eosin (Merck KGaA, Darmstadt, Germany).
DNA fragmentation assay
To investigate apoptosis in the histology specimen,
the In Situ Cell Death Detection kit, AP (Roche, Basel,
Switzerland) was used, which is based on the Terminal
deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL)-reaction. TUNEL-positive apoptotic cells were counted in
three representative independent microscopic fields (magnification,
200×) per tumor (Axioskope, Zeiss, Oberkochen, Germany).
Statistical analysis
For statistical analysis the Wilcoxon signed-rank
test was used. P-values were two-sided and P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient 1
This patient was a 43-year-old male, who had been
diagnosed with HPV-11-associated respiratory papillomatosis at the
age of 6 years. Between the ages of 8 and 13 years, multiple
interventions, including a temporary tracheostomy, had been
necessary and were followed by a stable disease phase for ~20
years. Since 1997, the disease had begun to progress and required
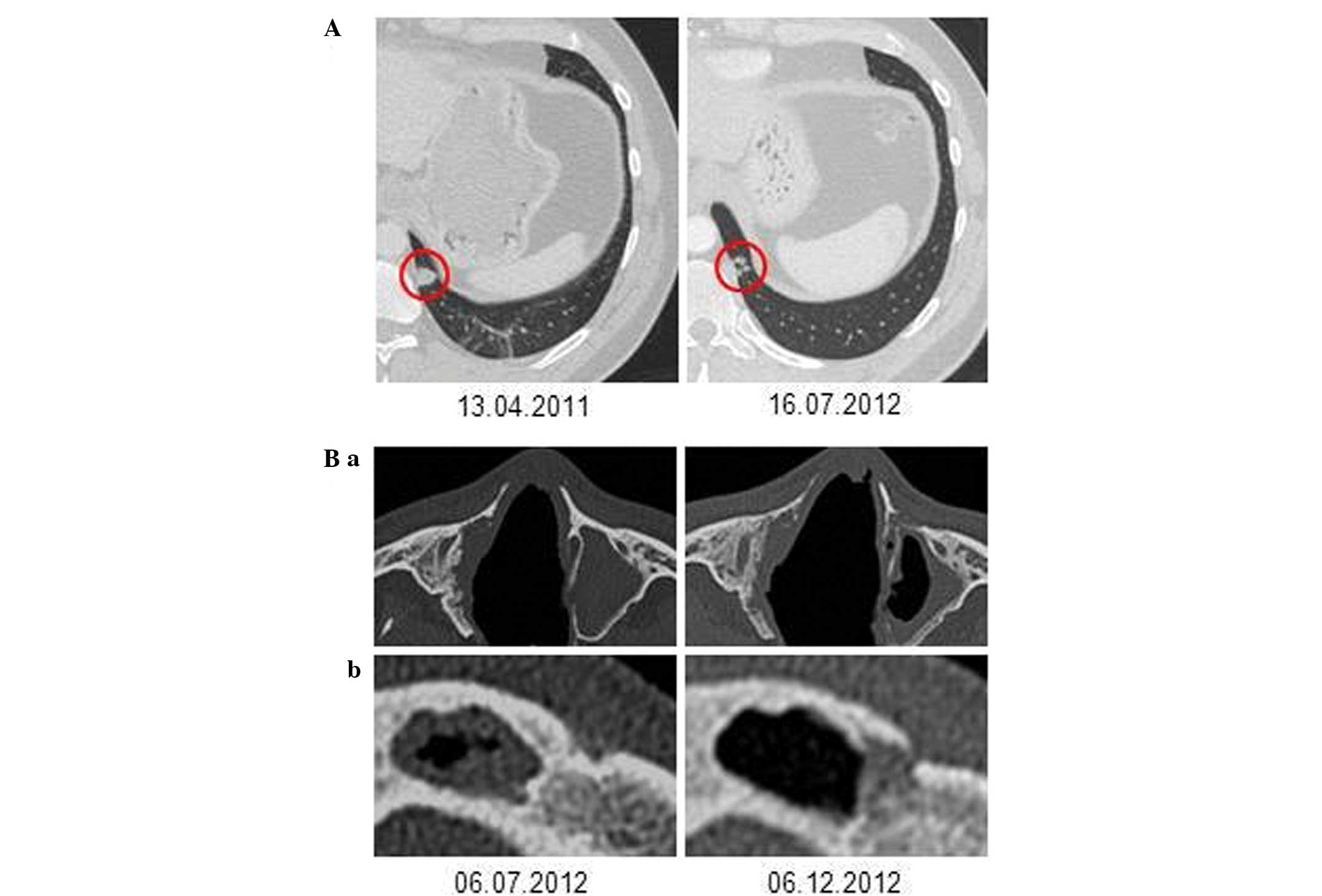
repeated interventions. In April 2011, multiple tracheal papilloma
manifestations led to a tracheal stenosis of upto 70%. CT scans
revealed numerous pulmonary nodes, cystic lesions and a tumor
measuring 2.5×2.6 cm in the middle lobe. Tracheal laser therapy
temporarily resulted in good short-term results and resection of
the middle lobe with lymphadenectomy led to the diagnosis of a
papilloma with transformation to squamous cell carcinoma
(T1bN0M0, TNM Classification for
Malignant Tumors) (10).
