Introduction
Cancer chemotherapy is frequently accompanied by
severe emesis. Cisplatin is an emetic, platinum-based
chemotherapeutic agent that has been widely used to treat various
malignancies. Emetic agents, including cisplatin, have been shown
to induce taste aversion, kaolin ingestion behavior (pica) and
anorexic behavior in a diverse range of species (1–3).
Rodents, such as rats, are incapable of vomiting, however,
investigations have demonstrated that rats readily exhibit
analogous behaviors in response to cisplatin administration. These
behaviors were associated with multiple neuropharmacological
mechanisms, including the dopamine D2 receptors in the
chemoreceptor trigger zone, the 5-HT3 receptors in the
visceral afferents of the stomach wall (4), the vagal afferents of the common
hepatic branch (5), the midbrain
and brainstem (6), the hippocampus,
hypothalamus, and medulla oblongata (7) and the emetic system of the lateral
parabrachial nucleus (8).
Therefore, the predominant species for investigating emesis has
been the laboratory rat (9–12).
Rats exhibit pica in response to stimuli, which
ordinarily induce emesis in species that posess an emetic reflex
(13), hence, kaolin consumption
has been evaluated as an index of cisplatin-induced emesis in rats.
It has also been demonstrated, using rotation stimulation, that
pica and conditioned taste aversion (CTA) are behavioral changes,
which are specific to rats (14).
Previously, cisplatin was used as the standard emetic agent in
studies of CTA learning in rats (15,16),
with saccharin solution consumption, to indicate CTA, used as an
alternative index of emesis in rats. Anorexia nervosa is one of the
most unwanted side-effects in cancer patients who are undergoing
chemotherapy. It was found that rats exhibited anorexic behavior in
response to cisplatin administration (5,17);
therefore, normal feed intake has been evaluated in the present
study as a third index of cisplatin-induced emesis in rats.
Cisplatin administration causes acute and delayed
nausea and vomiting via different mechanisms. Although the gastric
retention of solid material (18),
as well as radiological methods (19), have been reported as novel
indicators for predicting the potential of compounds that induce
emesis in non-vomiting rodents; pica, CTA and normal feed
consumption are commonly used as markers in investigations of
emesis. However, which of these markers is most suitable for
assessing the availability of cisplatin-induced acute or delayed
vomiting remains unclear. Thus, the aim of the current study was to
investigate which index, of kaolin, saccharin solution or normal
feed consumption, is the most suitable for evaluating the various
phases of cisplatin-induced emesis.
Materials and methods
Animals
Sixty adult Wistar strain rats (equal numbers of
males and females), weighing between 200 and 220 g, were purchased
from The Shanghai Slac Laboratory Animal Company Ltd. (Shanghai,
China) and acclimated to the laboratory environments for one week
prior to the investigation. Rats were housed in a polycarbonate
cage (45×35×25 cm), and maintained under controlled conditions of
temperature (22±2°C), relative humidity (55±10%) and lighting (12-h
light/dark cycle). All animals were allowed free access to kaolin,
saccharin solution and standard rat chow pellets (Shanghai Slac
Laboratory Animal Company Ltd.). The standard rat chow pellets,
kaolin and saccharin were all placed in separate containers
throughout the experiment to prevent them mixing and to enable the
more accurate calculation of weight. All cages were fitted with a
wire mesh floor to permit the collection of spilt kaolin and feed.
This study was approved by the ethics committee of the Second
Military Medical University (Shanghai, China).
Materials
Kaolin was prepared using a standard protocol from
the literature with slight modifications (13,20,21).
Briefly, 50 g pharmacological grade kaolin (China Clay [naturally
hydrated aluminum silicate]; Tianjin Third Chemical Reagent
Factory, Tianjin, China) was mixed with 1 g of acacia (gum arabic;
Guangfu Fine Chemical Research Institute, Tianjin, China) (50:1
ratio) with distilled water to form a thick paste. The paste was
extruded through a syringe onto wire mesh trays to dry partially
and was then introduced into a column, which was the same shape as
the rat chow pellets. The kaolin pellets were placed on plastics
trays and completely dried at room temperature for 72 h.
The saccharin (0.15% w/v) and granisetron solutions
were premixed using deionized water. Reagent grade granular sodium
saccharin anhydrous (Northern Food Co., Ltd., Tianjin, China) and
granisetron hydrochloride dispersible tablets (1 mg/tablet; Guangxi
Pubei Pharmaceutical Factory, Guangxi, China) were used.
Procedures
Male and female rats were randomly divided into
three groups; blank, control and granisetron, each consisting of 20
animals. Kaolin pellets and saccharin solution were placed on the
stainless steel grid cover of the cage for six days (days 1–6)
prior to cisplatin injection to allow the rats to adapt to its
presence, and all animals received intragastric administration of
physiological saline for three days (days 4–6; dosage, 2 ml/day),
prior to administration of the cisplatin injection. Between days 7
and 9, the granisetron group received intragastric administration
of the granisetron solution (2.7 mg/kg body weight; dosage, 2
ml/day), whilst the other two groups continued to receive
physiological saline. On day 7, cisplatin (6 mg/kg via
intraperitoneal [i.p.] injection) was administered to all rats,
with the exception of the blank group, 1 h subsequent to the
intragastric administration of granisetron. The kaolin, saccharin
solution and normal feed containers were removed each day (at 9:00
am). The kaolin and normal feed was collected and weighed, and the
weight of the bottle of saccharin was also determined. The quantity
of kaolin, normal feed and saccharin solution consumed during each
24 h period was determined by comparing the weights with the
initial weights. The following formula was used to determine the
ultimate weight value: Ultimate weight value (day n) = weight (day
n) − weight (day n+1). The total observation period was 72 h.
