Introduction
Intracranial hemangiopericytoma (HPC) is a rare
tumor well-known for clinically aggressive behavior in growth and
infiltration. HPC accounts for 0.4% of all primary central nervous
system tumors (1). HPC is
considered to arise from the pericytes of the capillaries and has
certain similar features to other types of tumor (2). The 2007 World Health Organization
(WHO) classification has divided intracranial HPC into two separate
categories: WHO Grade II HPC and WHO Grade III anaplastic HPC
(3). Surgical resection is the
standard treatment for HPC, however, the procedure presents a
challenge as it may lead to extensive blood loss (4). Previosu studies have indicated that
tumor recurrence is common in HPC patients and thus, radiotherapy
is used to treat recurrent HPC (5).
The radiological appearance of HPC resembles that of meningioma,
but the pathological features resemble those of solitary fibrous
tumors (6). The present study
describes five cases of intracranial HPC diagnosed by pathology
with the aim of comparing the radiological and pathological
features.
Materials and methods
Patient information
A total of five pathologically proven cases of
intracranial HPC were collected between May 2006 and March 2012 in
the Second Xiangya Hospital of Central South University (Changsha,
China). Of the five cases, four were classified as WHO Grade II and
one was classified as WHO Grade III. All cases had undergone
magnetic resonance imaging (MRI) examination. The images were
evaluated by two radiologists and the final diagnosis was confirmed
following evaluation of the specimens by two pathologists. The
procedures followed in the present study were in accordance with
the ethics committee of the Second Xiangya Hospital of Central
South University and written informed consent was obtained from all
patients. All the cases were followed up for between one and seven
years, and each case enrolled in this study was kept anonymous
following the retrieval of the follow-up information.
MRI
GE Signa 1.5 T superconducting MRI (GE Healthcare,
Little Chalfont, USA) was performed using a standard head coil,
with a thickness of 5 mm and a layer distance of 1.5 mm, using
spin-echo T1-weighted image [T1WI; repetition time (TR), 400–500
msec; echo time (TE), 15–30 msec] and fast spin-echo T2WI (TR,
3,000–4,500 msec; TE, 70–120 msec). Gadolinium diethylenetriamine
penta-acetic acid (Gd-DTPA) contrast agent was adopted at dose, 0.1
mmol/kg; injection flow rate, 3 ml/sec; and scan parameter, fat
suppression Flair T1WI (TR, 2,000–2,500 msec; TE, 7–13 msec).
Pathological and immunohistochemical
analysis
All cases underwent total resection of the tumor.
The specimen was fixed in 4% neutral formalin, dehydrated and
embedded in paraffin. Subsequently the sample was cut into 2.5 μm
slices and underwent routine hematoxylin-eosin staining and
immunohistochemical analysis of cluster of differentiation 34
(CD34), CD99, vimentin (Vim), S-100, epithelial membrane antigen
(EMA), glial fibrillary acidic protein (GFAP), Ki-67 and synapsin
(Syn) (Maxin-Bio, Co., Fuzhou, China) expression.
Results
Clinical findings
A total of five cases (three males and two females;
age range, 37–60 years) were enrolled. Headache (n=5) and dizziness
(n=4) were the most common presenting symptoms, followed by
vomiting (n=2), weakness (n=1) and blurred vision (n=1). The
routine laboratory findings were non-specific. During the follow-up
period, one case recurred within four years of tumor resection;
however, no cases developed metastases during the follow-up period.
The clinical findings of each case are listed in Table I.
 | Table IClinical findings from the five
intracranial hemangiopericytoma cases. |
Table I
Clinical findings from the five
intracranial hemangiopericytoma cases.
