Introduction
The mean platelet volume (MPV) is a platelet volume
index (1). Classically, MPV was
recognized as a characteristic of platelet activation. Larger
platelets are more reactive than smaller ones; therefore, their
release of chemical mediators is easier in response to endogenous
or exogenous stimuli (2). MPV has
been observed to correlate with various thromboembolic disorders,
and increased MPV associated with thromboembolism has been reported
in patients with myocardial infarction, cerebrovascular
thromboembolism, diabetes mellitus and smokers (3–6). In
addition, the decrease of MPV has been previously reported in a
cancer patient with thromboembolic events who was undergoing
chemotherapy with bevacizumab (7).
Recent studies have also revealed that the MPV and MPV/platelet
count (PC) ratio can predict the long-term mortality in patients
with advanced non-small cell lung cancer (NSCLC) (8).
Vascular endothelial growth factor (VEGF) is primary
target in the antiangiogenic treatment of solid tumors (9). Clinical trials have shown that
treatment with antiangiogenic agents, including sorafenib,
sunitinib, bevacizumab and pazopanib, in advanced renal cell
carcinoma (RCC) has exhibited consistent prolongation of the
progression-free survival and, in certain cases, overall survival
in treatment-naive and previously treated patients (10).
The inhibition of angiogenesis is associated with an
increased risk of arterial thromboembolic events (ATE) and venous
thromboembolic events (VTE) (11).
VEGF receptor tyrosine kinase inhibitors (TKIs) may modulate the
activation of systemic coagulation in cancer patients, rendering
them more susceptible to thromboembolism (12,13).
Clinical trials have reported that bevacizumab was significantly
associated with an increased risk of developing VTE in patients
with cancer (14). In this
analysis, the incidence of all-grade and high-grade VTE was 11.9
and 6.3%, respectively. A meta-analysis to assess the risk of ATE
reported that treatment with sunitinib and sorafenib is associated
with a three-fold increase in the risk of ATE, with an overall
incidence of 1.3% in patients with RCC (15).
The aim of the current study was to evaluate whether
antiangiogenic TKIs, such as sunitinib, sorefenib and pazopanib,
have an effect on MPV values in patients with metastatic RCC.
Materials and methods
Patients
A total of 45 patients with metastatic RCC were
reviewed from the Department of Oncology, Akdeniz University
Hospital (Antalya, Turkey) and the Department of Oncology, Afyon
Kocatepe University Ahmet Necdet Sezer Research and Practice
Hospital (Afyon, Turkey) between May 2009 and September 2013,
retrospectively. All patients received interferon-α (IFN-α) therapy
until progression or intolerance prior to treatment with TKIs.
Following the treatment with IFN-α, antiangiogenic TKIs (sunitinib,
sorafenib or pazopanib) were offered to the all patients. This
study was approved by the ethics committee of Akdeniz University
(Antalya, Turkey) and written informed consent was obtained from
all patients.
Treatment plan
In a treatment group of metastatic RCC patients, the
MPV values prior to treatment and after three months of treatment
with sunitinib, sorafenib and pazopanib were compared.
Additionally, the platelet levels at the baseline and three months
were compared.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 20.0 (IBM, Armonk, NY, USA). The variables were
investigated using visual (histograms and probability plots) and
analytical (Kolmogorov-Simirnov/Shapiro-Wilk test) methods to
determine whether the values were normally distributed. The data
are presented as the mean ± standard deviation for normally
distributed variables (MPV measurements). Paired Student’s t-test
was used to compare the measurements at two time points (baseline
and three months) for MPV and PC. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
The mean follow-up duration was 40.8 months. The
median age of the patients was 63 years (range, 41–90). Of the
patients, 75.6% were male and 24.4% were female; 77.8% of patients
used sunitinib, 11.1% used sorafenib and 11.1% used pazopanib. The
remaining characteristics of the patients prior to treatment are
shown in Table I.
 | Table IBaseline characteristics of
patients. |
Table I
Baseline characteristics of
patients.