Statistical analysis
Data are presented as the mean values ± standard
deviation. Kaolin, saccharin solution and normal feed consumption
were compared between the three groups using a two-way repeated
measures analysis of variance followed by Fisher’s least
significant difference multiple comparison tests. Data regarding
the heterogeneity of variance or abnormal distribution were
compared using a Kruskal-Wallis rank sum test followed by Nemenyi
multiple comparison tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
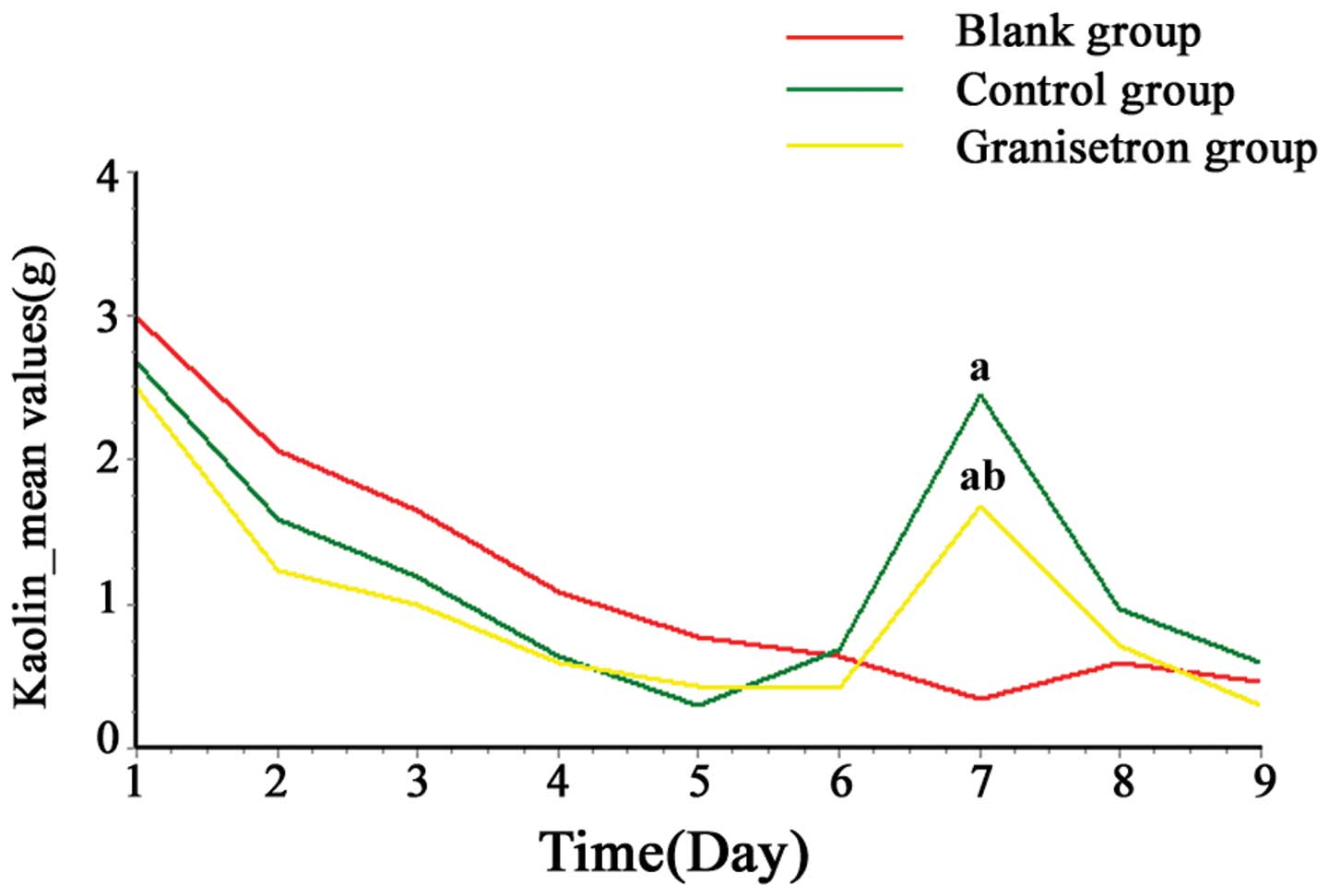
Kaolin consumption in rats
On days 1 and 2, all of the rats showed abnormal
pica behavior, however, kaolin consumption gradually returned to
the control level (~0.3–0.7 g/day), which was recorded prior to the
start of the experiment. No significant difference was identified
among the three groups between days 1 and 6 (P>0.05). As
demonstrated by Table I and
Fig. 1, following the cisplatin
injection, kaolin consumption in the control and granisetron groups
increased significantly when compared with that of the blank group
at 24 h (day 7; P<0.01), however not at 48 h (day 8; P=0.2658)
or 72 h (day 9; P=0.1222). The increase in kaolin consumption
peaked and was attenuated by granisetron 24 h after cisplatin
administration (day 7; P<0.05).