| Patient | Age | Sex | Recurrence | Headache | Dizziness | Vomiting | Weakness | Blurred vision |
|---|
| 1 | 48 | Male | No | Yes | Yes | Yes | No | Yes |
| 2 | 60 | Male | Yes | Yes | Yes | No | Yes | No |
| 3 | 56 | Female | No | Yes | No | No | No | No |
| 4 | 37 | Female | No | Yes | Yes | No | No | No |
| 5 | 41 | Male | No | Yes | Yes | Yes | No | No |
MRI findings
All cases were misdiagnosed as meningioma prior to
surgery. MRI revealed that all five cases had a single lesion (in
four cases located above the tentorium cerebelli; in one case
located under the tentorium cerebelli). The lesions were lobular,
measuring 3.0 to 7.5 cm with an iso-intense signal in T1WI and a
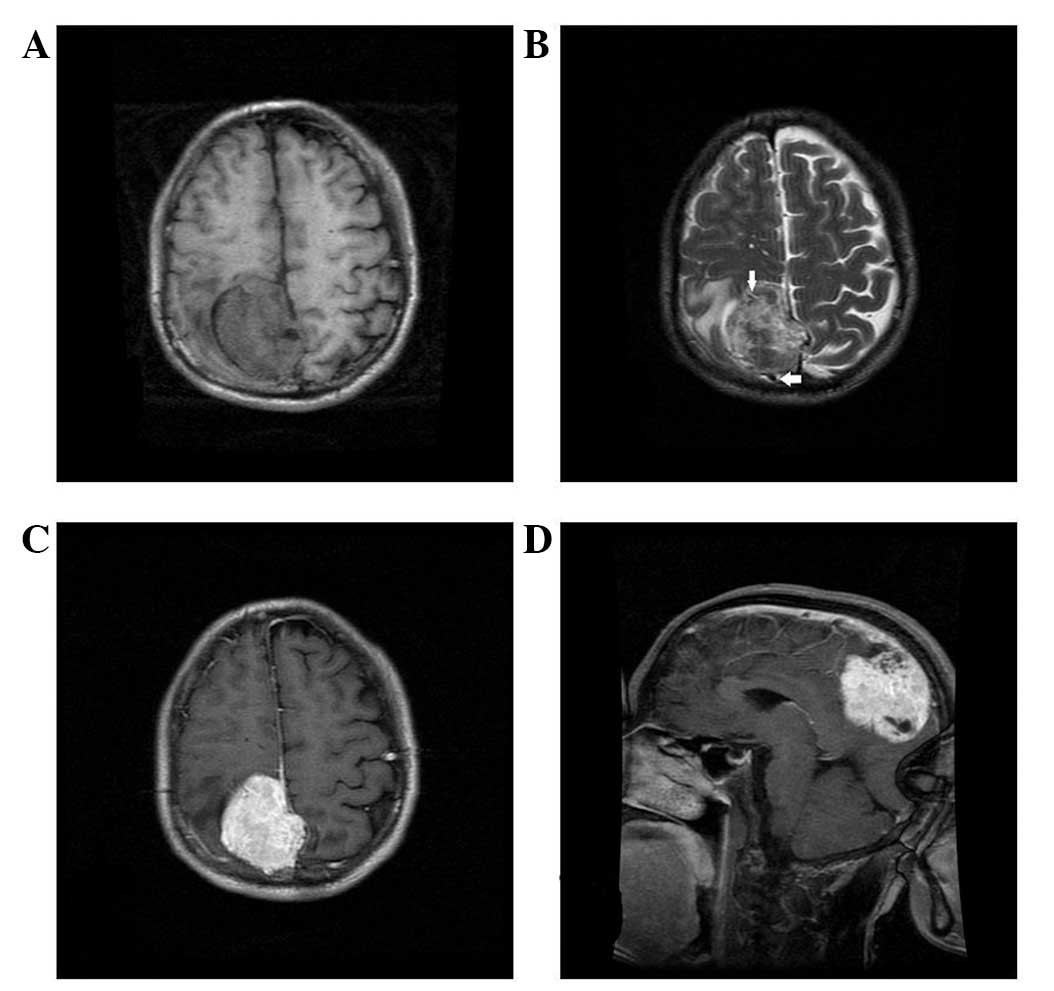
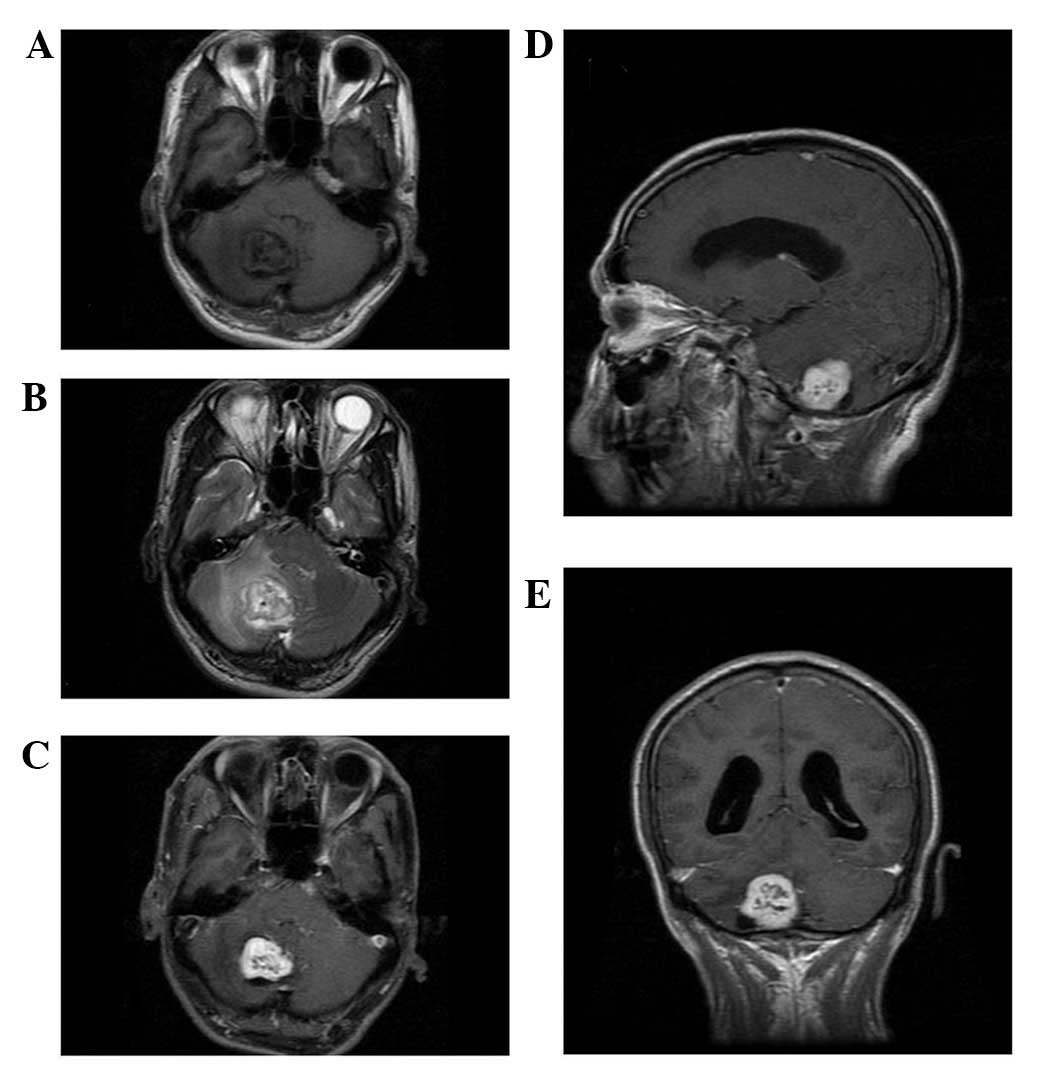
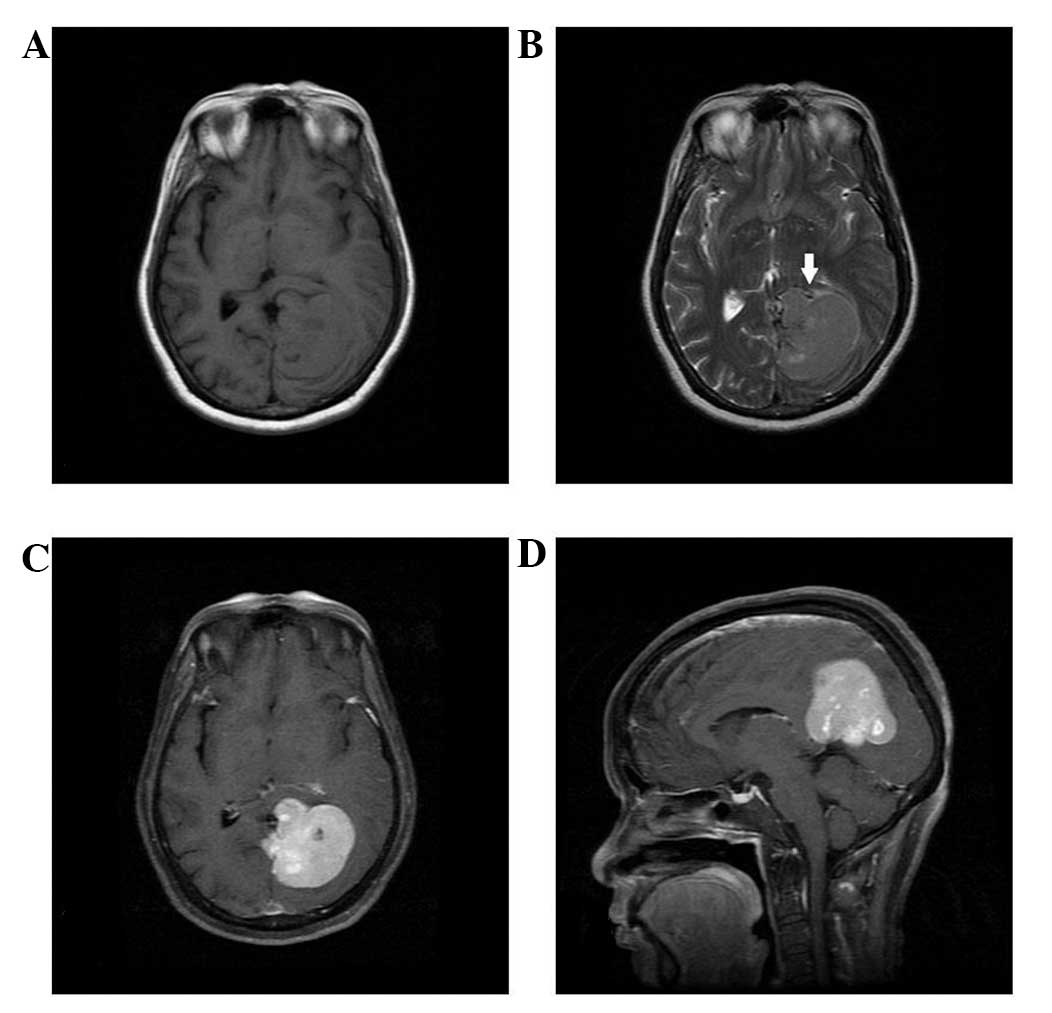
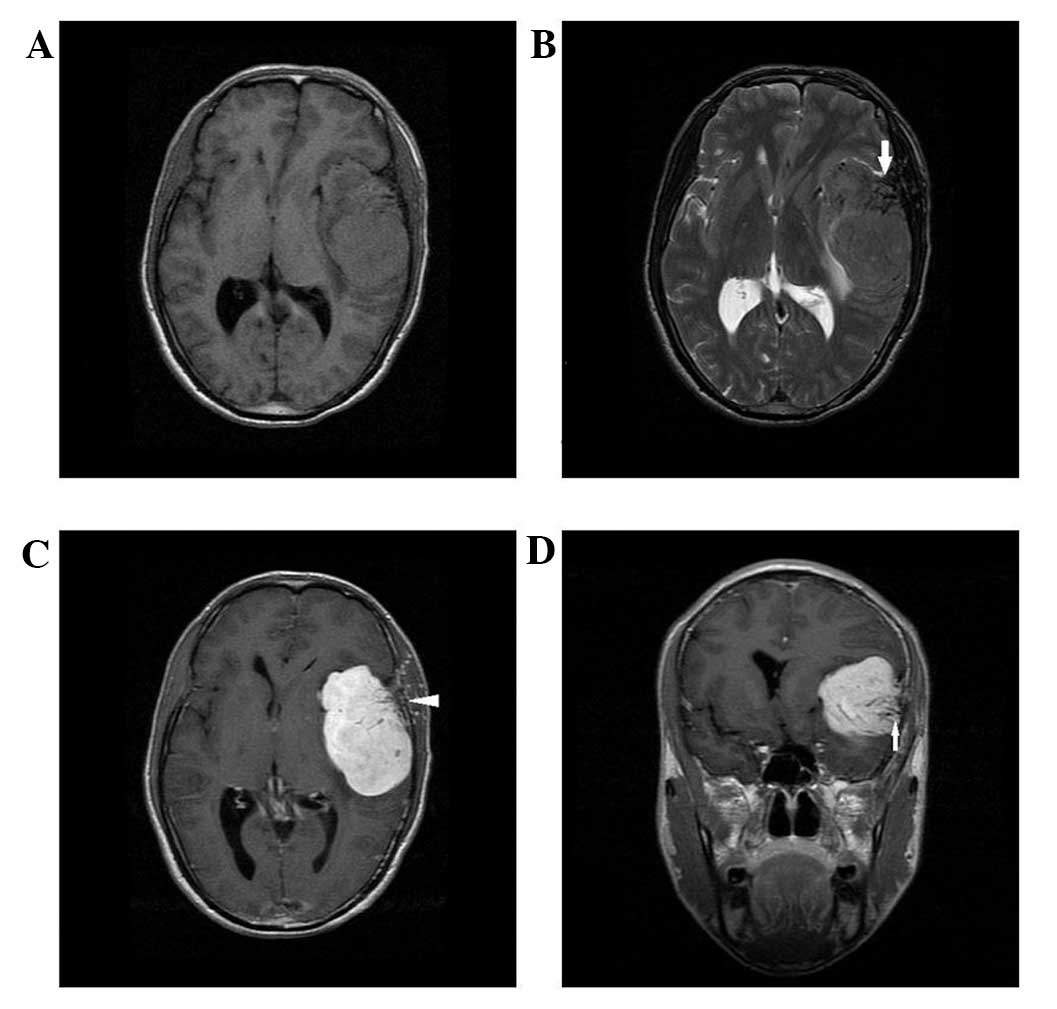
slightly long signal in T2WI on the unenhanced MRI scan (Figs. 1–5).
