Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer and the third leading cause of cancer-related
mortality worldwide (1). The
majority of cases arise in Asia and Africa, particularly in China
(2). Despite the extensive
application of intensive surveillance programs implemented over the
past few years, numerous patients are not diagnosed until the
disease is at an advanced stage, such that only palliative
treatment options are available.
Sorafenib, an orally active multikinase inhibitor,
is recommend as the standard treatment for advanced HCC [Barcelona
clinic liver cancer (BCLC) stage C] in western countries (3). A randomized controlled trial has
confirmed that sorafenib can prolong the median overall survival
(OS) compared with placebo in patients with advanced HCC (4). A further study confirmed the benefit
of sorafenib for patients from the Asia-Pacific region (5). However, the survival among patients
with advanced HCC remained modest, and the local tumor control rate
was low.
Transarterial chemoembolization (TACE) is the
standard treatment for intermediate-stage HCC (3). This procedure has a high local tumor
control rate and has been observed to enhance survival in patients
with intermediate HCC (6–8). However, the hypoxia caused by TACE in
surviving tumor cells leads to the release of angiogenic growth
factors, which contribute to tumor recurrence or metastases and a
poorer outcome (9,10). Sorafenib blocks tumor cell
proliferation by targeting Raf/MEK/ERK signaling at the Raf kinase
level, and exerts an antiangiogenic effect by targeting vascular
endothelial growth factor receptor-2 and -3, and platelet-derived
growth factor receptor-β tyrosine kinases (11). Therefore, the combination of TACE
with sorafenib may provide a benefit for patients with HCC. A
number of studies have reported that TACE combined with sorafenib
significantly prolonged the median OS time or time to progression
(TTP) for patients with unresectable HCC (12–14).
The majority of the patients included in these studies had
intermediate-stage HCC. TACE may also be used in advanced HCC with
conserved liver function (15). To
date, limited data has focused on the combination of TACE with
sorafenib for advanced hepatocellular carcinoma. The aim of the
current study was to investigate the OS and side effects of the
combination therapy with TACE and sorafenib in patients with
advanced HCC.
Patients and methods
Patient characteristics
This retrospective study was approved by the
Institutional Review Board of Shandong Cancer Hospital and
Institute (Jinan, China). A review of patients with advanced HCC
treated with TACE prior to sorafenib administration in Shandong
Cancer Hospital and Institute was undertaken. All patients were
diagnosed by histology, cytology or persistently elevated serum
α-fetoprotein (AFP) levels (>400 ng/ml; normal range, 0–7 ng/ml)
with typical imaging findings. All patients were staged according
to the BCLC staging classification (3). All patients had been previously
treated with at least one TACE session prior to sorafenib
administration. Written informed consent was obatined from all
patients.
Transarterial chemoembolization
TACE was performed according to the traditional
method (16). Chemotherapeutic
agents, 100–200 mg oxaliplatin and 750–1,000 mg fluorouracil
glycosides, were infused into feeding arteries of the tumor;
subsequently, 5–20 ml Lipiodol mixed with 30 mg epirubucin was
infused into the feeding arteries at a rate of 1 ml/min until
stasis flow in the tumor vascularity was achieved. The use of a
gelatin sponge was not required, as determined by the
interventional radiologist.
Sorafenib treatment
Sorafenib was administered 7–30 days following the
final TACE treatment. Patients received 400 mg sorafenib twice
daily at the beginning of treatment. A dose reduction from 400 to
200 mg of sorafenib twice daily was permitted when drug-related
adverse events were observed. When the adverse events subsided, the
decision to resume treatment with 400 mg sorafenib twice daily was
discussed between the patient and the physician, but was ultimately
made by the physician. On the observation of progressive disease,
the decision to continue treatment was discussed between the
patient and the physician, but was ultimately made by the patient.
If treatment was continued, informed consent was required, and
treatment with sorafenib would be maintained until a deterioration
in the Child-Pugh score to C or ECOG performance status (PS) score
to 4 was observed, or until the occurrence of intolerable adverse
events or mortality.