 | Table IKaolin consumption induced by
administration of cisplatin in rats. |
Table I
Kaolin consumption induced by
administration of cisplatin in rats.
| | Kaolin consumption
(g) |
|---|
| |
|
|---|
| Group | n | 0–24 h | 24–48 h | 48–72 h |
|---|
| Blank | 20 | 0.35±0.46 | 0.59±0.73 | 0.46±0.56 |
| Control | 20 | 2.45±1.46a | 0.96±0.86 | 0.59±0.48 |
| Granisetron | 20 | 1.68±0.56a,b | 0.72±0.55 | 0.30±0.18 |
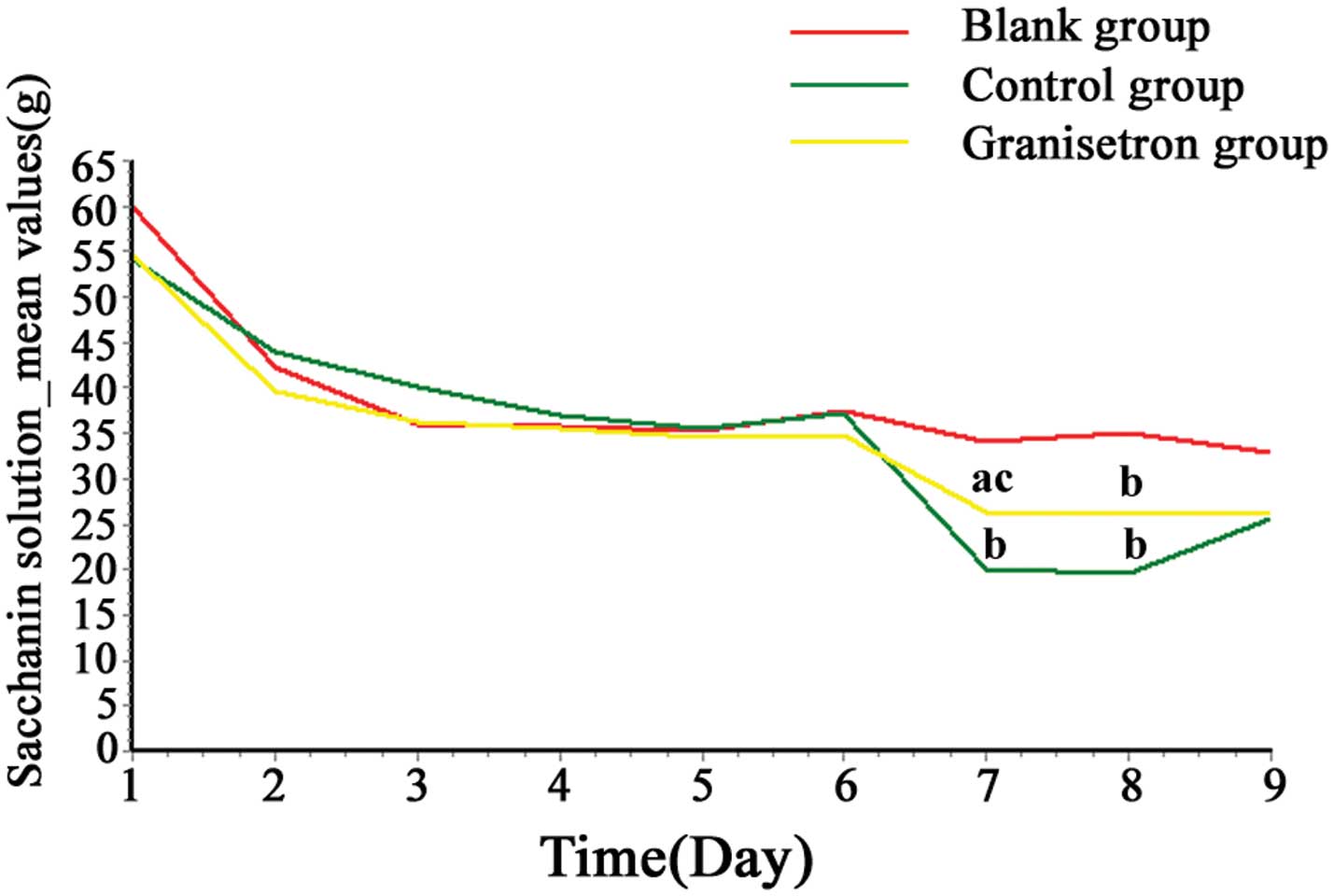
Saccharin solution consumption in
rats
On day 1, saccharin solution consumption increased
in all of the rats (~55–60 g/day), however, consumption returned to
control level (~35–45 g/day) from day 2. During the adaptation
period (days 1–6), there was no significant difference observed in
saccharin solution consumption between the three groups
(P>0.05). As demonstrated by Table
II and Fig. 2, following the
cisplatin injection, saccharin solution consumption in the control
and granisetron groups decreased significantly when compared with
the blank group at 24 and 48 h (day 7, P<0.05; day 8;
P<0.01), although not at 72 h (day 9; P>0.05). Furthermore
this decrease in saccharin solution consumption was attenuated by
granisetron at 24 h (day 7; P<0.05), although not at 48 h (day
8; P>0.05).
 | Table IISaccharin solution consumption induced
by administration of cisplatin in rats. |
Table II
Saccharin solution consumption induced
by administration of cisplatin in rats.
