Introduction
Gastric cancer is the second most common cause of
cancer-related mortality worldwide and is difficult to cure in
Western countries, mainly since the majority of patients present
with advanced disease (1).
According to the World Health Organization, >100 million
individuals are diagnosed worldwide every year and the three-year
retrospective survey of gastric cancer shows the rate to be 23.2%
in China (2). Malignancy is one of
the most significant biological characteristics of gastric cancer.
In total, >90% of gastric cancer patients suffer metastasis in
different organs, including the lymph nodes, liver and lungs
(3). Lymph node metastasis is
closely associated with the five-year survival rate of gastric
cancer. Certain studies have shown that the five-year survival rate
drops to 30.5% in patients with lymph node metastasis and rises to
86.1% in those without lymph node metastasis (4–5).
Surgery is the most commonly used treatment for
gastric tumors, but >50% of patients cannot rely on surgery to
remove all lesions, as their lymph node metastasis has progressed
outside the scope of surgical resection (6). At present, chemotherapy is the
conventional treatment for lymph node metastases, but it is of
detriment that the effect of chemotherapy drugs is not obvious and
that the high plasma concentrations often produce serious
side-effects. Therefore, the search for effective chemotherapy
drugs for gastric cancer to prevent lymphatic metastasis, improve
therapeutic efficacy and reduce toxicity is extremely important
(7).
Certain studies have shown that liposomes as drug
carriers can have affinity for tumor cells, changing the
distribution of drugs in tissues and selectively killing tumor
cells or restraining their reproduction (8). Liposomes are considered to be
efficient carriers due to certain advantages, including their
ability to incorporate water and lipid soluble agents, the presence
of a fluid liposomal membrane giving high versatility, their size
and their superficial charge (8).
Therefore, liposomes are able to improve therapeutic efficacy and
reduce the side-effects. Sterically stabilized liposomes are
generated as a result of the insertion of polyethylene glycol
(PEG)-derived phospholipids into the liposomal membrane (9–12).
Carboplatin CBP; cis-diammine
(1,1-cyclobutanedicarboxylato)-platinum (II)] is recommended for
the chemotherapy of numerous types of cancer, including gastric
tumors. Standard second-line regimens often include CBP, as it
exhibits non-cross resistance. However, the side-effects of CBP
include dose-related myelosuppression, with severe thrombocytopenia
and leucopenia. Thus, the ability to prepare PL-CBPs is important
to reduce or prevent its side-effects and improve its target
effect. Certain studies have reported that PL-CBPs coated with L-α
egg phosphatidylcholine and O-palmitoylpullulan enhance the
therapeutic effect and the stability of storage conditions.
However, there are no studies on the effect of PL-CBPs on lymph
node metastasis in a gastric cancer mouse model (13,14).
In the present study, PL-CBPs were prepared with
PEG-2000 and the PL-CBP characteristics, including their size and
stability, their release, entrapping and loading efficiencies, and
their antitumor and antimetastatic effects on the lymph nodes, were
analyzed.
Materials and methods
CBP (purity, >99%) was purchased from Kunming
Precious Metal Institute (Kunming, Yunnan, China). Injectable CBP
(100 mg/bottle) was purchased from Jinan Qilu Pharmaceutical
Company (Jinan, Shandong, China), while phosphatidylethanolamine
(PE) and cholesterol were purchased from Shanghai Dongshang Company
(Shanghai, China). PEG-2000 was purchased from Sigma-Aldrich (St.
Louis, MO, USA). All other chemicals, unless otherwise stated, were
obtained from Sigma-Aldrich.
PL-CBP preparation
Liposomes containing CBP were prepared according to
the thin film hydration method, as previously described (15). Briefly, lipids, including PE and
cholesterol (PE:cholesterol, 4:1), were dissolved in 30 ml
chloroform to form a mixture, and then the organic solvent was
removed by using rotary evaporation under reduced pressure to
obtain a film. The dry lipid film was hydrated with a solution of
CBP dissolved in 5% glucose (2 mg/ml). The dispersion of the lipid
was facilitated by mechanical agitation in an ultrasound bath for 1
min. The PEG-2000 dissolved in phosphate-buffered saline (PBS) with
the prepared lipid in an equal volume were mixed and placed at 4°C
for 60 min. Next, the same volume of PBS was added to the system at
4°C for 60 min.
Drug release
This study was carried out at 37°C, as it was used
for in vitro and in vivo studies. Next, 2 ml of
formulation mixture solution in a dialysis bag was incubated in 5%
glucose at 37°C, whilst being continuously agitated. Samples
collected at different times (0, 12, 24 and 48 h) were
ultrafiltered using the Genesys 10 ultra-violet/visible
spectrophotometry (Thermo Genesys 10; Thermo Scientific, Waltham,
MA, USA) at 235 nm. Release efficiency was expressed by the
equation: Release efficiency (%) = (release CBP in liquid/total CBP
in liposome) × 100. In addition, the particle size was also
characterized in these samples.