Follow-up
All patients were monitored monthly for the
occurrence of side effects, ECOG PS classification and Child-Pugh
score evaluation. The follow-up of survival was discontinued on
March 31, 2013, and patients that remained alive were censored at
that time point. The side effects of sorafenib were reported
according to the National Cancer Institute’s Common Terminology
Criteria for Adverse Events (version 3.0) (17).
Statistical analysis
The OS refers to the time between the first
administration of sorafenib to mortality by any cause or the final
follow-up. Survival analysis was estimated by the Kaplan-Meier
survival method. The Cox proportional-hazards regression model was
used to assess factors that were independently prognostic of OS.
Statistical analysis was performed with SPSS version 16.0 software
(SPSS Inc., Chicago, IL, USA).
Results
Characteristics of patients and
disease
In total, 38 patients were included in the study
between April 1st, 2009 and March 31st, 2012. The characteristics
of the 38 patients and their diseases including age, gender, ECOG
PS, Child-Pugh score and tumor size, as well as the presence of
portal vein thrombosis and/or metastasis are summarized in Table I.
 | Table IBaseline characteristics of the study
population. |
Table I
Baseline characteristics of the study
population.
| Patient
characteristics | n | % |
|---|
| Age, yearsa | 53.4 (35–68) |
| Gender |
| Male | 37 | 97.4 |
| Female | 1 | 2.6 |
| ECOG performance
status |
| 0 | 36 | 94.7 |
| 1 | 2 | 5.3 |
| Child-Pugh score |
| A | 32 | 84.2 |
| B | 6 | 15.8 |
| Etiology |
| Hepatisis B
virus | 38 | 100.0 |
| BCLC status |
| C | 38 | 100.0 |
| Diameter of main
tumor nodule, cma | 5.6 (3–10) |
| Portal vein
thrombosis | 26 | 68.4 |
| Distant
metastasis | 20 | 52.6 |
| Sessions of TACE
prior to sorafeniba | 4.3 (1–7) |
| Sessions of TACE post
sorafeniba | 0.9 (0–2) |
Survival
All 38 patients were subjected to follow-up. Seven
patients remained on sorafenib on March 31st, 2013, and were
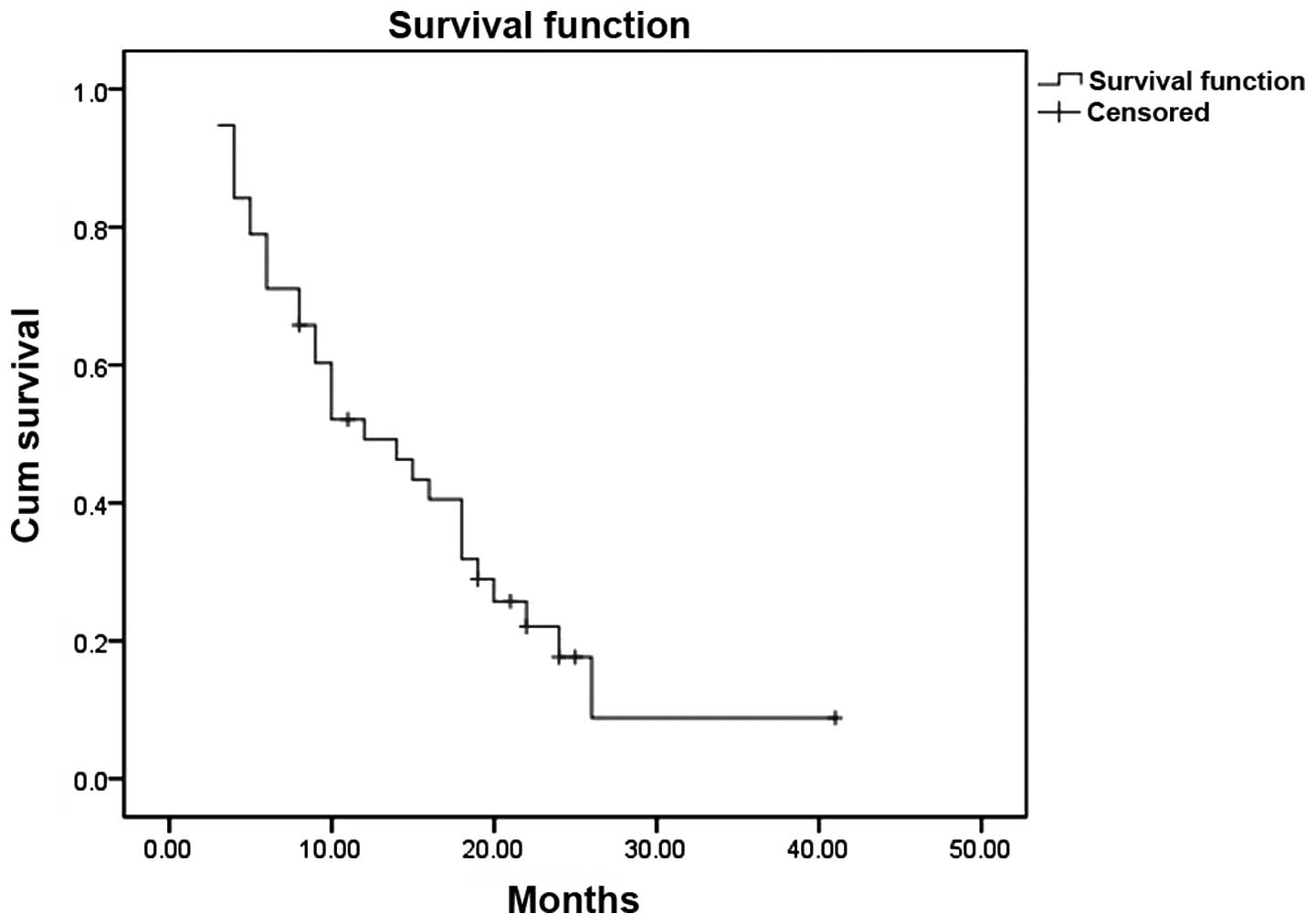
censored at that time point. The median OS time was 12 months (95%
confidence interval, 6.3–17.7 months) (Fig. 1). Compared with extrahepatic spread,