| | Saccharin solution
consumption (g) |
|---|
| |
|
|---|
| Group | n | 0–24 h | 24–48 h | 48–72 h |
|---|
| Blank | 20 | 34.18±9.99 | 34.95±11.41 | 32.78±9.65 |
| Control | 20 | 20.15±10.06b | 19.57±11.47b | 25.64±14.46 |
| Granisetron | 20 | 26.27±8.27a,c | 26.03±7.27b | 26.09±12.03 |
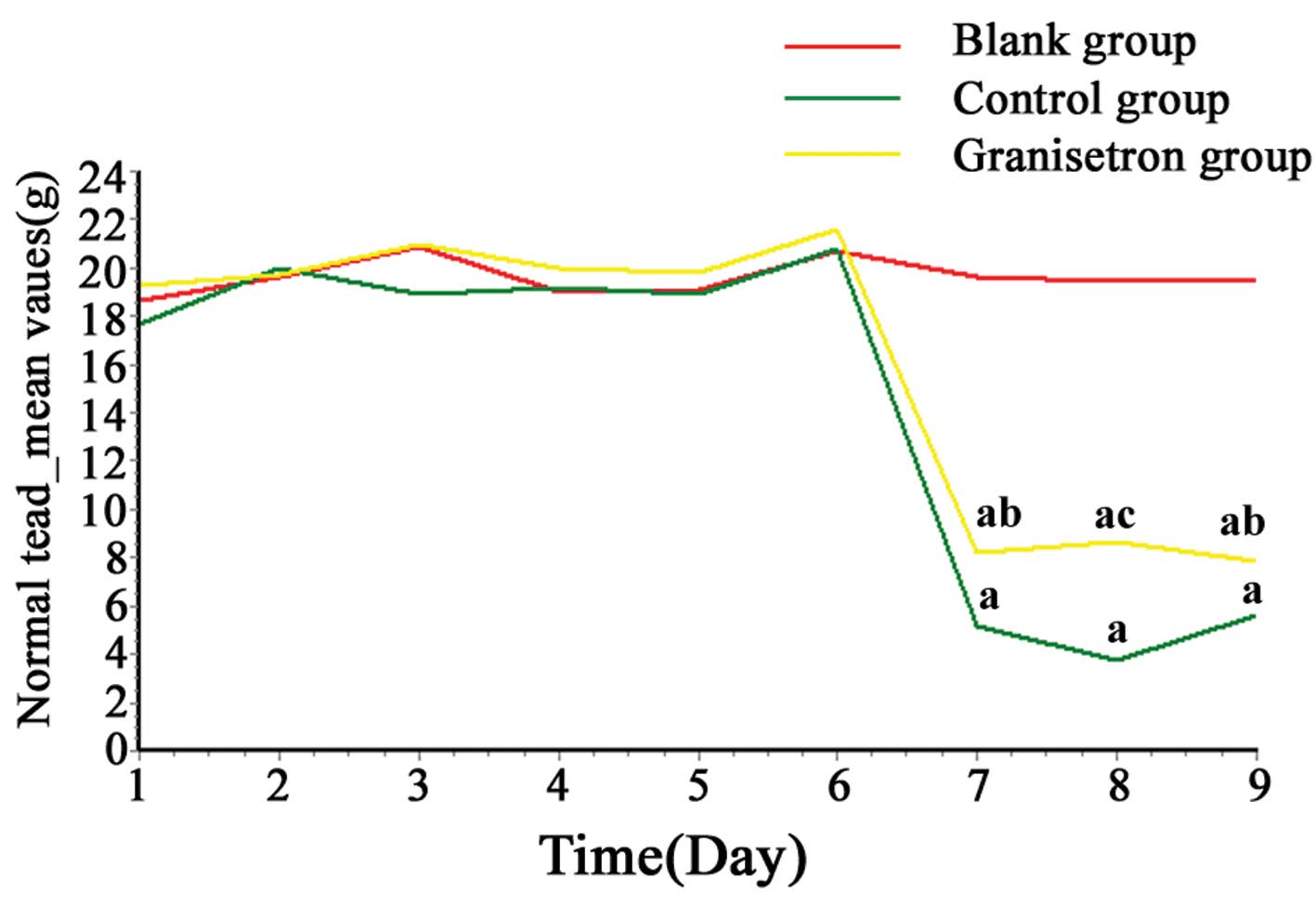
Normal feed consumption in rats
Between days 1 and 6, there was no significant
difference observed in normal feed consumption (P>0.05) between
the three groups. As demonstrated by Table III and Fig. 3, following the cisplatin injection,
normal feed consumption in the control and granisetron groups
decreased significantly, when compared with the blank group at 24,
48 and 72 h (days 7–9; all P<0.01). Furthermore, this decrease
was attenuated by granisetron administration during the whole
experimental period (P<0.05).
 | Table IIINormal feed consumption induced by
administration of cisplatin in rats. |
Table III
Normal feed consumption induced by
administration of cisplatin in rats.