Determination of encapsulation
efficiency
The detection of CBP was performed using an
ultraviolet (UV) wavelength at 279 nm. In this assay, full
wavelength scanning was used to detect the encapsulation
efficiency. The first step was to detect and calculate the standard
curve with the various concentrations of PL-CBP (0.01–0.25 mg/ml).
The liposome samples were then diluted with sodium chloride
injection fluid (0.9%). Next, the fluid was separated by gel
exclusion chromatography over Sephadex G-50 (General Electric
Company, Fairfield, CT, USA). The concentration of entrapped CBP
was determined by Genesys 10 ultra-violet/visible spectrophotometry
following lysis of the liposomes with anhydrous alcohol. The UV
absorbance of the sample solution was measured at 235 nm. Each
determination was made in triplicate. The encapsulation efficiency
was expressed by the following equation: Encapsulation (%) =
(QEn/Qtotal) × 100, where QEn is quantity of CBP (mg)/lipid (mg)
determined following removal of free drug, and Qtotal is the
quantity of CBP (mg)/lipid (mg) determined at the stage when the
drug was added to the lipid film.
Cell line
The human gastric cancer SGC-7901 cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA). The cells were grown in Dulbecco’s modified Eagle’s medium
containing 10% fetal bovine serum, 100 U/ml penicillin and 100
μg/ml streptomycin in a 37°C humidified incubator with 5%
CO2. The cells were subcultured at 80 to 90% confluence.
The cells used in this study were subjected to ≤20 cell
passages.
Tumor xenograft
A total of 30 male BALB/c nude mice with body
weights ranging between 18 and 22 g were injected with SGC-7901
cell line suspension in the right flank. After 7 days, the mice
were randomly divided into three groups (the control, CBP and
PL-CBP groups). CBP and PL-CBP were administered at 10 mg/kg for
two consecutive cycles of treatment, 5 days apart, to their
respective groups. In each group, two doses of 5 mg/kg were
administered every 48 h. The dose of liposomes was calculated based
on the quantity of encapsulated CBP (μg)/lipid (mg). At the end of
the study, the tumors were excised and weighed. Tumor size was
measured and volume was calculated according to the following
formula: Tumor volume (mm3) = d2 × D/2, where
d and D were the shortest and longest diameter, respectively. This
study was approved by the ethics committee of Fujian Medical
University (Fuzhou, China).
Food intake and body weight
Each cage of mice was provided daily with food, and
on the following day, the remainder was collected and weighed in
order to calculate daily food intake. Body weight was measured at
baseline and at the end of treatment.
Hematoxylin and eosin (HE) staining
Histological examination was performed by HE
staining. Tumor tissues were fixed with 10% buffered formalin for
24 h. Samples were then paraffin-embedded, sectioned and stained
with HE. Histopathological changes were observed under a light
microscope. According to the grading standards of lymph node
metastasis, the metastasis was divided into the following four
grades: 0, no tumor cells in the lymph nodes; +, numerous tumor
cells at the edge of the sinus; ++, tumor cells invade the middle
of the sinus; and +++, tumors occupy more than half of the
cross-section in the lymph node (16).
Analysis of Pt in tissue by atomic
absorption spectroscopy (AAS)
The level of Pt was determined in the tissues by
electrothermal atomization in an AAS 8000 graphite furnace (Renrui
Company, Shanghai, China). The tissue samples were analyzed
following enzymatic digestion and modification of the final matrix.
The tissue was dried at 100°C for 20 h and weighed. Subsequent to
wet combustion with 1.0 ml nitric acid and 0.5 ml perchloric acid,
all samples were diluted to 10 ml with extra-pure water (Milli-Q,
Nihon Millipore Kogyo KK, Tokyo, Japan), and the Pt content in the
tissue was measured. The furnace conditions and instrumental
parameters for the determination of Pt were 70–105°C for 60 sec,
1,400°C for 30 sec, 2,800°C for 10 sec and 2,900°C for 7 sec, and
argon was used as a carrier gas at a rate of 200 ml/min. The
results were expressed as the quantity of Pt (mg)/dried tissue
(g).