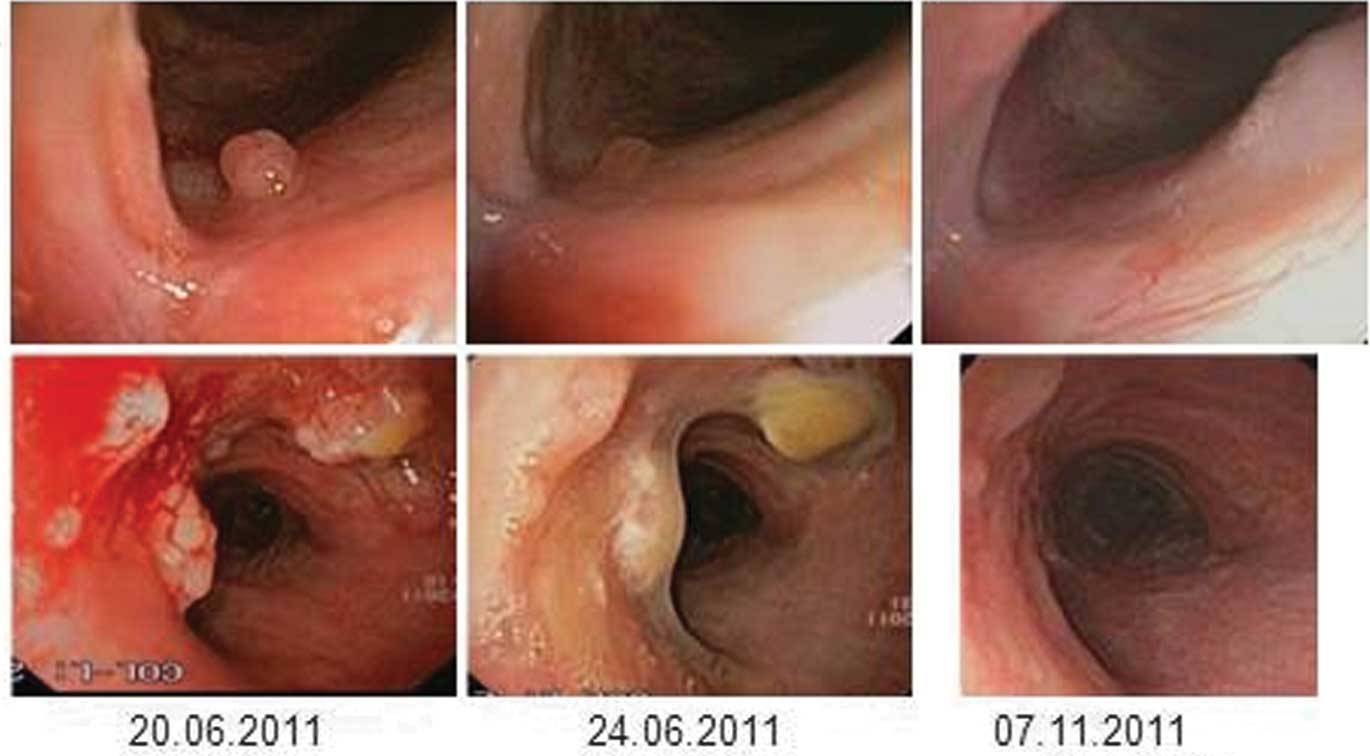
Four weeks later, a follow-up bronchoscopy
demonstrated progression of the tracheal papillomatosis and
clinical stridor was detected (Fig.