| | Normal feed
consumption (g) |
|---|
| |
|
|---|
| Group | n | 0–24 h | 24–48 h | 48–72 h |
|---|
| Blank | 20 | 19.61±5.56 | 19.49±5.05 | 19.46±5.44 |
| Control | 20 | 5.19±2.15a | 3.76±2.54a | 5.63±2.18a |
| Granisetron | 20 | 8.24±2.99a,b | 8.61±3.18a,c | 7.83±4.76a,b |
Discussion
Cisplatin is a chemotherapeutic agent, which is
limited in its therapeutic administration by the unwanted
side-effect of severe emesis. Cisplatin causes acute and delayed
vomiting, classifying it into the highest emetic risk group,
according to the American Society of Clinical Oncology guidelines
(22). Acute vomiting predominantly
occurs within a few minutes to several hours following cisplatin
administration and commonly resolves within the first 24 h. Delayed
vomiting develops in patients >24 h subsequent to cisplatin
administration and persists for six to seven days (22). Furthermore, it is generally accepted
that acute and delayed vomiting have different mechanisms. Acute
vomiting is predominantly due to the release of serotonin within
the gastrointestinal tract, whilst the delayed phase occurs
following the release of substance P into the brainstem (23,24).
In contrast to species that possess an emetic
reflex, rodents, such as rats exhibit pica in response to stimuli
that induce emesis. Pica is an illness-response behavior, where
consumption of non-nutritive substances, such as kaolin, is a
phenomenon of the emetic reflex (25,26).
Therefore, it has been proposed that pica may be analogous to
emesis and kaolin consumption may be an index by which emesis may
be evaluated in rats (25). It has
been identified that various substances may induce an increase in
kaolin consumption in rats, including oxycodone (27), apomorphine (4), cyclophosphamide, doxorubicin (28), cyclosporine A (29) and cisplatin (12,30,31).
Previous studies in rats have found that in anticancer therapeutic
agent-induced pica, the extent of kaolin intake and the duration of
the altered feeding behavior, are associated with the clinical
emetogenic potential of the therapeutic agent (32). Furthermore, intravenous or i.p.
administration of cisplatin has been shown to induce a
dose-dependent increase in kaolin consumption (28,33,34).
However, the mechanism by which cisplatin induces pica is
complicated and the duration of cisplatin-induced kaolin
consumption remains controversial. It has been reported that
administration of cisplatin at a dosage of 3 or 6 mg/kg
significantly increased kaolin consumption within 0–24 h of the
cisplatin injection, however, this significant increase did not
extend beyond 24 h (8,19,35).
Rudd et al (34), however,
identified that the duration of kaolin consumption was associated
with the dosage of cisplatin. For example, a low dose of cisplatin
(3 mg/kg via i.p. injection) induced kaolin consumption during the
0–24 and 48–72 h periods, whilst the highest dose of cisplatin (6
mg/kg via i.p. injection) only induced kaolin consumption during
the 0–24 h period. By contrast, considerable evidence indicates
that cisplatin may induce a long-term (up to 48 h) increase in pica
in rats (30,34,36–41),
for example Saeki et al (33) reported that cisplatin-induced kaolin
intake was observed for two days following cisplatin
administration. Tatsushima et al (42), Mehendale et al (38) and Aung et al (43) demonstrated that cisplatin-induced
kaolin intake could be induced for up to five days. De Jonghe et
al (44) demonstrated that on
days 1, 2, 4, 5 and 11 following a cisplatin injection, kaolin
intake was significantly higher than in the comparable group, which
was administered with saline; however, this increase significantly
peaked on day 1 (~4 g/day kaolin) compared with the subsequent days
(~1 g/day kaolin) (44). In the
current study, it was identified that kaolin consumption was
markedly enhanced one day after cisplatin administration (6 mg/kg),
although not for more than one day.
The 5-HT3 receptor antagonist,
ondansetron, has been identified as an inhibitor of
cisplatin-induced pica in rodents (11,30).
Saeki et al (33) reported
that although kaolin intake was observed for two days following
cisplatin administration, ondansetron only inhibited pica on the
first day. In the current study, the duration of pica (following
the cisplatin injection) did not exceed one day for the group
administered with cisplatin (the control group), however, similar
to ondansetron, granisetron inhibited cisplatin-induced pica on the
first day only (the granisetron group). Together, these results
indicate that kaolin consumption is the most appropriate method for
evaluating acute (rather than delayed) emesis as an index of
cisplatin-induced emesis, and that kaolin intake is only affected
by the 5-HT3 receptor antagonist on day 1, which is
consistent with the mechanism of acute emesis.
In animals, the combination of the taste and odor of
the majority of foods provides a strong conditioned stimulus that
can be utilised to elicit an appropriate response to any harmful
unconditioned stimuli that may follow (45). CTA is, therefore, a behavioral
response, which is essential to the survival of an individual.
Drinkable sweet solutions, such as saccharin and sucrose, are the
most widely used conditioned stimuli (46). Previously, CTA of rats was commonly
used in studies of motion sickness (14,47).
Emetic agents, such as lithium chloride, have been
shown to induce taste aversion in a diverse range of species
(48,49), thus, lithium chloride has been used
in hundreds of studies regarding CTA learning in rats (50,51).
Previous investigations have also demonstrated that apomorphine,
amphetamine and ethanol induce CTA in rats (52–54).