Tumor apoptosis
Tumor sections from the mice treated as
aforementioned were fixed with 4% paraformaldehyde for 48 h. The
5-μm thick sections of the tumor samples were analyzed by terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) staining using a TumorTACS in situ Apoptosis kit
(Roche Bioscience, Palo Alto, CA, USA) to detect fragmented DNA
according to the manufacturer’s instructions. Microscopic
immunohistochemical images were captured by an Olympus microscope
(Olympus Corporation, Tokyo, Japan) and a moticam 5000C camera from
Motic Instruments (Richmond, BC, Canada), and analyzed by Motic Med
6.0 software. The number of positive and total cells were counted
at five arbitrarily selected microscopic fields at ×100
magnification (each 7,050 μm2 in size). A dark brown
nucleus represented the positive apoptotic cells of the tumor,
observed due to TUNEL staining, and the apoptosis index (AI) was
calculated according to the following formula: AI = number of
positive cells/total number of cells.
Statistical analyses
Statistical analysis was performed with SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation. A two-sided α level (type I error rate) of
<0.05 was considered to indicate a statistically significant
result. One way analysis of variance was used to analyze the data
and Fisher’s protected least significant difference was used as a
post hoc test.
Results
Stability analysis
The stability analysis results are shown in Table I. The size and distribution of the
liposomes were relative to their stability. It was revealed that at
37°C, the lipsome size was similar at 0–48 h, as was expected. Once
the liposomes had been incubated at 37°C for 0, 12, 24 and 48 h,
the drug release efficiency was 0.7, 22.5, 48.7 and 65.1%. This
effect represents an advantage of CBP, as the drug would be more
stable in the blood circulation and could be released slowly at the
tumor site.
 | Table ISize and drug release of liposomal
formulationsa. |
Table I
Size and drug release of liposomal
formulationsa.
| Time, h | Size, nm | Re, % |
|---|
| 0 | 82.1±2.3 | 0.7±0.1 |
| 12 | 85.2±1.8 | 22.5±5.7 |
| 24 | 88.5±3.5 | 48.7±7.3 |
| 48 | 87.5±4.3 | 65.1±9.7 |
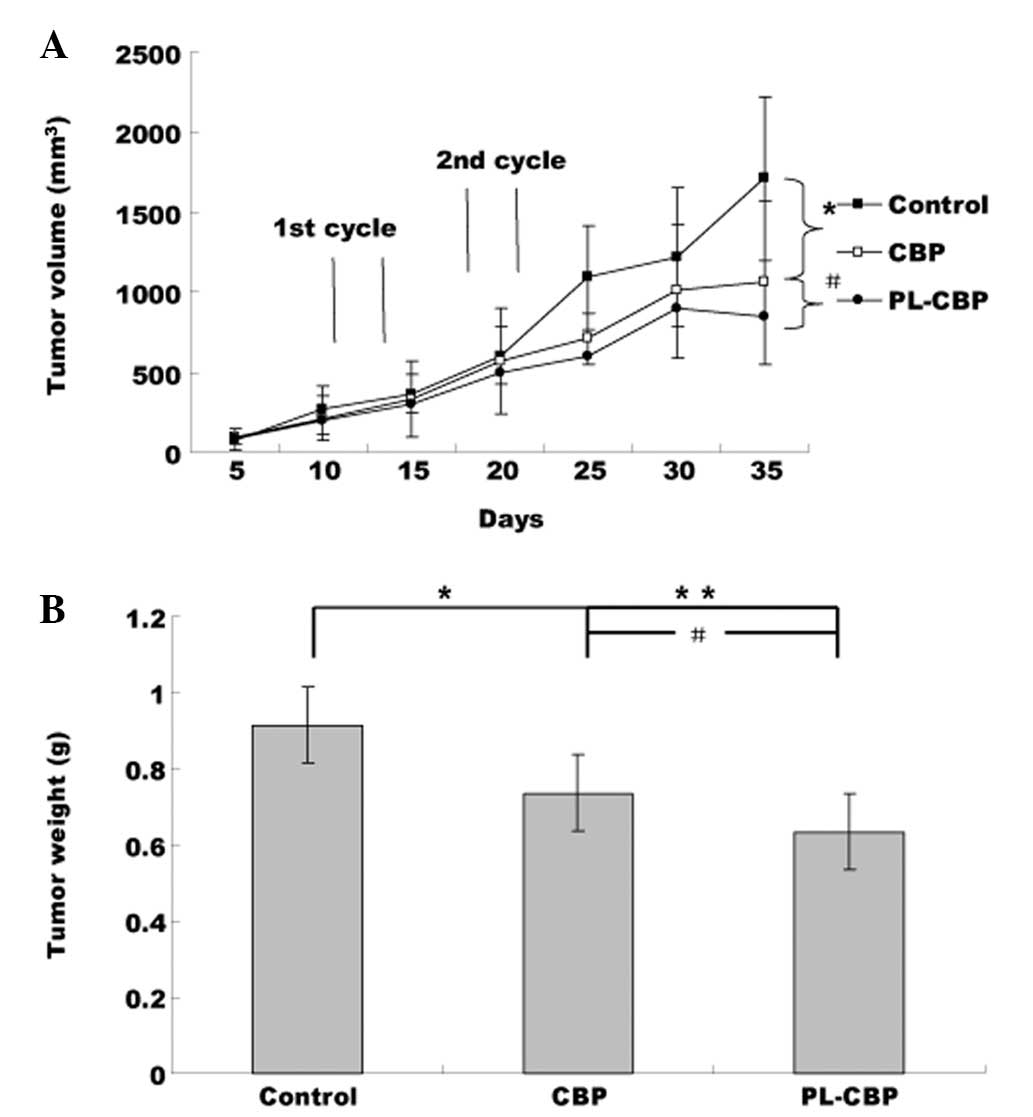
Antitumor effect
Fig. 1 shows that
the PLs suppressed tumor growth more efficiently than the control.