1). At that time, systemic treatment with bevacizumab was
initiated at a dose of 10 mg/kg. Three days after treatment
initiation a bronchoscopy indicated a significant response, with a
reduction of ~50% of the tracheal manifestations and the stridor
had disappeared completely. After 21 days, certain papillomas were
no longer present, and the dyspnea and hoarseness had improved
markedly, prompting the continuation of the bevacizumab therapy.
Due to the activity and good tolerability of bevacizumab, it was
decided that the patient could be treated at 14-day intervals.
Following eight cycles of treatment (day 139) a bronchoscopy
demonstrated only minimal residual disease (Fig. 1). The very good partial remission
(VGPR), which was also observed in the chest CT-scans (Fig. 2), showed ongoing stability for 27
months at the time of writing. The patient is well and has resumed
normal daily activities. The only treatment-associated side-effect
was mild hypertension, which was managed with a calcium antagonist.
Treatment intervals were extended to 4 months, and no surgical
interventions were necessary subsequent to treatment initiation
with bevacizumab.
Patient 2
This 49-year-old male was diagnosed with severe
laryngeal RRP in January 2011. At the initial diagnosis local
papilloma debulking was achieved using laser therapy. However, 6
months later, the papillomatosis relapsed and required a second
laser-based intervention. The disease progressed again and
bevacizumab treatment was initiated (dose, 10 mg/kg). An endoscopy
prior to treatment revealed exophytic papilloma growth in the right
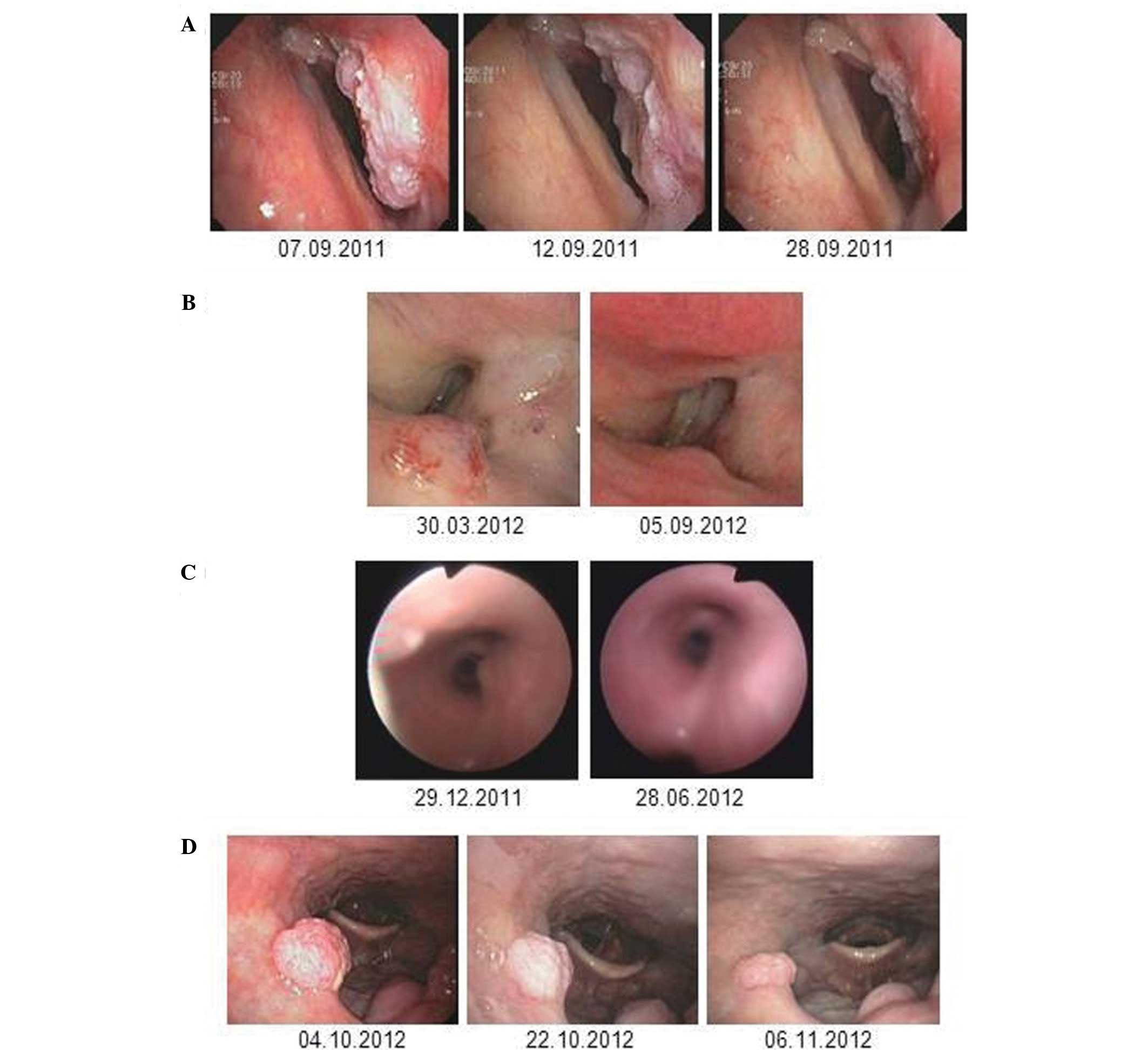
supraglottic area (Fig. 3A).
Pathological examination of biopsies taken from the area excluded
the presence of squamous cell carcinoma. An endoscopy on day four
after the first administration of bevacizumab demonstrated a rapid
response in the patient. A second control endoscopy, which was
performed immediately prior to the application of the second cycle
of bevacizumab on day 20, showed further regression of the
papilloma mass (Fig. 3A). In
parallel, hoarseness improved significantly within a few days and
the patient did not experience treatment-associated side-effects.
However, in this case, clinical worsening led to a re-evaluation,
including a CT scan prior to the third cycle of bevacizumab,