There have, however, been few investigations of cisplatin-induced
CTA in rats. Certain studies indicated that cisplatin was able to
induce CTA in rats at doses that were known to induce emesis in
other species; however, the responses were attenuated by
dexamethasone and the rats were resistant to treatment with the
5-HT3 receptor antagonists (15,16),
providing evidence that the 5-HT3 receptor antagonist is
not involved in CTA mechanisms in rats (55). In the current study, considerable
evidence indicated that rats were able to learn to avoid a taste,
as saccharin solution consumption was greatly decreased two days
subsequent to cisplatin administration. The current study did not
observe an inhibitory effect of granisetron in CTA beyond 24 h,
therefore, it was presumed that the 5-HT3 receptor
antagonist was involved in the CTA mechanisms in the acute, but not
in the delayed, emesis phase. Incidence of CTA in the delayed
emesis phase may be due to other reasons, which are consistent with
the mechanisms of delayed emesis. Furthermore, the duration of CTA
indicates that saccharin solution consumption is effective in the
evaluation of delayed emesis.
Anorexia nervosa is one of the most detrimental
gastrointestinal side-effects associated with chemotherapy and is,
therefore, used as an index of patient quality of life. A previous
study showed that rats exhibit anorexic behavior in response to the
administration of anticancer therapeutic agents and that the
incidence of anorexia nervosa is not associated with the emetogenic
potential of the rats (32).
Therefore, the intake of normal feed was examined as an indicator
of the emetic stimulus in rats (30,56,57).
In cisplatin-treated rats, intracerebroventricular ghrelin
administration (17), common
hepatic branch vagotomy (1) and
ondansetron (30) attenuated the
decrease in food intake. In the current study, it was found that
normal feed consumption decreased until at least the third day
following cisplatin administration. The present study also
indicated that reduced normal feed intake was attenuated by
administration of the 5-HT3 receptor antagonist,
granisetron during the whole experimental period. In conclusion,
the results of the present study demonstrated that anorexia nervosa
may be used to evaluate various phases of emesis, including the
acute and delayed phases, and is a particularly important index of
patient quality of life.
Acknowledgements
The author would like to thank Professor Wei
Pin-kang, Department of Traditional Chinese Medicine, Shanghai
Changzheng Hospital for his technical assistance and The Shanghai
Slac Laboratory Animal Company Ltd. (Shanghai, China) for providing
the animals.
References
|
1
|
Rudd JA, Jordan CC and Naylor RJ: The
action of the NK1 tachykinin receptor antagonist, CP 99,994, in
antagonizing the acute and delayed emesis induced by cisplatin in
the ferret. Br J Pharmacol. 119:931–936. 1996.
|
|
2
|
Gardner CJ, Armour DR, Beattie DT, et al:
GR205171: a novel antagonist with high affinity for the tachykinin
NK1 receptor, and potent broad-spectrum anti-emetic activity. Regul
Pept. 65:45–53. 1996.
|
|
3
|
Rudd JA and Naylor RJ: An interaction of
ondansetron and dexamethasone antagonizing cisplatin-induced acute
and delayed emesis in the ferret. Br J Pharmacol. 118:209–214.
1996.
|
|
4
|
Takeda N, Hasegawa S, Morita M and
Matsunaga T: Pica in rats is analogous to emesis: an animal model
in emesis research. Pharmacol Biochem Behav. 45:817–821. 1993.
|
|
5
|
De Jonghe BC and Horn CC:
Chemotherapy-induced pica and anorexia are reduced by common
hepatic branch vagotomy in the rat. Am J Physiol Regul Integr Comp
Physiol. 294:R756–R765. 2008.
|
|
6
|
Garcia J, Hankins WG and Rusiniak KW:
Behavioral regulation of the milieu interne in man and rat.
Science. 185:824–831. 1974.
|
|
7
|
Liu Y, Hamaue N, Endo T, Hirafuji M and
Minami M: 5-hydroxytryptamine (5-HT) concentrations in the
hippocampus, the hypothalamus and the medulla oblongata related to
cisplatin-induced pica of rats. Res Commun Mol Pathol Pharmacol.
113–114:97–113. 2003.
|
|
8
|
Horn CC, De Jonghe BC, Matyas K and
Norgren R: Chemotherapy-induced kaolin intake is increased by
lesion of the lateral parabrachial nucleus of the rat. Am J Physiol
Regul Integr Comp Physiol. 297:R1375–R1382. 2009.
|
|
9
|
Saito R and Takano Y: Easy method for
emesis using rats. Nihon Yakurigaku Zasshi. 127:461–466. 2006.(In
Japanese).
|
|
10
|
Mehendale SR, Aung HH, Yin JJ, et al:
Effects of antioxidant herbs on chemotherapy-induced nausea and
vomiting in a rat-pica model. Am J Chin Med. 32:897–905. 2004.
|
|
11
|
Yamamoto K, Matsunaga S, Matsui M, Takeda
N and Yamatodani A: Pica in mice as a new model for the study of
emesis. Methods Find Exp Clin Pharmacol. 24:135–138. 2002.
|
|
12
|
Takeda N, Hasegawa S, Morita M, et al:
Neuropharmacological mechanisms of emesis. II. Effects of
antiemetic drugs on cisplatin-induced pica in rats. Methods Find
Exp Clin Pharmacol. 17:647–652. 1995.
|
|
13
|
Liu YL, Malik N, Sanger GJ, Friedman MI
and Andrews PL: Pica - a model of nausea? Species differences in
response to cisplatin. Physiol Behav. 85:271–277. 2005.
|
|
14
|
Cai YL, Ma WL, Li M, et al: Behavioral
changes of rats under rotation stimulation. Space Med Med Eng
(Beijing). 18:98–101. 2005.(In Chinese).
|
|
15
|
Rudd JA, Ngan MP and Wai MK: 5-HT3
receptors are not involved in conditioned taste aversions induced
by 5-hydroxytryptamine, ipecacuanha or cisplatin. Eur J Pharmacol.