This inhibition reached statistical significance subsequent to the
second cycle. At 35 days, compared with the control group, the
tumor weight and tumor volume were reduced by 65% in the PL-CBP
group (Fig. 1, P<0.01) and by
50% in the CBP group (P<0.05 vs. control; P<0.05 vs.
PL-CBP).
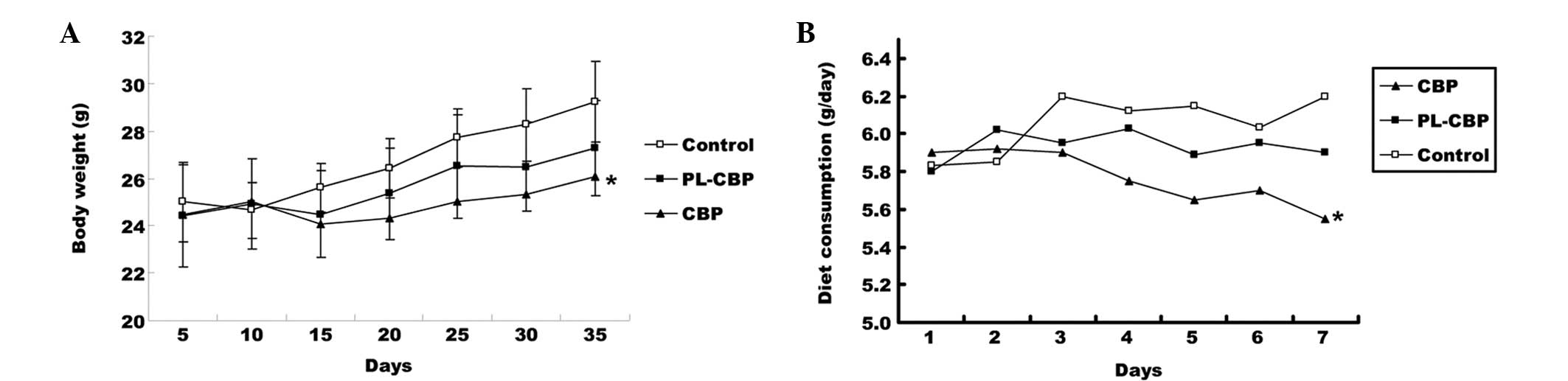
Average diet consumption and body
weight
There was no significant difference in the body
weight between the groups at baseline. The body weight per mouse
ranged between 24 and 29 g after 35 days. Mice in the PL-CBP and
CBP groups had a lower body weight compared with the control group,
and this difference was significant in the CBP group compared with
the control group (P<0.05; Fig.
2A). The average diet consumption was higher in the control
group than in the PL-CBP and CBP groups and this difference was
significant (P<0.05) between the CBP and control groups
subsequent to the first cycle (Fig.
2B). The PL-CBP group appeared to gain fewer side-effects
following treatment, while the CBP mice demonstrated impaired
movement, ruffled fur and a greater loss of body weight.