revealing destructive tumor growth that was potentially malignant
transformation in the subglottic area, while the supraglottic RRP
manifestation demonstrated a sustained VGPR. A laryngectomy was
performed and histology identified the co-existence of squamous
cell carcinoma and papilloma lesions. At present, the patient has
not yet demonstrated a relapse of RRP.
Patient 3
This patient was a 56-year-old female, who was
diagnosed with laryngeal and glottal RRP in 2009. Clinical symptoms
predominantly included hoarseness, an irritative cough and dyspnea.
Throughout the subsequent 3 years a total of 16 laser ablation
therapies were necessary at increasingly shorter intervals, with
the final interventions only leading to a short-term improvement of
the symptoms. Systemic therapy with bevacizumab was initiated in
March 2012. An endoscopy seven days after the first dose of
bevacizumab (10 mg/kg) revealed a partial response (PR) of the
supraglottic papilloma, while the manifestation on the right vocal
cord remained stable. After the second cycle, a further reduction
of the supraglottic lesion was observed. Clinically, the patient’s
voice became increasingly powerful and dyspnea on exertion
significantly improved shortly after treatment initiation.
Bevacizumab therapy was paused following four cycles and the
patient’s clinical condition remained stable for >9 months
(Fig. 3B). After this period the
patient developed symptoms of recurrence with an endoscopy
indicating recurrence of papilloma growth. Bevacizumab therapy was
resumed and resulted in clinical improvement within five days.
Again, an endoscopy on day 14 demonstrated the complete resolution
of the supraglottic papilloma. One infraglottic lesion, which had
not responded to the same extent was treated with additional laser
therapy and a diagnosis of malignant transformation was
excluded.
Patient 4
A 2-year-old female, who was diagnosed in 2007 with
HPV-11-associated RRP involving the larynx, trachea, deeper
bronchi, and the lung parenchyma required a tracheostomy in August
2008 and multiple laser ablation therapies until January 2012.
Adjuvant treatment attempts with interferon-alpha, cidofovir and
celecoxib did not result in disease control. In January 2012 laser
therapy of the large tracheal RRP manifestations was performed.