the Cox proportional-hazards regression model indicated that portal
vascular invasion was independently prognostic of OS.
Side effects
All patients experienced various toxicities at the
end of the first month of sorafenib treatment. The most common
toxicities were dermatological adverse effects (94.7%), diarrhea
(63.2%) and alopecia (26.3%). No grade 3 or 4 adverse events were
observed. At the end of month two, the sorafenib dose was reduced
to 200 mg twice daily in 32 patients due to toxicity. By the end of
month four, the majority of toxicities had been relieved following
suitable treatment and dose adjustment. Two patients resumed
treatment with 400 mg sorafenib twice daily and no repeated
aggravation of the toxicities was observed; however, the majority
of patients had to balance the repeated adverse effects and the
dose adjustment of sorafenib.
Discussion
TACE is the standard treatment for
intermediate-stage HCC according to the BCLC staging classification
(3). However, in Asia, the role of
TACE is further extended to include the treatment of advanced HCC.
The current study demonstrated that the combination treatment with
TACE and sorafenib led to a median OS time of 12 months for
hepatitis B virus-related advanced HCC. Compared with the OS time
of the Sorafenib Asia-Pacific (6.5 months) and SHARP trials (10.7
months), which used sorafenib monotherapy for untreated advanced
HCC (4,5), the survival benefit of sorafenib in
the current study is promising. This positive result may be due to
TACE treatment reducing the tumor burden by blocking angiogenesis
and killing tumor cells through the anticancer agents present,
prior to sorafenib administration. Repeated TACE may improve local
tumor control, however, it may also worsen liver function;
therefore, further treatment with TACE must be avoided if no
hypervascularity is observed. In the current study, the mean number
of TACE sessions prior to and following sorafenib administration
were 4.3 and 0.9, respectively. The reduced frequency of TACE
following, as compared with prior to, sorafenib administration, may
be due to the antiangiogenic effects of sorafenib.