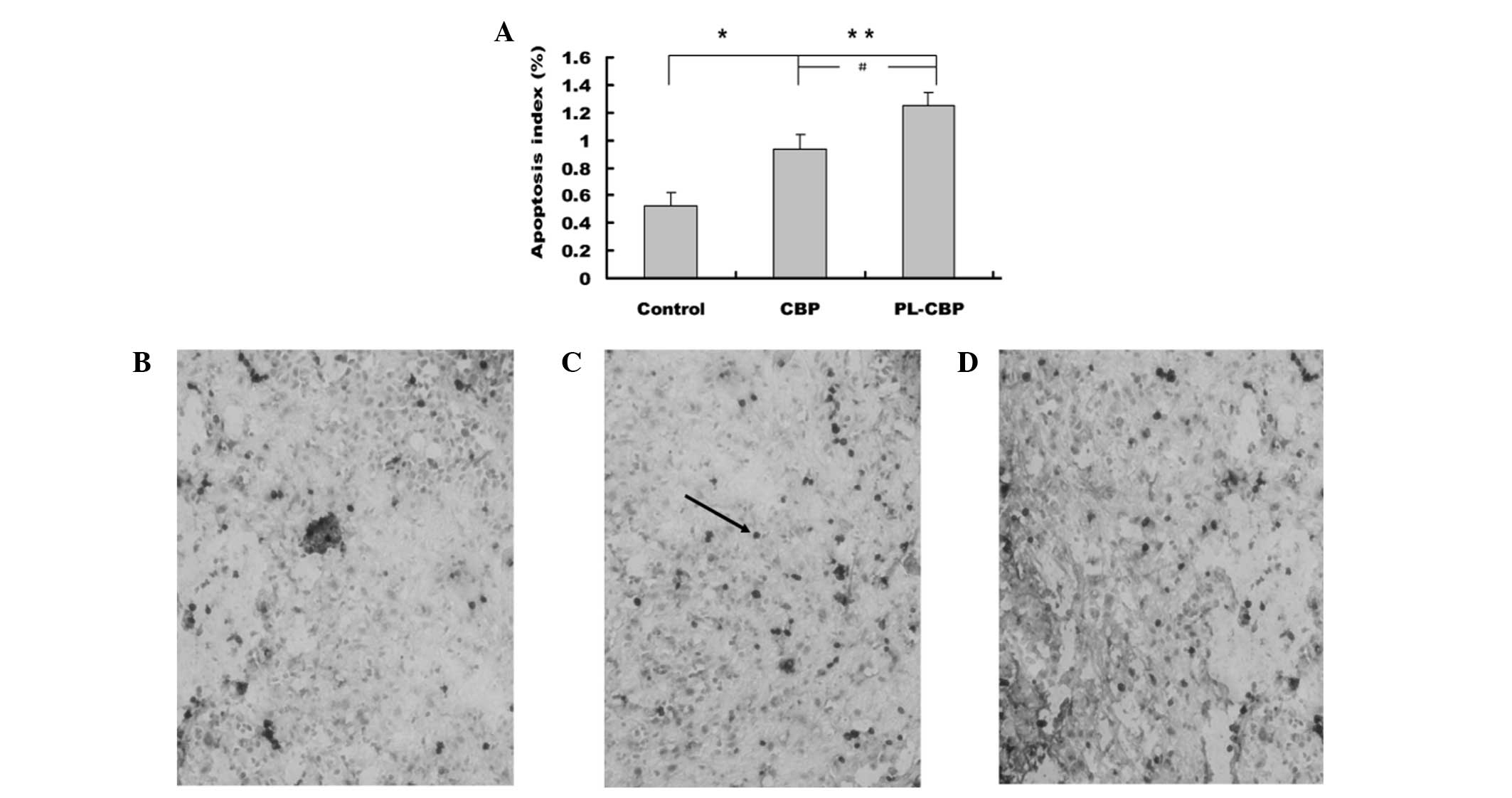
Tumor apoptosis
In the present study, the results showed that the AI
was significantly higher in the PL-CBP and CBP groups compared with
the control group (P<0.05 and P<0.01, respectively).
Representative images are shown in Fig.
3.