Fig. 3C shows the papilloma lesions
in the right main bronchus, which were not locally treated at that
time. Two life-threatening airway complications occurred during an
elective change of the tracheal cannula and in the context of a
tracheal laser intervention. To avoid re-growth of the papillomas
in the trachea and disease progression in the right main bronchus
the decision to initiate bevacizumab treatment (5 mg/kg every 2
weeks) was made following the laser treatment. After four cycles of
bevacizumab no further papilloma growth was observed in the trachea
and bronchi, and the treatment was paused (Fig. 3C). Monthly bronchoscopic assessments
did not indicate recurrent disease activity for 6 months. Five
months after bevacizumab discontinuation, an endoscopy showed
multilocular papilloma relapse in the trachea and bronchi, and a
fifth cycle of bevacizumab was administered. On day two after
administration, a notable response was observed and no further
local interventions for papillomatosis were necessary for more than
one year of treatment at three-monthly intervals. One solitary
manifestation at the distal end of the tracheal cannula was
clinically classified as a granuloma and had to be laser abraded
(histologically confirmed as papilloma) in May 2013.
Patient 5
A 32-year-old male was diagnosed with nasopharyngeal
papillomatosis and paranasal sinus papilloma in 2010. Over 24
months several interventions, including laser vaporization and
radical surgery via midfacial degloving had been necessary. In
February 2012 an attempt to treat the sinonasal inverted papilloma
with radiotherapy was made. However, three months later additional
laser therapy was required and was complicated by chronic bleeding
from the nasopharynx, which led to severe anemia (hemoglobin, 5.5
g/dl). A CT scan performed in July 2012 showed inverted papilloma
in all of the paranasal sinuses, including a complete obstructed
right frontal sinus, a subtotal obstruction of the left frontal
sinus and a complete obstruction of the left maxillary sinus. An
endoscopy revealed recurrent papilloma growth at the velum
(Fig. 3D) while the possibility of
deeper tracheobronchial manifestations could be excluded. It was
decided that this patient could be treated with bevacizumab at a
dose of 15 mg/kg every three weeks. An endoscopy on day four
following treatment initiation indicated an immediate response of
the papilloma manifestations that were located at the velum;
furthermore, a partial remission with a 50% reduction was observed
on day 20 (Fig. 3D). A CT scan
after three cycles of bevacizumab also revealed a response in the
left frontal sinus and the left maxillary sinus, while the right
frontal sinus continued to be obstructed (Fig. 2B). The patient experienced no
treatment-associated complications. After the third cycle no
further regression of the papilloma manifestations at the velum
occurred and the treatment was paused. Two months later, a routine
endoscopy showed papilloma progression again and a fourth cycle of
bevacizumab was administered, which resulted in immediate papilloma
regression.
Histopathological analyses
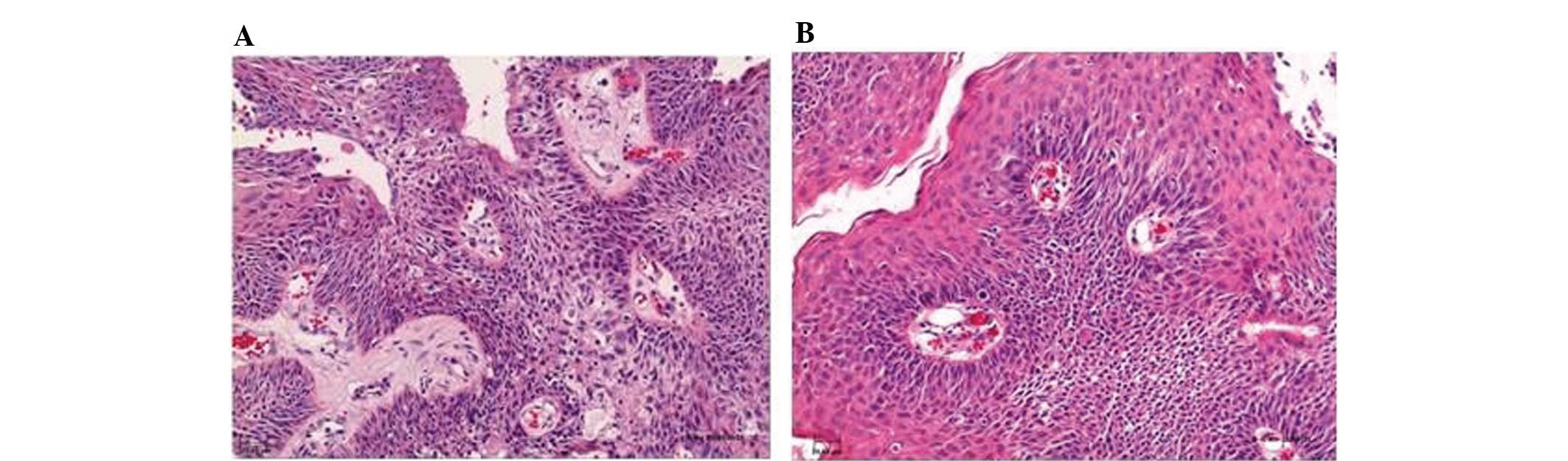
Histopathological analyses of pre- and
post-therapeutic papilloma biopsies showed regressive edema and
normalization of the vascular structures (Fig. 4). However, immunohistochemical
analyses of the VEGF and phosphorylated VEGFR-2 expression did not
show any changes following therapy with bevacizumab (data not
shown).