352:143–149. 1998.
|
|
16
|
Mele PC, McDonough JR, McLean DB and
O’Halloran KP: Cisplatin-induced conditioned taste aversion:
attenuation by dexamethasone but not zacopride or GR38032F. Eur J
Pharmacol. 218:229–236. 1992.
|
|
17
|
Yakabi K, Sadakane C, Noguchi M, et al:
Reduced ghrelin secretion in the hypothalamus of rats due to
cisplatin-induced anorexia. Endocrinology. 151:3773–3782. 2010.
|
|
18
|
Ando K, Takagi K and Tsubone H: Enhanced
gastric retention of solid resin beads as a marker for emetic
potential of agents in rats. J Toxicol Sci. 37:549–553. 2012.
|
|
19
|
Cabezos PA, Vera G, Castillo M, et al:
Radiological study of gastrointestinal motor activity after acute
cisplatin in the rat. Temporal relationship with pica. Auton
Neurosci. 141:54–65. 2008.
|
|
20
|
Mitchell D, Wells C, Hoch N, et al: Poison
induced pica in rats. Physiol Behav. 17:691–697. 1976.
|
|
21
|
Aung HH, Mehendale SR, Xie JT, Moss J and
Yuan CS: Methylnaltrexone prevents morphine-induced kaolin intake
in the rat. Life Sci. 74:2685–2691. 2004.
|
|
22
|
Kris MG, Hesketh PJ, Somerfield MR, et al;
American Society of Clinical Oncology. American Society of Clinical
Oncology guideline for antiemetics in oncology: update 2006. J Clin
Oncol. 24:2932–2947. 2006.
|
|
23
|
Rojas C, Li Y, Zhang J, et al: The
antiemetic 5-HT3 receptor antagonist Palonosetron inhibits
substance P-mediated responses in vitro and in vivo. J Pharmacol
Exp Ther. 335:362–368. 2010.
|
|
24
|
Darmani NA, Crim JL, Janoyan JJ, Abad J
and Ramirez J: A re-evaluation of the neurotransmitter basis of
chemotherapy-induced immediate and delayed vomiting: evidence from
the least shrew. Brain Res. 1248:40–58. 2009.
|
|
25
|
Yamamoto K, Ngan MP, Takeda N, Yamatodani
A and Rudd JA: Differential activity of drugs to induce emesis and
pica behavior in Suncus murinus (house musk shrew) and rats.
Physiol Behav. 83:151–156. 2004.
|
|
26
|
Vera G, Chiarlone A, Cabezos PA, et al:
WIN 55,212-2 prevents mechanical allodynia but not alterations in
feeding behaviour induced by chronic cisplatin in the rat. Life
Sci. 81:468–479. 2007.
|
|
27
|
Batra VR and Schrott LM: Acute oxycodone
induces the pro-emetic pica response in rats. J Pharmacol Exp Ther.
339:738–745. 2011.
|
|
28
|
Jeong SW, Cho JW, Hwang JS, et al: The
antiemetic effect of a novel tropisetron patch in anticancer
agents-induced kaolin pica model using rats. Environ Toxicol
Pharmacol. 20:167–174. 2005.
|
|
29
|
Fujisaki Y, Yamauchi A, Shuto H, et al:
Pharmacological characterization of cyclosporine A-induced kaolin
intake in rats. Pharmacol Biochem Behav. 70:267–271. 2001.
|
|
30
|
Malik NM, Liu YL, Cole N, Sanger GJ and
Andrews PL: Differential effects of dexamethasone, ondansetron and
a tachykinin NK1 receptor antagonist (GR205171) on
cisplatin-induced changes in behaviour, food intake, pica and
gastric function in rats. Eur J Pharmacol. 555:164–173. 2007.
|
|
31
|
Vera G, Chiarlone A, Martín MI and Abalo
R: Altered feeding behaviour induced by long-term cisplatin in
rats. Auton Neurosci. 126–127:81–92. 2006.
|
|
32
|
Yamamoto K, Nakai M, Nohara K and
Yamatodani A: The anti-cancer drug-induced pica in rats is related
to their clinical emetogenic potential. Eur J Pharmacol. 554:34–39.
2007.
|
|
33
|
Saeki M, Sakai M, Saito R, et al: Effects
of HSP-117, a novel tachykinin NK1-receptor antagonist, on
cisplatin-induced pica as a new evaluation of delayed emesis in
rats. Jpn J Pharmacol. 86:359–362. 2001.
|
|
34
|
Rudd JA, Yamamoto K, Yamatodani A and
Takeda N: Differential action of ondansetron and dexamethasone to
modify cisplatin-induced acute and delayed kaolin consumption
(‘pica’) in rats. Eur J Pharmacol. 454:47–52. 2002.
|
|
35
|
Cabezos PA, Vera G, Martín-Fontelles MI,
Fernández-Pujol R and Abalo R: Cisplatin-induced gastrointestinal
dysmotility is aggravated after chronic administration in the rat.