| Patients |
|---|
|
|
|---|
| Characteristics | n=45 | % |
|---|
| Gender, n |
| Female | 11 | 24.4 |
| Male | 34 | 75.6 |
| Age, years |
| Median | 63 |
| Range | 41–90 |
| Metastatic site,
n |
| Visceral | 24 | 53.3 |
| Bone | 8 | 17.8 |
| Bone and
visceral | 8 | 17.8 |
| Missing | 5 | 11.1 |
| ECOG performance
status, n |
| 0 | 5 | 13.2 |
| 1 | 14 | 36.8 |
| 2 | 11 | 28.9 |
| 3 | 8 | 21.1 |
| Tyrosine kinase
inhibitors, n |
| Sunitinib | 35 | 77.8 |
| Sorafenib | 5 | 11.1 |
| Pazopanib | 5 | 11.1 |
MPV values at baseline and three
months
MPV levels were normally distributed (P=0.372 and
P=0.615, respectively) according to the Shapiro-Wilk test
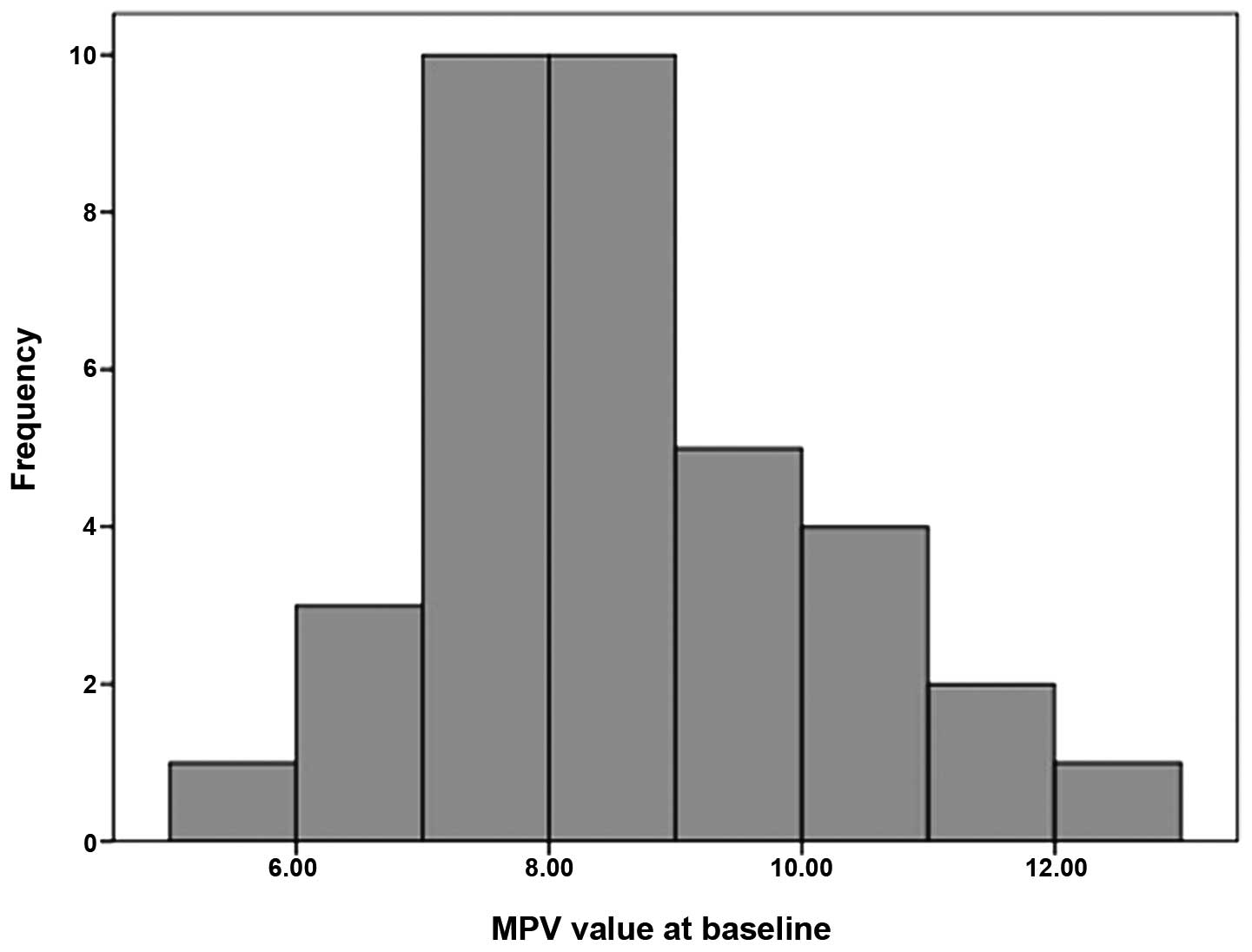
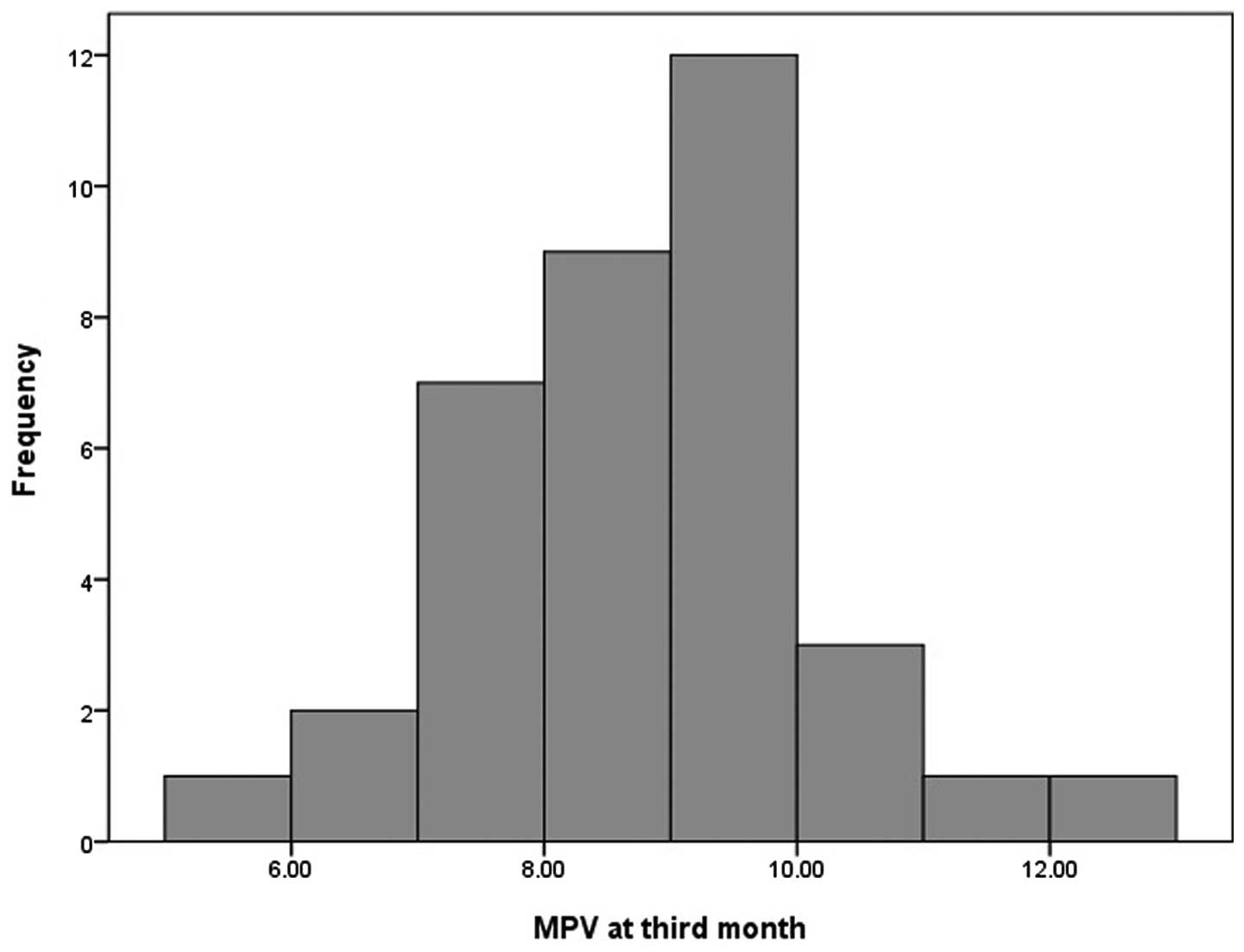
(n<50). Histograms for the MPV values are shown in Figs. 1 and 2. The mean MPV values at baseline and
three months were 8.53±1.44 and 8.75±1.30, respectively. MPV levels
increased with the treatment with TKIs; however, no statistically
significant difference was identified between MPV values at the
baseline and three months of the treatment (P=0.286). Conversely, a
statistically significant decrease in platelet levels was observed
following treatment (P=0.005). The values for PC and MPV are
presented in Table II.
 | Table IILevels of mean platelet volume and
platelet count. |
Table II
Levels of mean platelet volume and
platelet count.
| Parameters | Prior to treatment,
mean | Three months,
mean | P-value |
|---|
| Platelet count,
103/ml | 281.527±111.460 | 220.000±128.751 | 0.005 |
| Female | 293.750±91.610 | 223.875±201.447 | 0.167 |
| Male | 278.035±117.770 | 218.892±104.698 | 0.019 |
| Mean platelet volume,
fl | 8.52±1.43 | 8.75±1.30 | 0.286 |
| Female | 8.30±1.80 | 9.15±1.11 | 0.917 |
| Male | 8.57±1.34 | 8.63±1.34 | 0.738 |
Discussion
To the best of our knowledge, this study is the
first study to evaluate the MPV values in patients with metastatic
RCC, receiving antiangiogenic TKIs.