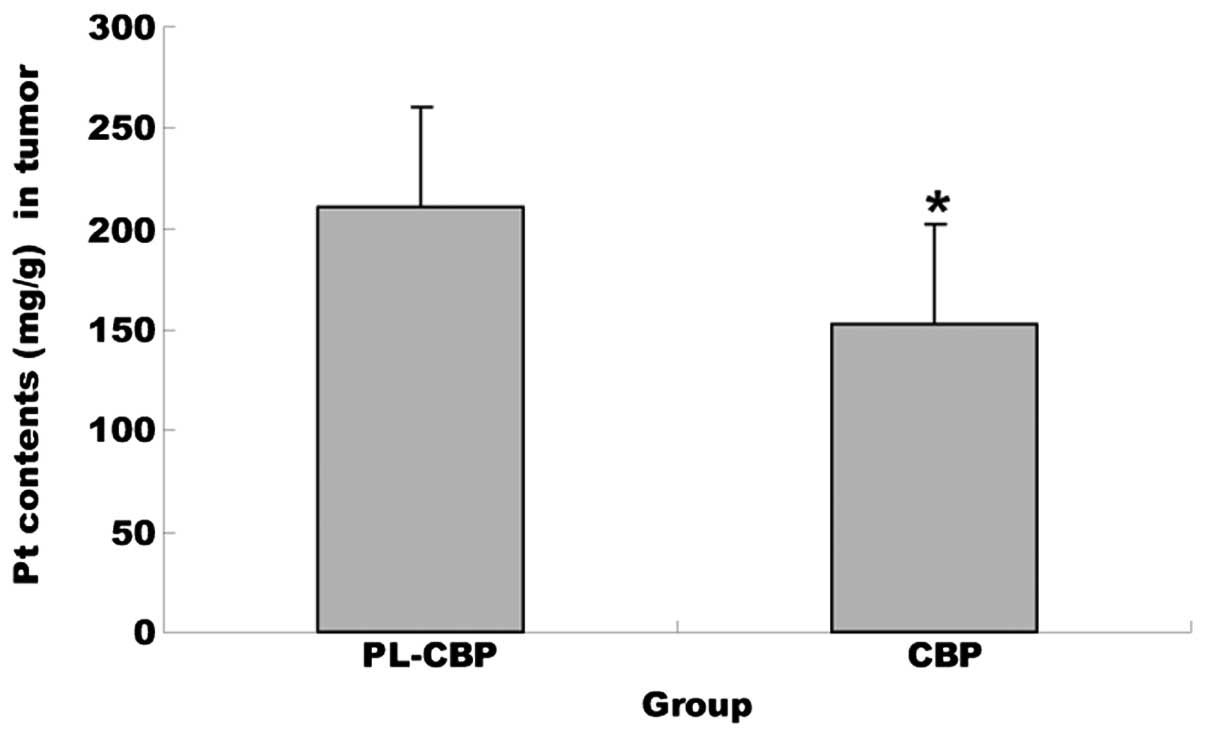
Pt accumulation in the tumor
Pt concentrations in each sample were calculated by
fitting linear regression lines to the points defined by the spiked
concentration values and the corresponding integrated peak areas.
In the tumor tissues, the concentration of Pt in the PL-CBP group
was much greater than that accumulated from the injected form
(P<0.01), which meant that the distribution of PL-CBP in the
tissues was improved significantly (Fig. 4).
Lymph node metastasis
The right submaxillary lymph node became swollen.
The volume of lymph in the PL-CBP group was smaller than that in
the CBP and control groups (PL-CBP vs. CBP, P<0.05; PL-CBP vs.
control, P<0.01). The tumor migrated to the lymph in the control
group, with grade +++ metastasis. The grade of metastasis in the
PL-CBP and CBP groups was 0/+ and +/++ (Table II; PL-CBP vs. CBP, P<0.05;
PL-CBP vs. control, P<0.01).
 | Table IIVolume and grade of lymph node
metastasisa. |
Table II
Volume and grade of lymph node
metastasisa.
| | Grade |
|---|
| |
|
|---|
| Group | Volume,
mm3 | 0 | + | ++ | +++ |
|---|
| Control | 98±54 | | | | 10 |
| PL-CBP | 37±24b | 6 | 4b | | |
| CBP | 12±10b | | 5 | 5c | |
Discussion
In the present study, a novel formula of PL-CBP was
developed in liposomal dosage form to improve its delivery
characteristics and inhibit the side-effects. Once CBP had been
encapsulated in the liposomes with PEG-2000, it exhibited a high
stability. Associated studies have indicated that PEG-modified
chemotherapy drugs can overcome any limitations, including short
circulation time and side-effects (16). The PEG-modification can confer
significant benefits on drugs, such as increasing their solubility
by the association with water molecules. The increased molecular
weight of the PEG-modified complex reduces the speed of kidney
clearance and induces few side-effects (17). Other than the use of the PEG-2000 as
a modifier of chemotherapy drugs, various lipids have been widely
used to form liposomes, including O-palmitoylpullulan,
phosphatidylserine, hydrogenated egg phosphatidylcholine, L-α egg
phosphatidylcholine, dipalmitoylphosphatidylcholine,
1,2-diacyl-glycero-3-phosphocholine/poly(ethyleneglycol)
2000-1,2-diacyl-glycero-3-phosphoethanolamine/cholesterol, and
distearoylphosphatidylcholine and hydrogenated phosphatidylinositol
(18–20). Key characteristics of these
liposomes include their decreased clearance and increased
accumulation at affected organ sites. This system can therefore
alter the biodistribution and pharmacokinetics of the encapsulated
drug (21).
Once the drugs had been modified in the present
study, the release efficiency was 65.1% after 48 h and the
entrapping efficiency was 85%. Water-soluble drugs can be enfolded
closely by the hydrophilic molecules of liposomes so that the drug
molecules can be released slowly in the body. Overall, low drug
loading and a low encapsulation efficacy are characteristics of
conventional liposomes produced for cisplatin (22,23).