Four cases presented with a cross-midline growth pattern (Figs. 1, 3–5) and
one case presented with a cross-lobe growth pattern (Fig. 2). One case exhibited dilatation of
the lateral ventricle as the tumor compressed the fourth ventricle
(Fig. 5). The adjacent bone was
destroyed in one case (Fig. 2).
Following injection of Gd-DTPA, no cases were found to exhibit the
dural tail sign. Heterogeneous enhancement was observed in all
cases (Figs. 1–5). Cystic degeneration, necrosis as well
as flow void were observed in all cases (Figs. 1–5).
The detailed MRI findings are listed in Table II.
 | Table IIMagnetic resonance imaging findings
from the five intracranial hemangiopericytoma cases. |
Table II
Magnetic resonance imaging findings
from the five intracranial hemangiopericytoma cases.
| Patient |
|---|
|
|
|---|
| Tumor
characteristic | 1 | 2 | 3 | 4 | 5 |
|---|
| Size | 4.1 cm | 5.3 cm | 4.0 cm | 7.5 cm | 3.0 cm |
| Margin | Well-defined | Ill-defined | Well-defined | Well-defined | Well-defined |
| Morphology | Lobulated | Lobulated | Lobulated | Lobulated | Lobulated |
| Location | Right occipital
region | Left temporal
region | Left occipital
region | Anterior skull
base | Infratentorial
posterior fossa |
| Growth pattern | Cross-midline | Cross-lobe | Cross-midline | Cross-midline | Cross-midline |
| Enhancement
pattern | Heterogeneous | Heterogeneous | Heterogeneous | Heterogeneous | Heterogeneous |
| Bony destruction | No | Yes | No | No | No |
| Dural tail sign | No | No | No | No | No |
| Narrow-based
attachment | Yes | Yes | Yes | Yes | Yes |
| Intratumoral
vessels | Yes | Yes | Yes | Yes | Yes |
Pathological and immunohistochemical
findings
Upon gross examination, the cut surfaces of the
tumors were gray in color and fish-like in texture. The boundaries
were clear with a complete or incomplete capsule. On microscopic
examination, the tumor cells were shown to exhibit diffuse growth
with abundant slit-shaped vessels in the central area. The cells
were of uniform size with obscured nucleoli (Fig. 6A and B). The nuclei of the tumor
cells were oval and mitotic figures were occasionally observed
(Fig. 6C). No case exhibited the
intranuclear inclusions that are relatively specific to meningioma.
Calcification was only found in one case. Immunohistochemical
analysis revealed a marked positive expression of CD34 (Fig. 6C), CD99 and Vim but negative
expression of EMA (Fig. 6D), S100
and GFAP. Proliferating cell nuclear antigen Ki-67
immunohistochemical staining revealed that <5% of cells
expressed Ki-67 in two cases and 5–10% of cells expressed Ki-67 in
three cases. Syn staining revealed no expression in all cases.