With regard to safety, the current study
demonstrated that sorafenib administration following TACE could be
tolerated in patients with advanced HCC, provided that dose
adjustment was permitted. The adverse events were deemed to be
predominantly sorafenib-related, as sorafenib was administered
following the completion of the TACE session. This was in
concordance with the results observed in western patients. However,
on comparison, the profile of adverse events differed to those
reported in western patients. In the study by Pawlick et al
(18), the most common toxicities
were fatigue (94%), anorexia (67%), alteration in liver enzymes
(64%) and dermatological adverse effects (48%), whilst in the
current study, the most common toxicities were dermatological
adverse effects (94.7%), diarrhea (63.2%) and alopecia (26.3%). %).
Fewer patients complained of fatigue and more hand-foot skin
reactions occurred in the present study. Additionally, no grade 3
or 4 toxicities were identified in our study, which may have been
due to the high-rate of sorafenib dose reduction. In the current
study, 84.2% of patients reduced the dose of sorafenib in month
two, while Dufour et al (19) reported that the dose of sorafenib
was reduced in only 21.4% of patients. The majority of toxicities
were relieved following the dose reduction and were not aggravated
further, even the dose of sorafenib was increased to 400 mg twice
daily. This also indicated that sorafenib was well tolerated in
patients with HCC following TACE treatment.
One aspect of the current study that must be
considered is that all patients were treated with sorafenib,
despite radiographic progression, until a deterioration was
observed in the patient Child-Pugh score to C or the ECOG PS score
to 4, or until the occurrence of intolerable adverse events or
mortality. This differs from the study by Sansonno et al
(14), in which sorafenib
administration was discontinued in the instance of disease
progression. With informed consent, sorafenib treatment was
continued in the current study following disease progression, due
to the lack of secondary treatment options for advanced HCC and the
assumed survival benefit. However, it is uncertain whether the
benefit of sorafenib remains following disease progression;
therefore, further investigation is required. Furthermore, for
advanced HCC patients treated with sorafenib following TACE, portal
vascular invasion was identified as an independent risk factor,
while extrahepatic spread was not an independent risk factor. This
implies that for advanced HCC, patients who do not exhibit portal
vascular invasion would be more likely to benefit from sorafenib as
adjuvant therapy following TACE.
Three strategies have been identified for the
combination of sorafenib with TACE: The sequential approach, the
interrupted approach and the continuous approach. Certain studies
have reported that TACE combined with sorafenib in an interrupted
or continuous approach significantly prolonged the median OS time
(12,13). However, the outcome of the
sequential approach for sorafenib combined with TACE is
controversial. Kudo et al (20) reported that sorafenib did not
significantly prolong TTP or OS in patients with unresectable HCC
who responded to TACE. Conversely, Sansonno et al (14) reported that a conventional TACE
procedure followed by sorafenib treatment resulted in a
significantly longer TTP in patients with intermediate-stage
hepatitis C virus-related HCC. In the sequential approach,
sorafenib is used as an adjuvant therapy when the TACE sessions
have been completed. The results from the current study indicate
that sorafenib as an adjuvant therapy following TACE holds promise
as a useful strategy for the treatment of patients with advanced
HCC.
In conclusion, treatment with sorafenib combined
with TACE exhibited a median survival time of 12 months in patients
with advanced HCC. The Cox proportional-hazards regression model
indicated that portal vascular invasion was independently
prognostic of OS. The survival benefit of sorafenib combined with
TACE for advanced HCC is promising, with no intolerable adverse
events, providing that dose adjustment is permitted.
References
|
1
|
Kudo M: The 2008 Okuda lecture: Management
of hepatocellular carcinoma: from surveillance to molecular
targeted therapy. J Gastroenterol Hepatol. 25:439–452. 2010.
|
|
2
|
Yuen MF, Hou JL and Chutaputti A: Asia
Pacific Working Party on Prevention of Hepatocellular Carcinoma:
Hepatocellular carcinoma in the Asia pacific region. J
Gastroenterol Hepatol. 24:346–353. 2009.
|
|
3
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: an update. Hepatology. 53:1020–1022.