In the presence of increased MPV levels, wide
platelets with dense granules, containing increased thromboxane A2
may be observed in the blood. The in vitro response to
adenosine 50-diphosphate and collagen, as well as a tendency
towards aggregation, are also increased (16). Several reports have indicated that
an elevation of MPV is closely associated with the severity and
prognosis of cerebra-and cardiovascular disorders (16,17).
Osada et al (18) showed
that the MPV was higher in patients with gastric cancer than in
control patients. Another two trials demonstrated that the MPV and
MPV/PC ratio were elevated in patients with hepatocellular
carcinoma and NSCLC (7,19). By contrast, a study by Mutlu et
al (20) analyzed the MPV in
patients with metastatic colon cancer who were treated with
bevacizumab. A decrease in PC and MPV was identified during the
treatment period (8). Recently
Braekkan et al (21)
investigated MPV as a potential risk factor for VTE. The results
demonstrated that patients with an MPV of >9.5 had a
significantly (1.5-fold) increased risk of VTE, compared with an
MPV of <8.5. Antiplatelet drugs reduce the risk of arterial
cardiovascular events and VTE (21). MPV levels have been shown to be
decreased in patients with cancer in clinical trials (8,20). In
the current study, the MPV exhibited a tendency to be increased in
patients with metastatic RCC.
Bevacizumab is an antiangiogenic agent that has
exhibited activity as a cancer treatment; however, significant
adverse events, including hemorrhage and thrombosis, have also been
observed during treatment. A previous study demonstrated a decrease
in MPV levels in cancer patients who use chemotherapy regimens with
bevacizumab (7). The evidence for
the use of aspirin prophylaxis for ATE for patients using
bevacizumab is conflicting. Scappatici et al (22) reported marginally more grade 3 and 4
bleeding events among aspirin users on bevacizumab than in the
control subjects (3.7 vs. 1.8%). Conversely, a pooled analysis of
low-dose aspirin for primary prophylaxis for ATEs in patients
undergoing chemotherapy with bevacizumab did not identify any
increased bleeding risk (23).
Tebbutt et al (24)
demonstrated that the rate of ATE was moderately higher in patients
on aspirin in combination with bevacizumab. A clinical study
demonstrated a decrease in MPV during the treatment period with
bevacizumab (7). In the current
study, the MPV value was further increased in patients with
metastatic RCC. This result may be due to the different mechanisms
of action of bevacizumab and antiangiogenic TKIs.
According to the results of this study, MPV levels
were increased by the treatment with TKIs after three months;
however, the difference was not statistically significant. Further
studies are required to validate the use of TKIs to increase the
MPV values, which act as indicators of thrombocytic reactivity. We
hypothesize that the use of aspirin for thromboprophaxis may be of
additional benefit to these patients.
References
|
1
|
Thompson CB and Jakubowski JA: The
pathophysiology and clinical relevance of platelet heterogeneity.
Blood. 72:1–8. 1988.
|
|
2
|
Thompson CB, Jakubowski JA, Quinn PG,
Deykin D and Valeri CR: Platelet size and age determine platelet
function independently. Blood. 63:1372–1375. 1984.
|
|
3
|
Smith NM, Pathansali R and Bath PM:
Platelets and stroke. Vasc Med. 4:165–172. 1999.
|
|
4
|
Endler G, Klimesch A, Sunder-Plassmann H,
et al: Mean platelet volume is an independent risk factor for
myocardial infarction but not for coronary artery disease. Br J
Haematol. 117:399–404. 2002.
|
|
5
|
O’Malley T, Langhorne P, Elton RA and
Stewart C: Platelet size in stroke patients. Stroke. 26:995–999.