Summary of clinical efficacy
The clinical efficacy of systemic bevacizumab
treatment is summarized in Table
II. Bevacizumab induced PR or VGPR of papilloma manifestations
in 5/5 patients. When comparing the cumulative number of local
interventions during the 12-month period prior to bevacizumab
treatment initiation with the 12 month period following bevacizumab
treatment, the number of interventions was reduced from 18 to one
(P=0.042, Wilcoxon signed-rank test).
 | Table IISummary of the clinical efficacy of
systemic bevacizumab treatment. |
Table II
Summary of the clinical efficacy of
systemic bevacizumab treatment.
| Case | Best response | Freedom from
progression following treatment discontinuation (months) | Second response
following treatment discontinuation and progression | No. of interventions
in 12 months |
|---|
|
|---|
| Prior to
bevacizumab | Following
bevacizumab |
|---|
| 1 | VGPR | >4 | N.a. | 3 | 0 |
| 2 | PR | N.a. | N.a. | 2 | 1a |
| 3 | VGPR | 9 | Yes | 6 | 0 |
| 4 | VGPR | 5 | Yes | 3 | 0 |
| 5 | PR | 2 | Yes | 4 | 0 |
| All | - | - | - | 18 | 1b |
Discussion
The induction of papilloma regression is presented
in five consecutive patients with progressive RRP who, following
various previous treatment attempts, were administered with
systemic bevacizumab therapy at our institution. Previously,
systemic administration of bevacizumab has been hypothesized as an
effective adjuvant treatment modality following local intervention.
It was reported that bevacizumab may have delayed relapse in one
case of RRP (7) and was reported to
potentially improve photoangiolytic laser therapy, when used
concomitantly and applied topically (8,9). In
addition, epidermal growth factor receptor inhibition using
gefitinib or erlotinib demonstrated efficacy in two cases of RRP
(11,12).
An immediate and sustained therapeutic effect of
systemically administered bevacizumab was observed in all five RRP
patients in the present case series. Notably, it was possible to
document the response to treatment bronchoscopically as early as
within a few days following the first infusion of bevacizumab.
Continued anti-VEGF treatment resulted in sustained PR or VGPRs of
tracheal or laryngeal papilloma manifestations. Remission has been
sustained in one patient via administration of bevacizumab at
prolonged treatment intervals of 3–4 months. Three patients, who
exhibited disease progression following discontinuation of
bevacizumab, subsequently responded again following the second
cycle of treatment. Notably, none of the patients showed papilloma
progression whilst treatment was ongoing. Patient 2 required a
laryngectomy due to malignant transformation. The condition of
patient 3 was clinically stable for 9 months following bevacizumab
treatment, however, papilloma regrowth was observed following
treatment discontinuation. After this re-growth, re-treatment led
to a complete resolution of the supraglottic lesions. In this
patient, an infraglottic lesion did not respond to the same extent
as the other lesions and was abrased; a diagnosis of malignant
transformation was excluded. In patient 4, with former multilocular
tracheobronchial and lung parenchyma involvement, a solitary
manifestation, which was clinically interpreted as granuloma
associated with mechanical irritation by the distal end of the
tracheal cannula, had to be abrased. However, histological
assessment indicated that this particular manifestation was
papilloma growth.