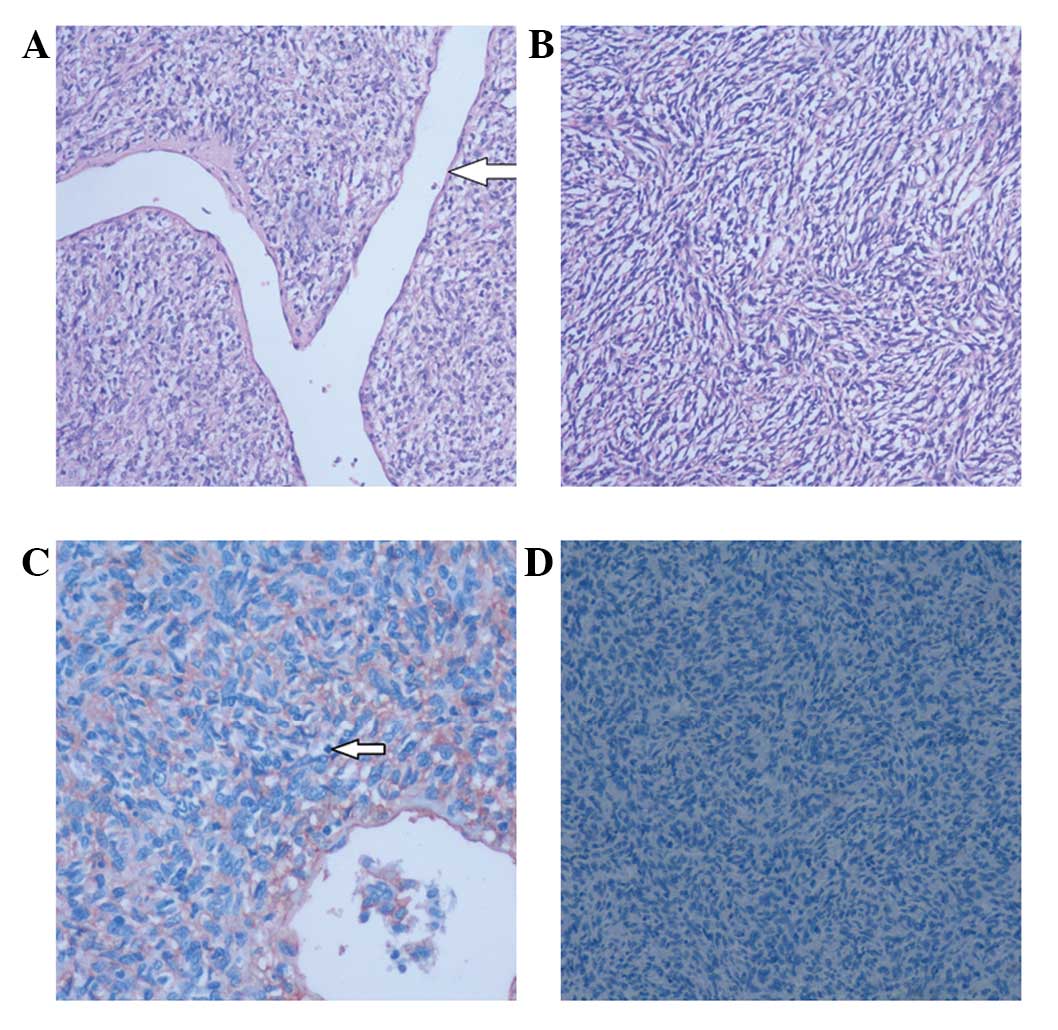 | Figure 6Patient 3.Pathological features of
intracranial hemangiopericytoma. (A) On microscopic examination,
tumor cells exhibit a diffuse growth pattern with abundant
slit-shaped vessels in the central area (arrow). (B) The tumor
cells are uniform in size with obscured nucleoli. (C) The nuclei of
the tumor cells are oval and mitotic figures are occasionally
observed (arrow). Immunohistochemical analysis reveals (C) marked
positive expression of cluster of differentiation 34, but (D)
negative expression of epithelial membrane antigen [stain, (A and
B) hematoxylin and eosin, (C) cluster of differentiation 34, (D)
epithelian membrane antigen; magnification, A, ×100; B, ×100; C,
×200; D, ×200]. |
Discussion
Intracranial HPC is a rare tumor with aggressive
behavior. HPC is usually known to occur in the musculoskeletal
system and has been less frequently reported to occur in the
central nervous system. The morbidity of HPC accounts for <1% of
all intracranial tumors and ~2–4% of all meningeal tumors,
worldwide (7). Owing to the unknown
origin, HPC was hypothesized to originate from the meninges and
thus was previously considered to be a subtype of meningioma.