2011.
|
|
4
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, et al: SHARP Investigators Study Group:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008.
|
|
5
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, et al: Efficacy and safety of sorafenib in patients in the
Asia-Pacific region with advanced hepatocellular carcinoma: a phase
III randomised, double-blind, placebo-controlled trial. Lancet
Oncol. 10:25–34. 2009.
|
|
6
|
Cheng HY, Wang X, Chen D, Xu AM and Jia
YC: The value and limitation of transcatheter arterial
chemoembolization in preventing recurrence of resected
hepatocellular carcinoma. World J Gastroenterol. 11:3644–3646.
2005.
|
|
7
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, et al; Barcelona Liver Cancer Group. Arterial
embolisation or chemoembolisation versus symptomatic treatment in
patients with unresectable hepatocellular carcinoma: a randomised
controlled trial. Lancet. 359:1734–1739. 2002.
|
|
8
|
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM,
Poon RT, et al: Randomized controlled trial of transarterial
lipiodol chemoembolization for unresectable hepatocellular
carcinoma. Hepatology. 35:1164–1171. 2002.
|
|
9
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, et al: Transcatheter arterial
chemoembolization (TACE) in hepatocellular carcinoma (HCC): the
role of angiogenesis and invasiveness. Am J Gastroenterol.
103:914–921. 2008.
|
|
10
|
Shim JH, Park JW, Kim JH, An M, Kong SY,
Nam BH, et al: Association between increment of serum VEGF level
and prognosis after transcatheter arterial chemoembolization in
hepatocellular carcinoma patients. Cancer Sci. 99:2037–2044.
2008.
|
|
11
|
Wilhelm SM, Adnane L, Newell P, et al:
Preclinical overview of sorafenib, a multikinase inhibitor that
targets both Raf and VEGF and PDGF receptor tyrosine kinase
signaling. Mol Cancer Ther. 7:3129–3140. 2008.
|
|
12
|
Cabrera R, Pannu DS, Caridi J, Firpi RJ,
Soldevila-Pico C, Morelli G, et al: The combination of sorafenib
with transarterial chemoembolisation for hepatocellular carcinoma.
Aliment Pharmacol Ther. 34:205–213. 2011.
|
|
13
|
Qu XD, Chen CS, Wang JH, Yan ZP, Chen JM,
Gong GQ, et al: The efficacy of TACE combined sorafenib in advanced
stages hepatocellullar carcinoma. BMC Cancer. 12:2632012.
|
|
14
|
Sansonno D, Lauletta G, Russi S, Conteduca
V, Sansonno L and Dammacco F: Transarterial chemoembolization plus
sorafenib: a sequential therapeutic scheme for HCV-related
intermediate-stage hepatocellular carcinoma: a randomized clinical
trial. Oncologist. 17:359–366. 2012.
|
|
15
|
Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY,
Chen MS and Shi M: Transarterial chemoembolization for unresectable
hepatocellular carcinoma with portal vein tumor thrombosis: a
prospective comparative study. Ann Surg Oncol. 18:413–420.
2011.
|
|
16
|
Guan YS, He Q and Wang MQ: Transcatheter
arterial chemoembolization: history for more than 30 years. ISRN
Gastroenterol. 2012:4806502012.
|
|
17
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003.
|
|
18
|
Pawlik TM, Reyes DK, Cosgrove D, Kamel IR,
Bhagat N and Geschwind JF: Phase II trial of sorafenib combined
with concurrent transarterial chemoembolization with drug-eluting
beads for hepatocellular carcinoma. J Clin Oncol. 29:3960–3967.
2011.
|
|
19
|
Dufour JF, Hoppe H, Heim MH, Helbling B,
Maurhofer O, Szucs-Farkas Z, et al: Continuous administration of
sorafenib in combination with transarterial chemoembolization in
patients with hepatocellular carcinoma: results of a phase I study.
Oncologist. 15:1198–1204. 2010.
|
|
20
|
Kudo M, Imanaka K, Chida N, Nakachi K, Tak
WY, Takayama T, et al: Phase III study of sorafenib after
transarterial chemoembolisation in Japanese and Korean patients
with unresectable hepatocellular carcinoma. Eur J Cancer.
47:2117–2127. 2011.
|