At the time of writing the longest period of
progression-free survival observed during follow-up is 27 months in
patient 1, who is receiving maintenance therapy. Notably, in the
present case series, it was possible to document the responses to
bevacizumab therapy with regard to lung parenchyma involvement and
papillomas of the paranasal sinuses, which are usually not
accessible to local interventions (Fig.
2).
The rapid response to bevacizumab was accompanied by
a disappearance of the perivascular edema in papilloma lesions as
revealed by histopathological analyses of the pre- and
post-treatment papilloma lesions. This finding is consistent with
the antibody-mediated inhibition of VEGF-induced vascular
permeability (13). DNA
fragmentation assays that were performed on papilloma tissues to
detect apoptosis did not indicate significant differences prior to
and following the administration of bevacizumab. This indicates
that the observed therapeutic effect is predominantly mediated by
modulation of the vasculature and not by induction of
apoptosis.
In conclusion, the experience of the five present
patients suggests that systemic bevacizumab presents a highly
effective treatment option for RRP, offering a unique opportunity
even for patients with non-accessible bronchial lesions, lung
parenchyma involvement, papillomas of the paranasal sinuses, or for
patients at high risk for voice mutilation by interventional
therapies. These findings indicate that systemic bevacizumab
treatment may have the potential to alter the current management of
RRP. Whether the treatment will have an impact on the frequency of
malignant transformation in papillomatosis remains unclear and
VEGF-targeted therapeutic strategies require further investigation
in clinical trials.
References
|
1
|
Glikman D and Baroody FM: Images in
clinical medicine. Recurrent respiratory papillomatosis with lung
involvement. New Engl J Med. 352:e222005.
|
|
2
|
Derkay CS and Wiatrak B: Recurrent
respiratory papillomatosis: a review. Laryngoscope. 118:1236–1247.
2008.
|
|
3
|
Kimberlain DW: Current status of antiviral
therapy for juvenile-onset recurrent respiratory papillomatosis.
Antiviral Res. 63:141–151. 2004.
|
|
4
|
Gélinas JF, Manoukian J and Côté A: Lung
involvement in juvenile onset recurrent respiratory papillomatosis:
a systematic review of the literature. Int J Pediatr
Otorhinolaryngol. 72:433–452. 2008.
|
|
5
|
Chadha NK and James A: Adjuvant antiviral
therapy for recurrent respiratory papillomatosis. Cochrane Database
Syst Rev. 2010.CD0050532010.
|
|
6
|
Rahbar R, Vargas SO, Folkman J, et al:
Role of vascular endothelial growth factor-A in recurrent
respiratory papillomatosis. Ann Otol Rhinol Laryngol. 114:289–295.
2005.
|
|
7
|
Nagel S, Busch C, Blankenburg T and
Schütte W: Treatment of respiratory papillomatosis - a case report
on systemic treatment with bevacizumab. Pneumologie. 63:387–389.
2009.(In German).
|
|
8
|
Zeitels SM, Barbu AM, Landau-Zemer T, et
al: Local injection of bevacizumab (Avastin) and angiolytic KTP
laser treatment of recurrent respiratory papillomatosis of the
vocal folds: a prospective study. Ann Otol Rhinol Laryngol.
120:627–634. 2011.
|
|
9
|
Rogers DJ, Ojha S, Maurer R and Hartnick
CJ: Use of adjuvant intralesional bevacizumab for aggressive
respiratory papillomatosis in children. JAMA Otolaryngol Head Neck
Surg. 139:496–501. 2013.
|
|
10
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th edition. John Wiley
& Sons; UK: 2009
|
|
11
|
Limsukon A, Susanto I, Hoo GW, et al:
Regression of recurrent respiratory papillomatosis with celecoxib
and erlotinib combination therapy. Chest. 136:924–926. 2009.
|
|
12
|
Bostrom B, Sidman J, Marker S, et al:
Gefitinib therapy for life-threatening laryngeal papillomatosis.
Arch Otolaryngol Head Neck Surg. 131:64–67. 2005.
|
|
13
|
Ferrara N: VEGF as a therapeutic target in
cancer. Oncology. 69(Suppl 3): S11–S16. 2005.
|