However, HPC is currently hypothesized to originate from Zimmermann
pericytes (8).
Intracranial HPC usually occurs more commonly in
males than females and the average age of presentation in patients
with HPC was identified to range between 38 and 42 years in a
previous series (9), which is
similar to the findings of the present study. The symptoms of this
type of tumor are non-specific and, furthermore, are similar to
those of other types of tumors, such as meningeal meningoma
(10). In general, HPC symptoms
depend on the tumor size, extent and position. In the present
cohort, the main symptoms were headache (n=5), dizziness (n=4),
vomiting (n=2), weakness (n=1) and blurred vision (n=1). Routine
laboratory tests revealed no specific findings.
On gross pathological examination, the lesion may
manifest as a solitary nodule with a complete or incomplete
capsule. In a study conducted by Zhou et al (11) examining 39 cases intracranial HPC
and anaplastic HPC, the majority of the anaplastic HPC cases
presented with an incomplete capsule and ill-defined boundary, but
intracranial HPC had a complete capsule and clear boundary. The
results of the present study are similar to those findings. Another
previous study demonstrated that HPC more commonly occurs in the
frontoparietal region (12).
However, the present study observed no specific tumor location in
all patients.
Intracranial HPC has a rich blood supply; marked
heterogeneous enhancement was detected in the cases in the present
study, which may also be explained by the pathological
characteristics. On microscopic examination, the tumor cells
exhibited diffuse growth patterns with abundant slit-shaped
vessels. Intratumoral vessels were detected in all cases, which
were indicated by flow voids on the MRI scans. This feature may be
characteristic of HPC. In the present study, mitotic figures were
occasionally detected. This feature indicates that intracranial HPC
exhibits an aggressive behavior that results in recurrence and
metastasis.
A previous study reported that HPC cells were
strongly immunopositive for Vim, but negative for EMA, with CD34
expression focally positive and the endothelial cells always
positive for CD34 (13). The
results of the present study concurred with these findings.
To produce a correct preoperative diagnosis of
intracranial HPC is difficult. In the present study, all cases were
misdiagnosed as meningioma prior to surgery. Furthermore, the MRI
features of HPC appear similar to those of meningioma. However,
certain specific signs of intracranial HPC that are different from
those of meningioma were identified in the present study. For
example, flow void appears to be more common in intracranial HPC
than in meningioma, as intracranial HPC has a richer blood supply
and abundant slit-shaped vessels. In the present study, the growth
patterns of intracranial HPC were as follows: Crossing the midline
(n=4) and crossing the lobe (n=1) with a lobulated shape, which
indicated that the intracranial lesions exhibited an invasive
growth pattern. Compared with intracranial HPC, the growth pattern
of meningioma appears to be more localized and the shape more
regular. Furthermore, intracranial HPC exerts a destructive effect
on the adjacent bone, unlike meningioma, which exerts a
hyperplastic effect (14). This
feature indicates that intracranial HPC exhibits a marked
propensity for invasiveness. In addition, no case exhibited the
dural tail sign in the present study. A previous study reported
that dural tail sign was associated with the long-term response to
the stimulation of the meninges by the tumor (15). Intracranial HPC is classified as WHO
Grade II or III, and exhibits a rapid tumor growth rate and high
malignant characteristics, therefore the dural tail sign is less
common. Furthermore, a narrow dural attachment is another feature
that differentiates intracranial HPC from meningioma (16). Intracranial HPC exhibits a narrow
dural attachment, which is due to the malignant behavior of the
tumor. However, meningioma commonly has a wide dural
attachment.
Surgical resection of the tumor is the primary
treatment choice in order to obtain a definitive diagnosis as well
as to relieve symptoms (4). A
cohort study conducted by Kumar et al (17) suggested that the main therapy for
intracranial HPC was gross total resection combined with
postoperative radiotherapy. In the present study, all cases
underwent surgical resection combined with radiotherapy. In the
follow-up period, only one case recurred within four years. Thus, a
long-term follow-up is reasonable for the timely detection of
recurrence.
In conclusion, intracranial HPC exhibits particular
characteristics of WHO Grade II or III tumors, which are similar to
those of meningioma. However, certain features may aid in
differentiating intracranial HPC from meningioma. The flow void is
a relatively specific sign common in intracranial HPC due to the
rich blood supply. The growth pattern of intracranial HPC appears
to be irregular with a lobulated shape. Adjacent bone erosion may
also occasionally be identified in patients with intracranial HPC.