Comparison with pica. Neurogastroenterol Motil. 22:797–805.
2010.
|
|
36
|
Horn CC, Ciucci M and Chaudhury A: Brain
Fos expression during 48 h after cisplatin treatment: neural
pathways for acute and delayed visceral sickness. Auton Neurosci.
132:44–51. 2007.
|
|
37
|
Wang CZ, Basila D, Aung HH, et al: Effects
of Ganoderma lucidum extract on chemotherapy-induced nausea
and vomiting in a rat model. Am J Chin Med. 33:807–815. 2005.
|
|
38
|
Mehendale S, Aung H, Wang A, et al:
American ginseng berry extract and ginsenoside Re attenuate
cisplatin-induced kaolin intake in rats. Cancer Chemother
Pharmacol. 56:63–69. 2005.
|
|
39
|
Du J, Li P, Sun X and Zhang M:
Ameliorative effect of Armillariella tabescens on
cisplatin-induced gastrointestinal tract reaction in the rat.
Zhonghua Zhong Liu Za Zhi. 33:579–582. 2011.(In Chinese).
|
|
40
|
Qian Q, Chen W, Guo C, et al:
Xiao-Ban-Xia-Tang inhibits cisplatin-induced pica by down
regulating obestatin in rats. J Ethnopharmacol. 135:186–193.
2011.
|
|
41
|
Wang CZ, Fishbein A, Aung HH, et al:
Polyphenol contents in grape-seed extracts correlate with antipica
effects in cisplatin-treated rats. J Altern Complement Med.
11:1059–1065. 2005.
|
|
42
|
Tatsushima Y, Egashira N, Matsushita N, et
al: Pemirolast reduces cisplatin-induced kaolin intake in rats. Eur
J Pharmacol. 661:57–62. 2011.
|
|
43
|
Aung HH, Dey L, Mehendale S, et al:
Scutellaria baicalensis extract decreases cisplatin-induced
pica in rats. Cancer Chemother Pharmacol. 52:453–458. 2003.
|
|
44
|
De Jonghe BC, Lawler MP, Horn CC and
Tordoff MG: Pica as an adaptive response: Kaolin consumption helps
rats recover from chemotherapy-induced illness. Physiol Behav.
97:87–90. 2009.
|
|
45
|
Cairnie AB and Leach KE: Dexamethasone: a
potent blocker for radiation-induced taste aversion in rats.
Pharmacol Biochem Behav. 17:305–311. 1982.
|
|
46
|
Geerse GJ, van Gurp LC, van Wijk DC,
Wiegant VM and Stam R: Duodenal pain and spinal morphine induce
conditioned taste aversion in rats. Physiol Behav. 91:310–317.
2007.
|
|
47
|
Wu XW, Xin B, Zou JF, et al: Effect of
rotation stimulation on the anesthetic sensitivity of sevoflurane
in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 28:114–117.
2012.(In Chinese).
|
|
48
|
Garcia J, Rusiniak KW and Brett LP:
Conditioning food-illness aversions in wild animals: Caveant
canonici. Operant-Pavlovian Interactions. Davis H and Hurwitz H:
Lawrence Erlbaum Associates; Hillsdale, NJ: pp. 45–86. 1977
|
|
49
|
Smith JE, Friedman MI and Andrews PL:
Conditioned food aversion in Suncus murinus (house musk
shrew) - a new model for the study of nause in a species with an
emetic reflex. Physiol Behav. 73:593–598. 2001.
|
|
50
|
Cross-Mellor SK, Ossenkopp KP, Piomelli D
and Parker LA: Effects of the FAAH inhibitor, URB597, and
anandamide on lithium-induced taste reactivity responses: a measure
of nausea in the rat. Psychopharmacology (Berl). 190:135–143.
2007.
|
|
51
|
Riley AL and Freeman KB: Conditioned taste
aversion: a database. Pharmacol Biochem Behav. 77:655–656.
2004.
|
|
52
|
McAllister KH and Pratt JA: GR205171
blocks apomorphine and amphetamine-induced conditioned taste
aversions. Eur J Pharmacol. 353:141–148. 1998.
|
|
53
|
Anderson RI, Agoglia AE, Morales M,
Varlinskaya EI and Spear LP: Stress, κ manipulations, and aversive
effects of ethanol in adolescent and adult male rats. Neuroscience.
249:214–222. 2013.
|
|
54
|
Shinpo K, Hirai Y, Maezawa H, Totsuka Y
and Funahashi M: The role of area postrema neurons expressing
H-channels in the induction mechanism of nausea and vomiting.
Physiol Behav. 107:98–103. 2012.
|
|
55
|
Bienkowski P, Kuca P, Piasecki J and
Kostowski W: 5-HT3 receptor antagonist, tropisetron, does not
influence ethanol-induced conditioned taste aversion and
conditioned place aversion. Alcohol. 14:63–69. 1997.
|
|
56
|
Raghavendran HR, Rekha S, Shin JW, et al:
Effects of Korean ginseng root extract on cisplatin-induced emesis
in a rat-pica model. Food Chem Toxicol. 49:215–221. 2011.
|
|
57
|
Liu YL, Malik NM, Sanger GJ and Andrews
PL: Ghrelin alleviates cancer chemotherapy-associated dyspepsia in
rodents. Cancer Chemother Pharmacol. 58:326–333. 2006.
|