Conventional liposomes are liposomes with varying lipid
compositions. Compositions that are very high in
phosphatidylcholine and cholesterol are typically the most commonly
used (24). It has been suggested
in previous studies that phosphatidylethanolamine transfers across
mammalian cell membranes more easily than phosphatidylcholine
(25). This may contribute to the
strong ability to carry cisplatin into cells.
Liposomes targeting tumor cells have shown
considerably greater cytotoxicity. This is due to an increased
bioavailability once the liposomes have been transported to the
cytoplasm, where the liposomal membrane is broken down by
degradative enzymes and the drug is released (24). In the present study, the results
showed that administering PL-CBP to SGC-7901 gastric tumor
cell-bearing mice was therapeutically more effective and resulted
in less side-effects compared with free CBP. Next, it was found
that PL-CBP induced tumor cell apoptosis and resulted in
accumulation of a large amount of Pt in the tumors. This may be the
reason for the apparent antitumor effect in the PL-CBP group.
Earlier studies demonstrated that the tumor accumulation of
liposomal drugs correlated with their circulation time in the blood
(25). Later studies found that
liposome accumulation in tumors was not only correlated with blood
circulation levels, but also with the size of the liposomes
(26). Another study reported that
doxorubicin encapsulation in 100-nm liposomes coated with PEG
resulted in increased blood levels and an enhanced survival time in
the circulation (25). The
formulation used in the present study was by far the most efficient
system, and increased the levels of CBP in the tumors. The main
side-effects of CBP are its toxicity to the kidney and damage to
the marrow, with severe thrombocytopenia and leucopenia (27). The results presented in the present
study show that PL-CBP can increase bioavailability and may
increase the therapeutic efficacy.
The extent of lymph node metastasis is the most
important prognostic factor, as analyzing node metastasis has
become the leading method for assessing the extent of disease,
determining the prognosis in gastric cancer patients and affecting
therapy strategies (28). In the
present study, lymph node metastasis was evaluated according to the
assessing standard (16). The
results showed that the lymph node metastasis in the PL-CBP group
was the lowest among the three groups.
Overall, the study showed that the characteristics
of PL-CBP with PEG-2000 were stable and indicated an obvious
antitumor and antimetastasis effect in the gastric cell-bearing
mice. In future studies, we aim to further detect the biological
mechanism behind the antitumor and antimetastasis effects of
PL-CBP.
Acknowledgements
This study was supported by the Youth Research
Project of Fujian Province Department of Health (2007-2-25).
Abbreviations:
|
PEG
|
polyethylene glycol
|
|
PL-CBP
|
PEGylated carboplatin liposomes
|
|
CBP
|
carboplatin
|
|
PEG-2000
|
polyethylene glycol-2000
|
|
HE
|
hematoxylin and eosin
|
|
AAS
|
atomic absorption spectroscopy
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling
|
|
AI
|
apoptosis index
|
References
|
1
|
Bittoni A, Scartozzi M, Giampieri R,
Faloppi L, Bianconi M, Mandolesi A, et al: Clinical evidence for
three distinct gastric cancer subtypes: time for a new approach.
PLoS One. 8:e785442013.
|
|
2
|
Hyun MH, Lee CH, Kim HJ, Tong Y and Park
SS: Systematic review and meta-analysis of robotic surgery compared
with conventional laparoscopic and open resections for gastric
carcinoma. Br J Surg. 100:1566–1578. 2013.
|
|
3
|
Sarvaiya PJ, Guo D, Ulasov I, Gabikian P
and Lesniak MS: Chemokines in tumor progression and metastasis.
Oncotarget. 4:2171–2185. 2013.
|
|
4
|
Endo S, Nishikawa K, Yamada T, Nakagawa T,
Fushimi H, Chihara T, et al: Our experience of treating
undifferentiated gastric carcinoma: report of four cases. Surg
Today. Nov 20–2013.(Epub ahead of print).
|
|
5
|
Hayakawa Y, Fox JG, Gonda T, Worthley DL,
Muthupalani S and Wang TC: Mouse models of gastric cancer. Cancers
(Basel). 5:92–130. 2013.
|
|
6
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.
|
|
7
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.
|
|
8
|
Dal Lago L, D’Hondt V and Awada A:
Selected combination therapy with sorafenib: a review of clinical
data and perspectives in advanced solid tumors. Oncologist.