In addition, a narrow dural attachment suggests a diagnosis of
intracranial HPC rather than meningioma. Nevertheless, imaging
alone should not be used to diagnose intracranial HPC; pathological
examination is required for confirmation.
Abbreviations:
|
HPC
|
hemangiopericytoma
|
|
MRI
|
magnetic resonance imaging
|
|
Vim
|
vimentin
|
|
EMA
|
epithelial membrane antigen
|
|
GFAP
|
glial fibrillary acidic protein
|
|
WHO
|
World Health Organization
|
References
|
1
|
Rutkowski MJ, Jian BJ, Bloch O, et al:
Intracranial hemangiopericytoma: clinical experience and treatment
considerations in a modern series of 40 adult patients. Cancer.
118:1628–1636. 2012.
|
|
2
|
Holland H, Livrea M, Ahnert P, et al:
Intracranial hemangiopericytoma: Case study with cytogenetics and
genome wide SNP-A analysis. Pathol Res Pract. 207:310–316.
2011.
|
|
3
|
Kleihues P and Cavenee WK: World Health
Organization Classification of Tumours. Pathology and Genetics of
Tumours of the Nervous System. IARC Press; Lyon: 2000
|
|
4
|
Fountas KN, Kapsalaki E, Kassam M, et al:
Management of intracranial meningeal hemangiopericytoma: outcome
and experience. Neurosurg Rev. 29:145–153. 2006.
|
|
5
|
Olson C, Yen CP, Schlesinger D and Sheehan
J: Radiosurgery for intracranial hemangiopericytoma: outcomes after
initial and repeat Gamma Knife surgery. J Neurosurg. 112:133–139.
2010.
|
|
6
|
Hori E, Kurimoto M, Fukuda O, et al:
Recurrent intracranial solitary fibrous tumor initially diagnosed
as hemangiopericytoma. Brain Tumor Pathol. 24:31–34. 2007.
|
|
7
|
Kumar R and Wani AA: Unusual tumors of the
posterior fossa skull base. Skull Base. 16:75–84. 2006.
|
|
8
|
Stout AP and Murray MR:
Hemangiopericytoma: a vascular tumor featuring Zimmerman’s
pericytes. Ann Surg. 116:26–33. 1942.
|
|
9
|
Brunori A, Delitala A, Oddi G and
Chiappetta F: Recent experience in the management of meningeal
hemangiopericytomas. Tumori. 83:856–861. 1997.
|
|
10
|
Schiariti M, Goetz P, El-Maghraby H,
Tailor J and Kitchen N: Hemangiopericytoma: long-term outcome
revisited clinical article. J Neurosurg. 114:747–755. 2011.
|
|
11
|
Zhou JL, Liu JL, Zhang J and Zhang M:
Thirty-nine cases of intracranial hemangiopericytoma and anaplastic
hemangiopericytoma: A retrospective review of MRI features and
pathological findings. Eur J Radiol. 81:3504–3510. 2012.
|
|
12
|
Wu W, Shi JX, Cheng HL, et al:
Hemangiopericytomas in the central nervous system. J Clin Neurosci.
16:519–523. 2009.
|
|
13
|
Alén JF, Lobato RD, Gómez PA, et al:
Intracranial Hemangiopericytoma: Study of 12 Cases. ACTA Neurochir.
143:575–586. 2001.
|
|
14
|
Shetty PM, Moiyadi AV and Sridhar E:
Primary CNS hemangiopericytoma presenting as an intraparenchymal
mass - case report and review of literature. Clin Neurol Neurosurg.
112:261–264. 2010.
|
|
15
|
Spatola C and Privitera G: Recurrent
intracranial hemangiopericytoma with extracranial and unusual
multiple metastases: case report and review of the literature.
Tumori. 90:265–268. 2004.
|
|
16
|
Bonde VR and Goel A: Two patients with
intracavernous hemangiopericytoma. J Clin Neurosci. 16:330–333.
2009.
|
|
17
|
Kumar N, Kumar R, Kapoor R, et al:
Intracranial meningeal hemangiopericytoma: 10 years experience of a
tertiary care institute. ACTA Neusrochir. 154:1647–1651. 2012.
|