1995.
|
|
6
|
Tschoepe D, Roesen P, Esser J, et al:
Large platelets circulate in an activated state in diabetes
mellitus. Semin Thromb Hemost. 17:433–438. 1991.
|
|
7
|
Mutlu H, Berk V, Karaca H, et al:
Treatment regimen with bevacizumab decreases mean platelet volume
in patients with metastatic colon cancer. Clin Appl Thromb Hemost.
18:546–548. 2012.
|
|
8
|
Inagaki N, Kibata K, Tamaki T, Shimizu T
and Nomura S: Prognostic impact of the mean platelet
volume/platelet count ratio in terms of survival in advanced
non-small cell lung cancer. Lung Cancer. 83:97–101. 2013.
|
|
9
|
Willett CG, Boucher Y, di Tomaso E, et al:
Direct evidence that the VEGF-specific antibody bevacizumab has
antivascular effects in human rectal cancer. Nat Med. 10:145–147.
2004.
|
|
10
|
Cohen RB and Oudard S: Antiangiogenic
therapy for advanced renal cell carcinoma: management of
treatment-related toxicities. Invest New Drugs. 30:2066–2079.
2012.
|
|
11
|
Elice F, Rodeghiero F, Falanga A and
Rickles FR: Thrombosis associated with angiogenesis inhibitors.
Best Pract Res Clin Haematol. 22:115–128. 2009.
|
|
12
|
Kuenen BC, Levi M, Meijers JC, et al:
Analysis of coagulation cascade and endothelial cell activation
during inhibition of vascular endothelial growth factor/vascular
endothelial growth factor receptor pathway in cancer patients.
Arterioscler Thromb Vasc Biol. 22:1500–1505. 2002.
|
|
13
|
Kuenen BC: Analysis of prothrombotic
mechanisms and endothelial perturbation during treatment with
angiogenesis inhibitors. Pathophysiol Haemost Thromb. 33:13–14.
2003.
|
|
14
|
Nalluri SR, Chu D, Keresztes R, Zhu X and
Wu S: Risk of venous thromboembolism with the angiogenesis
inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA.
300:2277–2285. 2008.
|
|
15
|
Choueiri TK, Schutz FA, Je Y, Rosenberg JE
and Bellmunt J: Risk of arterial thromboembolic events with
sunitinib and sorafenib: a systematic review and meta-analysis of
clinical trials. J Clin Oncol. 28:2280–2285. 2010.
|
|
16
|
Greisenegger S, Endler G, Hsieh K, et al:
Is elevated mean platelet volume associated with a worse outcome in
patients with acute ischemic cerebrovascular events? Stroke.
35:1688–1691. 2004.
|
|
17
|
Smith NM, Pathansali R and Bath PM:
Platelets and stroke. Vasc Med. 4:165–172. 1999.
|
|
18
|
Osada J, Rusak M, Kamocki Z, Dabrowska MI
and Kedra B: Platelet activation in patients with advanced gastric
cancer. Neoplasma. 57:145–150. 2010.
|
|
19
|
Cho SY, Yang JJ, You E, et al: Mean
platelet volume/platelet count ratio in hepatocellular carcinoma.
Platelets. 24:375–377. 2012.
|
|
20
|
Mutlu H, Artis TA, Erden A and Akca Z:
Alteration in mean platelet volume and platicrit values in patients
with cancer that developed thrombosis. Clin Appl Thromb Hemost.
19:331–333. 2013.
|
|
21
|
Braekkan SK, Mathiesen EB, Njølstad I,
Wilsgaard T, Størmer J and Hansen JB: Mean platelet volume is a
risk factor for venous thromboembolism: the Tromsø Study, Tromsø,
Norway. J Thromb Haemost. 8:157–162. 2010.
|
|
22
|
Scappaticci FA, Skillings JR, Holden SN,
et al: Arterial thromboembolic events in patients with metastatic
carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer
Inst. 99:1232–1239. 2007.
|
|
23
|
Hambleton J, Skillings J, Kabbinavar F, et
al: Safety of low-dose aspirin (ASA) in pooled analysis of 3
randomized, controlled trials (RCTs) of bevacizumab (BV) with
chemotherapy (CT) in patients (pts) with metastatic colorectal
cancer (mCRC). J Clin Oncol. 23:16S(abstr 3554). 2005.
|
|
24
|
Tebbutt NC, Murphy F, Zannino D, et al:
Australasian Gastro-Intestinal Trials Group: Risk of arterial
thromboembolic events in patients with advanced colorectal cancer
receiving bevacizumab. Ann Oncol. 22:1834–1838. 2011.
|