13:845–858. 2008.
|
|
9
|
Moghimi SM and Szebeni J: Stealth
liposomes and long circulating nanoparticles: critical issues in
pharmacokinetics, opsonization and protein-binding properties. Prog
Lipid Res. 42:463–478. 2003.
|
|
10
|
Gabizon AA, Shmeeda H and Zalipsky S: Pros
and cons of the liposome platform in cancer drug targeting. J
Liposome Res. 16:175–183. 2006.
|
|
11
|
Hofheinz RD, Gnad-Vogt SU, Beyer U and
Hochhaus A: Liposomal encapsulated anti-cancer drugs. Anticancer
Drugs. 16:691–707. 2005.
|
|
12
|
Allen TM, Hansen C, Martin F, Redemann C
and Yau-Young A: Liposomes containing synthetic lipid derivatives
of poly(ethylene glycol) show prolonged circulation half-lives in
vivo. Biochim Biophys Acta. 1066:29–36. 1991.
|
|
13
|
Boulikas T and Vougiouka M: Recent
clinical trials using cisplatin, carboplatin and their combination
chemotherapy drugs (Review). Oncol Rep. 11:559–595. 2004.
|
|
14
|
Kurt E, Cubukcu E, Karabulut B, Olmez OF,
Kurt M, Avci N, et al: A multi-institutional evaluation of
carboplatin plus docetaxel regimen in elderly patients with
advanced gastric cancer. J BUON. 18:147–153. 2013.
|
|
15
|
Bangham JA and Lea EJ: The interaction of
detergents with bilayer lipid membranes. Biochim Biophys Acta.
511:388–396. 1978.
|
|
16
|
Bollschweiler E, Hölscher AH, Metzger R,
Besch S, Mönig SP, Baldus SE and Drebber U: Prognostic significance
of a new grading system of lymph node morphology after neoadjuvant
radiochemotherapy for esophageal cancer. Ann Thorac Surg.
92:2020–2027. 2011.
|
|
17
|
Romberg B, Hennink WE and Storm G:
Sheddable coatings for long-circulating nanoparticles. Pharm Res.
25:55–71. 2008.
|
|
18
|
Song H, Zhang J, Han Z, Zhang X, Li Z,
Zhang L, et al: Pharmacokinetic and cytotoxic studies of pegylated
liposomal daunorubicin. Cancer Chemother Pharmacol. 57:591–598.
2006.
|
|
19
|
Frank MM: The reticuloendothelial system
and bloodstream clearance. J Lab Clin Med. 122:487–488. 1993.
|
|
20
|
Kaneda Y and Tabata Y: Non-viral vectors
for cancer therapy. Cancer Sci. 97:348–354. 2006.
|
|
21
|
Brannon-Peppas L and Blanchette JO:
Nanoparticle and targeted systems for cancer therapy. Adv Drug
Deliv Rev. 56:1649–1659. 2004.
|
|
22
|
Newman MS, Colbern GT, Working PK, Engbers
C and Amantea MA: Comparative pharmacokinetics, tissue
distribution, and therapeutic effectiveness of cisplatin
encapsulated in long-circulating, pegylated liposomes (SPI-077) in
tumor-bearing mice. Cancer Chemother Pharmacol. 43:1–7. 1999.
|
|
23
|
Xiao C, Qi X, Maitani Y and Nagai T:
Sustained release of cisplatin from multivesicular liposomes:
potentiation of antitumor efficacy against S180 murine carcinoma. J
Pharm Sci. 93:1718–1724. 2004.
|
|
24
|
Drummond DC, Meyer O, Hong K, Kirpotin DB
and Papahadjopoulos D: Optimizing liposomes for delivery of
chemotherapeutic agents to solid tumors. Pharmacol Rev. 51:691–743.
1999.
|
|
25
|
Sleight RG and Pagano RE: Transbilayer
movement of a fluorescent phosphatidylethanolamine analogue across
the plasma membranes of cultured mammalian cells. J Biol Chem.
260:1146–1154. 1985.
|
|
26
|
Parr MJ, Masin D, Cullis P and Bally MB:
Accumulation of liposomal lipid and encapsulated doxorubicin in
murine Lewis lung carcinoma: the lack of beneficial effects by
coating liosomes with poly(ethylene glycol). J Pharmacol Exp Ther.
280:1319–1327. 1997.
|
|
27
|
Wagstaff AJ, Ward A, Benfield P and Heel
RC: Carboplatin: A preliminary review of its pharmacodynamic and
pharmacokinetic properties and therapeutic efficacy in the
treatment of cancer. Drugs. 37:162–190. 1989.
|
|
28
|
Aurello P, D’Angelo F, Rossi S, Bellagamba
R, Cicchini C, Nigri G, et al: Classification of lymph node
metastases from gastric cancer: comparison between N-site and
N-number systems. Our experience and review of the literature. Am
Surg. 73:359–366. 2007.
|